Pharmacotherapy Relapse Prevention in Body Dysmorphic Disorder: A Double-Blind, Placebo-Controlled Trial
Abstract
Objective:
Body dysmorphic disorder is common, distressing, and often severely impairing. Serotonin reuptake inhibitors appear efficacious, but the few existing pharmacotherapy studies were short term (≤4 months), and no relapse prevention studies or continuation phase studies have been conducted to the authors’ knowledge. The authors report results from the first relapse prevention study in body dysmorphic disorder.
Method:
Adults (N=100) with DSM-IV body dysmorphic disorder received open-label escitalopram for 14 weeks (phase 1); 58 responders were then randomized to double-blind continuation treatment with escitalopram versus switch to placebo for 6 months (phase 2). Reliable and valid outcome measures were utilized.
Results:
In phase 1, 67.0% of treated subjects and 81.1% of subjects who completed phase 1 responded to escitalopram. Body dysmorphic disorder severity (in both the intent-to-treat and the completer groups) and insight, depressive symptoms, psychosocial functioning, and quality of life significantly improved from baseline to end of phase 1. In phase 2, time to relapse was significantly longer with escitalopram than with placebo treatment (hazard ratio=2.72, 95% CI=1.01–8.57). Phase 2 relapse proportions were 18% for escitalopram and 40% for placebo. Among escitalopram-treated subjects, body dysmorphic disorder severity significantly decreased over time during the continuation phase, with 35.7% of subjects showing further improvement. There were no significant group differences in body dysmorphic disorder severity or insight, depressive symptoms, psychosocial functioning, or quality of life.
Conclusions:
Continuation-phase escitalopram delayed time to relapse, and fewer escitalopram-treated subjects relapsed than did placebo-treated subjects. Body dysmorphic disorder severity significantly improved during 6 additional months of escitalopram treatment following acute response; more than one-third of escitalopram-treated subjects experienced further improvement.
Body dysmorphic disorder, an often severe disorder, consists of distressing or impairing preoccupations with nonexistent or slight defects in appearance (e.g., “scarred” skin), plus repetitive behaviors performed in response to appearance concerns (e.g., mirror checking, excessive grooming). Body dysmorphic disorder has a prevalence of 1.7%−2.4% (1–4), causes substantial impairment in psychosocial functioning (5), and is associated with high rates of suicidality (6).
However, few pharmacotherapy studies have been conducted in body dysmorphic disorder, and all contained relatively small sample sizes (in the largest study, 34 patients received active medication [7]). Only one double-blind, placebo-controlled trial (N=67) has been done (7), which reported that fluoxetine was more efficacious than placebo. In a blinded crossover trial (N=29), the serotonin reuptake inhibitor (SRI) clomipramine was more efficacious than desipramine (8). Four open-label trials (Ns of 15 to 30) also found that selective serotonin reuptake inhibitors (SSRIs) are often efficacious (9–12). To our knowledge, no studies have prospectively examined the risk of relapse following discontinuation of efficacious medication or have examined medication response during continuation treatment (following acute treatment). Such studies are needed because many patients with body dysmorphic disorder receive pharmacotherapy (most often SRIs) (13) and because the disorder is often chronic, necessitating longer-term treatment (13).
Two studies that examined relapse after SRI treatment were limited by small samples, lack of placebo, unblinded evaluation of outcome, and imprecise definition of relapse (using only the Clinical Global Impressions Scale [CGI] [14]). In one, a small chart-review study from a clinical practice, 83% (N=31) of patients who discontinued an effective SRI experienced relapse of body dysmorphic disorder (15). In the other, a 16-week open-label fluvoxamine study, six responders who were subsequently treated in a clinical practice discontinued fluvoxamine; all relapsed from within 4 days to about 2 months (11). In both studies, subjects were assessed only at clinic visits, thus precluding precise assessment of time to relapse, and secondary outcomes were not assessed.
Here we report on the first prospective pharmacotherapy relapse prevention study in body dysmorphic disorder. In phase 1, subjects received 14 weeks of acute open-label escitalopram. In phase 2, responders were randomized to 6 months of continued escitalopram treatment or switch to placebo. The study’s primary aim was to compare time to relapse (and relapse rates) in phase 2; we hypothesized that escitalopram responders who continued escitalopram for 6 additional months would have a longer time to relapse and a lower relapse rate than subjects who switched to placebo. Secondary and exploratory phase 2 hypotheses were that subjects randomized to 6 months of continuation escitalopram treatment would perform better than placebo-treated subjects on secondary outcome measures and would significantly improve with continued escitalopram treatment.
Phase 1 (acute open-label phase) outcomes were of interest because few pharmacotherapy studies have been done in body dysmorphic disorder, and all used relatively small samples (7–12). Furthermore, no study has examined remission (as opposed to response or improvement) with pharmacotherapy. Phase 1 hypotheses were that body dysmorphic disorder symptoms and insight, depressive symptoms, functioning, and quality of life would significantly improve after 14 weeks of open-label escitalopram, and that subjects with delusional body dysmorphic disorder beliefs would be as likely as those with nondelusional beliefs to respond to escitalopram.
Method
Subjects
This study was conducted at two academic sites: Rhode Island Hospital (formerly at Butler Hospital) at Brown University and Massachusetts General Hospital. The following inclusion criteria were used for participants: age ≥18; diagnosis of DSM-IV body dysmorphic disorder (including delusional body dysmorphic disorder) for ≥6 months; baseline total score ≥24 on the Yale-Brown Obsessive-Compulsive Scale Modified for Body Dysmorphic Disorder (BDD-YBOCS) (16, 17); score of at least moderate on the CGI severity scale (14); ability to communicate meaningfully with the investigators; and competency to provide written informed consent. The following exclusion criteria were used: current clinically significant suicidality or a suicide attempt within the past year; need for inpatient or partial hospital treatment; current or past DSM-IV bipolar disorder or psychotic disorder; DSM-IV alcohol or drug dependence or abuse in the past 3 months, or a positive result on a urine drug screen for any illicit substances of abuse; mental retardation, dementia, brain damage, or other cognitive impairment that would interfere with participation; body image concerns accounted for primarily by an eating disorder; body dysmorphic disorder criteria not met if weight concerns were excluded; use of psychotropic medication or herbal preparations with alleged behavioral effects during the study or for 2 weeks (6 weeks for fluoxetine) before the baseline assessment; use of investigational medication within 3 months of baseline or use of a depot antipsychotic within 6 months of baseline; previous allergic reaction to escitalopram or citalopram; current cognitive-behavioral therapy (CBT); presence of significant or unstable medical illness; history of a seizure disorder; females who were pregnant, breastfeeding, or sexually active and not using adequate contraception; and presence of behavior that would interfere with protocol cooperation.
The protocol and informed-consent documents were approved by the hospitals’ institutional review boards. All participants provided written informed consent after receiving a complete description of the study. An independent data and safety monitoring board provided oversight.
Study Design and Procedures
During screening, psychiatric and medical histories (using the Body Dysmorphic Disorder Form [e.g., 7]) were conducted, a urine pregnancy test and a urine toxicology screen were obtained, and the Structured Clinical Interview for DSM-IV, Patient Version (18–20) was administered.
Phase 1.
Participants received open-label escitalopram monotherapy for 14 weeks: 10 mg/day during weeks 1–3, 20 mg/day during weeks 4–6, and 30 mg/day thereafter. A lower dosage was allowed to improve tolerability. Phase 1 assessments (below) were conducted at baseline, weekly during weeks 1–4, and biweekly during weeks 6–14. Escitalopram response was defined as ≥30% reduction in the BDD-YBOCS score from baseline that persisted for at least two consecutive assessments and through the end of phase 1 (or through the last visit, for early terminators).
Phase 2.
Nonresponders were removed from the study at the end of phase 1. Subjects who completed phase 1, responded to escitalopram, and agreed to continue study participation entered phase 2. Phase 2 participants were randomly assigned to 6 additional months of treatment with continuation monotherapy with escitalopram or discontinuation of escitalopram (via tapering by 10 mg/day per week) and substitution with pill placebo. Randomization was stratified by body dysmorphic disorder–related insight and by presence of major depressive disorder. Phase 2 assessments were conducted at randomization and biweekly thereafter.
Throughout phase 2, subjects randomized to escitalopram received the dosage prescribed at the end of phase 1. However, those taking 10–20 mg/day at the end of phase 1 could increase their dosage to 30 mg/day during phase 2 if symptoms worsened before relapse; conversely, if side effects occurred at 30 mg/day, the dosage could be lowered to 20 mg/day.
Relapse of body dysmorphic disorder was defined as a 50% or greater loss of BDD-YBOCS improvement that had occurred during phase 1, plus a BDD-YBOCS score >20 (which corresponds to full-criteria body dysmorphic disorder), plus a rating of “much worse” or “very much worse” on the CGI for body dysmorphic disorder symptoms. We developed this definition after consulting the literature on other disorders, primarily obsessive-compulsive disorder (OCD), because of these disorders’ similarities (21) and because the BDD-YBOCS is derived from the YBOCS (22, 23). However, there was no consensus on the definition of relapse in the OCD literature (24), so we developed a rigorous, clinically meaningful definition that was informed by this literature. Relapse criteria needed to be met for 2 consecutive weeks, although for safety reasons, subjects could be withdrawn after meeting relapse criteria for only 1 week (and they were considered to have relapsed). Relapsing participants were referred for doctor’s choice rescue treatment by a psychiatrist not otherwise involved in the study. Additional psychotropic medication was limited to zolpidem at 5–10 mg/h.s. for insomnia and sildenafil at up to 100 mg three times a week for treatment-emergent sexual dysfunction.
Assessments
Independent evaluators who did not provide study treatment administered the following reliable and valid measurements. The BDD-YBOCS, a 12-item semistructured clinician-administered scale adapted from the YBOCS (16, 17), rated past-week disorder severity. The CGI improvement scale, a global rating scale that ranges from 1 (very much improved) to 7 (very much worse) (14), assessed body dysmorphic disorder symptoms and overall symptoms. The CGI for body dysmorphic disorder symptoms and the overall (global) CGI were secondary outcome measures in phase 1, and the CGI for body dysmorphic disorder symptoms determined relapse in phase 2. The Brown Assessment of Beliefs Scale, a seven-item semistructured clinician-administered scale, assessed past-week body dysmorphic disorder–related insight and delusional beliefs dimensionally (25, 26). It also classified false beliefs (e.g., “I look like a monster”) as delusional or nondelusional. The 17-item Hamilton Depression Rating Scale (HAM-D) assessed current severity of depressive symptoms (27). The Range of Impaired Functioning Tool assessed psychosocial functioning; it consists of 5- to 7-point rater-administered subscales for work, household duties, student work, relationships with family and friends, recreation, life satisfaction, and global social adjustment (28). The Quality of Life Enjoyment and Satisfaction Questionnaire Short Form assessed quality of life across social, leisure, household, work, emotional well-being, physical, and school parameters (29). The Psychiatric Status Rating Scale for Body Dysmorphic Disorder, a 7-point scale, reflects whether body dysmorphic disorder symptoms meet full DSM-IV criteria or are in full or partial remission (13, 30). In phase 1, this rating scale was used to examine remission from body dysmorphic disorder, and in phase 2 it was used to examine further improvement (by one or more points) during continuation treatment with escitalopram.
Measurements were administered at all visits, except that the functional impairment measure and the quality of life questionnaire were administered only at the phase 1 baseline and termination visits and at the phase 2 midpoint and termination visits. Interrater reliability (based on intraclass correlations) on the BDD-YBOCS and Brown Assessment of Beliefs Scale for all scale items and total score was >0.9.
At all visits, vital signs were obtained, and medication compliance was monitored by patient inquiry, returned pill count, and a drug accountability form. Any adverse physical symptoms since the last visit were rated for severity, action taken, outcome, and seriousness. Urine drug screen and pregnancy tests were repeated at the end of phase 1.
Data Analysis
Analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, N.C.), and employed a two-sided alpha=0.05 type I error rate. Major variables were screened for inconsistent or abnormal values, and continuous measures were assessed for skewness and outliers. Transformations to improve normality were applied. Differences in baseline covariates were tested using Fisher’s exact test (categorical) and Student’s t test (continuous). In phase 1, estimated power was >95% to detect a medium effect size for the change in BDD-YBOCS score over time. Changes in scores were compared with a null value using a series of Student’s t tests. Secondary analyses quantified the proportion (and 95% Wald CIs) of subjects who were much or very much improved (CGI improvement score of 1 or 2) and who attained remission from body dysmorphic disorder (Psychiatric Status Rating Scale score of 1 or 2) by week 14 or by an early termination visit. Cox proportional hazards regression examined predictors of response to open-label escitalopram, with estimated power from 76% to ≥90% to detect medium to large effect sizes. We planned to enroll enough subjects in phase 1 to obtain 58 responders for randomization in phase 2. For the primary aim in phase 2 of examining group differences in time to relapse (and proportion relapsing), Kaplan-Meier survival curves were generated, and Cox proportional hazards regression was employed (with the likelihood ratio p value reported). In phase 2, data from assessments made after a patient entered rescue treatment were excluded from analyses. A priori, we determined that randomization of 58 subjects to phase 2 would yield 80% power to detect a hazard ratio as small as 3.0 over 6 months of follow-up. For secondary analyses of change in the BDD-YBOCS score, as well as changes in secondary outcome measures (the Brown Assessment of Beliefs Scale, the HAM-D, the functional impairment measure, and the quality of life questionnaire), we employed generalized linear regression models and regressed the change score (baseline to last visit) against the treatment group. For large effect sizes (d=0.8), estimated power for a two-tailed test (alpha=0.05) was ≥74% for the BDD-YBOCS, and for the other secondary outcome measures, using a Bonferroni-adjusted alpha of 0.0125, estimated power was 31%−74% to detect medium to large effect sizes. During 6 months of continuation escitalopram treatment, we estimated the proportion (and 95% Fisher’s exact CIs) of subjects who further improved by one or more points on the Psychiatric Status Rating Scale; power to detect significant improvement in 40% or more of subjects was 99% (http://web1.sph.emory.edu/cdckms/proportion-ext-Mid-Pnew.html). We examined site differences in the effect of treatment by including site and the interaction between treatment and site in regression models. Within the escitalopram and placebo groups, Student’s t tests were used to compare changes in BDD-YBOCS total scores during phase 2 with a null value.
Results
Enrollment and Sample Description
Across the two sites, 173 subjects were enrolled (Figure 1). Seventy-three subjects did not pass screening. One hundred subjects received initial open-label escitalopram treatment in phase 1. Table 1 summarizes the participants’ demographic and clinical characteristics; no significant site differences existed.
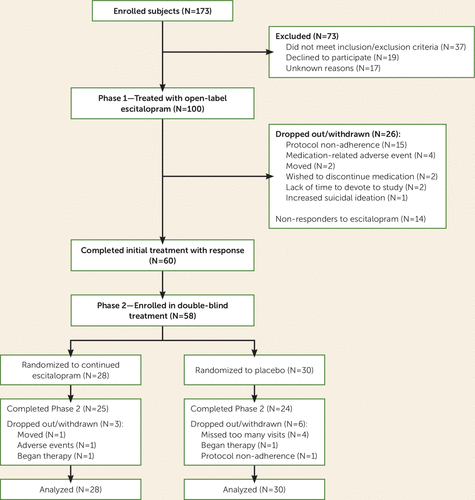
FIGURE 1. Flow Diagram of Subject Enrollment and Progress Through the Study
| Phase II | ||||||
|---|---|---|---|---|---|---|
| Variable | Phase I (N=100) | Escitalopram (N=28) | Placebo (N=30) | |||
| N | % | N | % | N | % | |
| Female | 64 | 64.0 | 20 | 71.4 | 20 | 66.7 |
| Race | ||||||
| American Indian or Alaskan Native | 2 | 2.0 | 0 | 0.0 | 1 | 3.3 |
| Asian | 2 | 2.0 | 1 | 3.6 | 0 | 0.0 |
| Black/African American | 7 | 7.0 | 1 | 3.6 | 1 | 3.3 |
| Native Hawaiian or other Pacific Islander | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| White | 84 | 84.0 | 24 | 85.7 | 27 | 90.0 |
| More than one race | 5 | 5.0 | 2 | 7.1 | 1 | 3.3 |
| Hispanic or Latino ethnicity | 12 | 12.0 | 4 | 14.3 | 5 | 16.7 |
| Body dysmorphic disorder age at onset <18 yearsb | 69 | 70.4 | 21 | 77.8 | 20 | 66.7 |
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 33.5 | 12.4 | 37.3 | 12.4 | 31.8 | 13.5 |
| BDD-YBOCS score at randomizationc | 10.3 | 7.5 | 13.8 | 6.4 | ||
TABLE 1. Baseline Demographic and Clinical Characteristics, by Study Phasea
Acute, Open-Label Phase (Phase 1)
A total of 74.0% (N=74) of subjects completed 14 weeks of escitalopram treatment in phase 1. Body dysmorphic disorder symptom response was achieved by 67.0% (95% CI=57.8–75.7) (N=67) of all treated subjects (in the intent-to-treat population) and by 81.1% (95% CI=72.2–90.0) (N=60) of the 74 subjects who completed phase 1. Based on CGI improvement scores for body dysmorphic disorder symptoms, 71.1% (95% CI=62.1–80.2) (N=69) of the 97 subjects with a postbaseline assessment were much or very much improved by their last phase 1 visit. Full remission from body dysmorphic disorder was attained by 20.0% (95% CI=12.2–27.8) (N=20) of intent-to-treat subjects and by 25.7% (95% CI=15.7–35.6) (N=19) of subjects who completed phase 1. Median time to first response was 7.9 weeks (95% CI=6.9–8.9). The mean escitalopram dose at the last phase 1 visit was 26.2 mg/day (SD=7.2, range=5–30) (two subjects received less than 10 mg/day).
Significant improvement from baseline to the last phase 1 visit was attained on the BDD-YBOCS, the Brown Assessment of Beliefs Scale, the HAM-D, the functional impairment measure, and the quality of life questionnaire (all p values <0.0001). Mean BDD-YBOCS scores decreased from 32.7 (SD=5.4) at baseline to 16.9 (SD=10.2) at the last phase 1 visit among the 97 subjects with a postbaseline assessment (average decrease of 48.7%), and these scores decreased to 14.9 (SD=9.3) at week 14 in the population who completed phase 1 (average decrease of 54.4%).
Based on the CGI global improvement score, 67.0% (95% CI=57.7–76.4) (N=65) of subjects were much or very much improved by their last postbaseline phase 1 visit. Response rates did not significantly differ between subjects with delusional and those with nondelusional body dysmorphic disorder, but there was a trend for the 74 subjects with nondelusional beliefs to be more likely to respond to escitalopram than the 26 subjects with delusional beliefs (70.3% and 57.7%, respectively; hazard ratio=0.58, 95% CI=0.33–1.04, p=0.054). The only baseline variable that predicted response of body dysmorphic disorder was presence of a personality disorder (hazard ratio=0.58, 95% CI=0.36–0.94, p=0.030). Response to acute open-label escitalopram did not significantly differ by baseline body dysmorphic disorder severity or duration, depressive symptoms, gender, or minority status.
Relapse Prevention Efficacy (Phase 2)
Fifty-eight participants were randomized to double-blind continuation treatment with escitalopram or to discontinuation of escitalopram and substitution with placebo (Figure 1). Table 1 presents participants’ demographic and clinical characteristics at randomization; no significant differences existed by treatment. Phase 2 was completed by 89.3% (N=25) of escitalopram-treated subjects and by 80.0% (N=24) of placebo-treated subjects (n.s.).
Time to relapse of body dysmorphic disorder was significantly longer with escitalopram than with placebo (hazard ratio=2.72, 95% CI=1.01–8.57, p=0.049) (Figure 2). By the end of phase 2, relapse proportions were 40% for the placebo group compared with 18% for the escitalopram group. There were no statistically significant between-group treatment differences on secondary outcome measures or site differences.
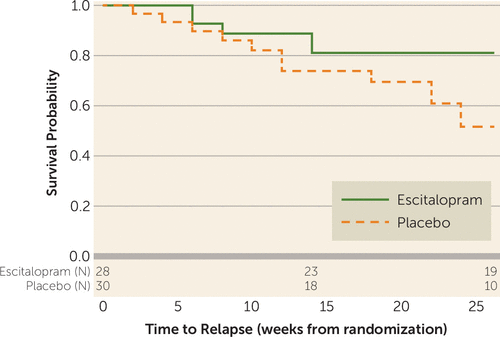
FIGURE 2. Time to Relapse by Phase 2 Treatment Arm, With Number of Subjects at Risk
Continuation Treatment Efficacy
Among the 28 subjects randomized to continuation escitalopram, BDD-YBOCS scores decreased significantly during phase 2 (mean=4.1 points, p=0.036). Among escitalopram-treated subjects, 35.7% (95% CI=18.0–53.5) (N=10) had a further decrease in the Psychiatric Status Rating Scale score. The mean escitalopram dose at the end of phase 2 was 28.7 mg/day (SD=4.6, range=7.5–30.0) (one subject received less than 10 mg/day).
Safety and Tolerability
Phase 1.
Among intent-to-treat subjects, 89% (N=89) experienced at least one treatment-emergent adverse event (regardless of relationship to treatment). Adverse events that occurred in at least 5% of subjects and were considered at least possibly related to study medication were fatigue (N=37), nausea (N=30), sexual dysfunction (N=28), dry mouth (N=25), insomnia (N=25), headache (N=22), appetite changes (N=20), agitation (N=10), flatulence (N=7), hypersomnia (N=6), sweating (N=6), dizziness (N=5), yawning (N=5), and irritability (N=5).
Phase 2.
Overall, 25.0% (N=7) of escitalopram-treated subjects and 46.7% (N=14) of placebo-treated subjects experienced at least one adverse event considered at least possibly related to study medication (Fisher’s exact p=0.11). No serious adverse events occurred during either treatment phase.
Discussion
Among subjects who responded to acute-phase escitalopram, continued escitalopram treatment significantly delayed time to relapse compared with treatment with placebo, thus confirming our primary hypothesis. Moreover, relapse occurred in more than twice as many placebo-treated subjects as escitalopram-treated subjects. We believe that these differences are clinically meaningful. These findings are particularly relevant because body dysmorphic disorder is often chronic (13, 30).
In the only previously published relapse study—a chart-review study in a clinical practice—discontinuation of an effective SRI was followed by relapse (rated “much” or “very much worse” on the CGI) in 83% (N=31) of cases (15), a considerably higher rate than in the present study (40%). However, we assessed relapse over 6 months, whereas the chart-review study assessed relapse between the time an SRI was started and the last clinic visit, which varied considerably among subjects (the mean follow-up duration was not reported). Furthermore, the two studies have methodological differences, including different outcome measures and blind assessments every 2 weeks in the present study, compared with less frequent, unblinded assessments in the chart-review study. The prior study’s higher relapse rate may also be attributable in part to patients’ knowledge that active medication was being discontinued, unlike the current study.
Our secondary and exploratory hypothesis concerning changes in clinical symptoms, functioning, and quality of life during phase 2 was not confirmed. Studies with larger sample sizes are needed to determine whether group differences might emerge over a longer period following medication discontinuation.
Consistent with our hypothesis, during the 6 months of phase 2, BDD-YBOCS scores significantly improved with continued escitalopram treatment; 35.7% of subjects improved by at least 1 point on the 7-point Psychiatric Status Rating Scale. This finding is consistent with clinical observations that patients may improve further with SSRI continuation following acute response (31). Such improvement has been found in studies of OCD, depression, and other disorders (32, 33).
Our open-label phase 1 results confirm findings from all prior treatment studies, indicating that acute SRI treatment is efficacious for a majority of patients. The present study’s intent-to-treat response rate (67%) and degree of improvement on the BDD-YBOCS are similar to those in previous, smaller acute-phase studies (the number of SSRI-treated subjects ranged from 15 to 34), which had intent-to-treat response rates ranging from 53% to 83% (7, 9–12).
Consistent with our hypothesis and with previous research, body dysmorphic disorder–related insight, depressive symptoms, psychosocial functioning, and quality of life also significantly improved with open-label escitalopram (9–12). The mean phase 1 escitalopram dosage (26.2 mg/day, SD=7.2) is higher than dosages often used to treat anxiety disorders and depression but is similar to dosages usually recommended for OCD and those typically used for body dysmorphic disorder (31, 34, 35). No dose-finding studies have been conducted in body dysmorphic disorder; however, clinical observations and retrospective data from an observational study (31, 36) suggest that relatively high SRI doses (sometimes higher than those used in this study) are often needed to effectively treat this disorder.
Our finding that individuals with delusional body dysmorphic disorder beliefs were as likely as those with nondelusional beliefs to respond to escitalopram is consistent with previous SRI studies (7–11). It is also consistent with data from a range of diagnostic validators indicating that delusional and nondelusional body dysmorphic disorder constitute the same disorder (37, 38), as specified in DSM-5. However, unlike previous studies, we found a trend for those with nondelusional body dysmorphic disorder beliefs to be more likely to respond to escitalopram.
Limitations of the present study include statistical power that was too limited to examine predictors of relapse or to test group differences for other reported results (e.g., within a type of adverse event). Analysis of multiple outcomes in phase 1 may have inflated type I error. Also, while use of placebo is a gold-standard approach with many methodological advantages, it does not mimic what occurs in clinical practice, where patients know whether they have discontinued a medication. Knowledge of medication discontinuation may possibly be associated with a higher relapse rate than observed in this study. Furthermore, our findings may not be fully generalizable to certain clinical settings or populations. For example, suicidality and substance use disorders appear common in individuals with body dysmorphic disorder (6, 39), yet we excluded individuals with a current or recent substance use disorder from this efficacy study. We also excluded more highly suicidal individuals because of potential risks of discontinuing efficacious medication, and we excluded more severely ill patients who required concomitant psychotherapy or a higher level of care. Study strengths include the fact that, to our knowledge, this is the first relapse prevention study in body dysmorphic disorder; it contained the largest sample of any treatment study in this disorder thus far; and it used randomization, placebo, and blind and rigorous assessment of outcomes at specified intervals. Additional studies with larger sample sizes and longer follow-up are needed. Research is also needed to investigate whether treatment with CBT for body dysmorphic disorder will decrease the risk of relapse when an effective medication is discontinued.
Patient Perspectives
Patient 1
“Ms. M,” a 53-year-old married white insurance agent, was preoccupied for 15–16 hours a day with her “asymmetrical” eyes and “thin” hair, which she believed looked “abnormal.” In reality, her eyes and hair looked normal. These concerns were “severely distressing.” She spent many hours a day checking her appearance in mirrors, comparing with others, asking her husband if she looked okay, and pulling on her eyelids to “even up” her eyes. She tried to hide her eyes by wearing glasses and covering them with her hair. Her appearance preoccupations decreased her concentration and productivity at work. Because she did not want to be seen, she avoided most social situations, activities such as food shopping, and physical intimacy with her husband. Her distress over her appearance caused depressed mood and frequent suicidal ideation. A blepharoplasty, facelift, 20 sessions of psychotherapy, and treatment in a partial hospital program did not improve her symptoms. Trials of fluvoxamine at 50 mg/day for less than a week and of escitalopram at 20 mg/day for 6 months, as well as fluoxetine, venlafaxine, paroxetine, citalopram, lorazepam, and clonazepam at unknown dosages, were not helpful, although medication adherence was poor.
At study baseline, Ms. M was diagnosed with body dysmorphic disorder and current major depressive disorder. Her score on the 48-point BDD-YBOCS was 31, indicating moderate to severe body dysmorphic disorder (a cutoff point of 20 indicates current body dysmorphic disorder). After 12 weeks of escitalopram, which was gradually increased to a dosage of 30 mg/day, her BDD-YBOCS score was 19; she now thought about her appearance for less than 1 hour a day and reported only mild distress and moderate impairment in functioning. She went out of the house to socialize and do errands, but her appearance concerns still diminished her work productivity. After randomization to placebo, her symptoms gradually worsened; she relapsed after 3 months, at a BDD-YBOCS score of 32. She now thought about her appearance for 12–13 hours a day, and her appearance concerns caused severe distress and impairment in functioning (for example, she avoided 75%–80% of social activities).
Patient 2
“Mr. N,” a single white 37-year-old electrician, had been preoccupied with his “nonexistent” chin, generally “ugly” face, and “huge” nose since age 15. He thought about his appearance for several hours a day and frequently compared his appearance with that of others. He held his head at certain angles so his perceived flaws would be less noticeable. His appearance preoccupations decreased his concentration and work productivity; because he felt embarrassed about how he looked, he avoided work colleagues, work meetings, and some social situations, and he did not date. His appearance concerns caused suicidal ideation. Treatment with 20 sessions of individual psychotherapy, 10 sessions of family therapy, and fluoxetine at 20 mg/day for 4 weeks were not beneficial. Treatment with 150 mg/day of sertraline for several years substantially improved his symptoms, but some symptoms remained.
At study baseline, Mr. N was diagnosed with body dysmorphic disorder and past major depressive disorder. His BDD-YBOCS score was 25 (mild to moderate body dysmorphic disorder). After 12 weeks of treatment with escitalopram, which was gradually increased to a dosage of 30 mg/day, his BDD-YBOCS score was 4. He had no appearance preoccupations or associated distress or impairment; his score of 4 was accounted for by absent insight (he was still completely convinced that he looked “deformed and abnormal”). After randomization to 6 months of continued escitalopram treatment, he continued to do well; at study termination, his BDD-YBOCS score was still 4.
1 : The prevalence of body dysmorphic disorder: a population-based survey. Psychol Med 2006; 36:877–885Crossref, Medline, Google Scholar
2 : Updates on the prevalence of body dysmorphic disorder: a population-based survey. Psychiatry Res 2010; 178:171–175Crossref, Medline, Google Scholar
3 : The prevalence of body dysmorphic disorder in the United States adult population. CNS Spectr 2008; 13:316–322Crossref, Medline, Google Scholar
4 : Prevalence of body dysmorphic disorder among Swedish women: a population-based study. Compr Psychiatry 2015; 58:108–115Crossref, Medline, Google Scholar
5 : Functional impairment in body dysmorphic disorder: a prospective, follow-up study. J Psychiatr Res 2008; 42:701–707Crossref, Medline, Google Scholar
6 : Suicidality in body dysmorphic disorder: a prospective study. Am J Psychiatry 2006; 163:1280–1282Link, Google Scholar
7 : A randomized placebo-controlled trial of fluoxetine in body dysmorphic disorder. Arch Gen Psychiatry 2002; 59:381–388Crossref, Medline, Google Scholar
8 : Clomipramine vs desipramine crossover trial in body dysmorphic disorder: selective efficacy of a serotonin reuptake inhibitor in imagined ugliness. Arch Gen Psychiatry 1999; 56:1033–1039Crossref, Medline, Google Scholar
9 : An open-label study of citalopram in body dysmorphic disorder. J Clin Psychiatry 2003; 64:715–720Crossref, Medline, Google Scholar
10 : An open-label study of escitalopram in body dysmorphic disorder. Int Clin Psychopharmacol 2006; 21:177–179Crossref, Medline, Google Scholar
11 : Efficacy and safety of fluvoxamine in body dysmorphic disorder. J Clin Psychiatry 1998; 59:165–171Crossref, Medline, Google Scholar
12 : Fluvoxamine in the treatment of body dysmorphic disorder (dysmorphophobia). Int Clin Psychopharmacol 1996; 11:247–254Crossref, Medline, Google Scholar
13 : A 4-year prospective observational follow-up study of course and predictors of course in body dysmorphic disorder. Psychol Med 2013; 43:1109–1117Crossref, Medline, Google Scholar
14 : ECDEU Assessment Manual for Psychopharmacology. Washington, DC, US Department of Health, Education, and Welfare, 1976Google Scholar
15 : Effectiveness of pharmacotherapy for body dysmorphic disorder: a chart-review study. J Clin Psychiatry 2001; 62:721–727Crossref, Medline, Google Scholar
16 : A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacol Bull 1997; 33:17–22Medline, Google Scholar
17 : Psychometric evaluation of the Yale-Brown Obsessive-Compulsive Scale Modified for Body Dysmorphic Disorder (BDD-YBOCS). JOCRD 2014; 3:205–208Google Scholar
18 : The Structured Clinical Interview for DSM-III-R (SCID): I: History, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
19 : The Structured Clinical Interview for DSM-III-R (SCID): II. Multisite test-retest reliability. Arch Gen Psychiatry 1992; 49:630–636Crossref, Medline, Google Scholar
20 : Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II). Washington, DC, American Psychiatric Association Publishing, 1997Google Scholar
21 : Should an obsessive-compulsive spectrum grouping of disorders be included in DSM-V? Depress Anxiety 2010; 27:528–555Crossref, Medline, Google Scholar
22 : The Yale-Brown Obsessive Compulsive Scale: I. Development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Medline, Google Scholar
23 : The Yale-Brown Obsessive Compulsive Scale: II. Validity. Arch Gen Psychiatry 1989; 46:1012–1016Crossref, Medline, Google Scholar
24 : Standard criteria for relapse are needed in obsessive-compulsive disorder. Depress Anxiety 2005; 21:1–8Crossref, Medline, Google Scholar
25 : The Brown Assessment of Beliefs Scale: reliability and validity. Am J Psychiatry 1998; 155:102–108Link, Google Scholar
26 : Psychometric evaluation of the Brown Assessment of Beliefs Scale in body dysmorphic disorder. J Nerv Ment Dis 2013; 201:640–643Crossref, Medline, Google Scholar
27 : The modified Hamilton Rating Scale for Depression: reliability and validity. Psychiatry Res 1985; 14:131–142Crossref, Medline, Google Scholar
28 : The Range of Impaired Functioning Tool (LIFE-RIFT): a brief measure of functional impairment. Psychol Med 1999; 29:869–878Crossref, Medline, Google Scholar
29 : Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol Bull 1993; 29:321–326Medline, Google Scholar
30 : A 12-month follow-up study of the course of body dysmorphic disorder. Am J Psychiatry 2006; 163:907–912Link, Google Scholar
31 : Understanding Body Dysmorphic Disorder: An Essential Guide. New York, Oxford University Press, 2009Google Scholar
32 : Relapse prevention and residual symptoms: a closer analysis of placebo-controlled continuation studies with escitalopram in major depressive disorder, generalized anxiety disorder, social anxiety disorder, and obsessive-compulsive disorder. J Clin Psychiatry 2010; 71:121–129Crossref, Medline, Google Scholar
33 : Continuation treatment of OCD: double-blind and open-label experience with fluoxetine. J Clin Psychiatry 1994; 55(Suppl:69–76):77–78Google Scholar
34 : Body dysmorphic disorder, in Handbook on Obsessive-Compulsive and Related Disorders. Edited by Phillips KA, Stein DJ. Washington, DC, American Psychiatric Association Publishing, 2015, pp 57–98Google Scholar
35 : Obsessive-compulsive disorder, in Handbook on Obsessive-Compulsive and Related Disorders. Edited by Phillips KA, Stein DJ. Washington, DC, American Psychiatric Association Publishing, 2015, pp 25–56Google Scholar
36 : Pharmacotherapy for body dysmorphic disorder: treatment received and illness severity. Ann Clin Psychiatry 2006; 18:251–257Crossref, Medline, Google Scholar
37 : Delusional versus nondelusional body dysmorphic disorder: recommendations for DSM-5. CNS Spectr 2014; 19:10–20Crossref, Medline, Google Scholar
38 : Delusional versus nondelusional body dysmorphic disorder: clinical features and course of illness. J Psychiatr Res 2006; 40:95–104Crossref, Medline, Google Scholar
39 : Substance use disorders in individuals with body dysmorphic disorder. J Clin Psychiatry 2005; 66:309–316Crossref, Medline, Google Scholar



