Inflammation: Depression Fans the Flames and Feasts on the Heat
Abstract
Depression and inflammation fuel one another. Inflammation plays a key role in depression’s pathogenesis for a subset of depressed individuals; depression also primes larger cytokine responses to stressors and pathogens that do not appear to habituate. Accordingly, treatment decisions may be informed by attention to questions of how (pathways) and for whom (predispositions) these links exist, which are the focus of this article. When combined with predisposing factors (moderators such as childhood adversity and obesity), stressors and pathogens can lead to exaggerated or prolonged inflammatory responses. The resulting sickness behaviors (e.g., pain, disturbed sleep), depressive symptoms, and negative health behaviors (e.g., poor diet, a sedentary lifestyle) may act as mediating pathways that lead to further, unrestrained inflammation and depression. Depression, childhood adversity, stressors, and diet can all influence the gut microbiome and promote intestinal permeability, another pathway to enhanced inflammatory responses. Larger, more frequent, or more prolonged inflammatory responses could have negative mental and physical health consequences. In clinical practice, inflammation provides a guide to potential targets for symptom management by signaling responsiveness to certain therapeutic strategies. For example, a theme across research with cytokine antagonists, omega-3 fatty acids, celecoxib, and exercise is that anti-inflammatory interventions have a substantially greater impact on mood in individuals with heightened inflammation. Thus, when inflammation and depression co-occur, treating them in tandem may enhance recovery and reduce the risk of recurrence. The bidirectional links between depression, inflammation, and disease suggest that effective depression treatments could have a far-reaching impact on mood, inflammation, and health.
Depression and inflammation are intertwined, fueling and feeding off each other. This bidirectional loop, in which depression facilitates inflammatory responses and inflammation promotes depression, has clear health consequences. Heightened inflammation characterizes a number of disorders and systemic diseases, including cardiovascular disease, diabetes, metabolic syndrome, rheumatoid arthritis, asthma, multiple sclerosis, chronic pain, and psoriasis; each of these also features an elevated risk for depression (1, 2).
Three meta-analyses have highlighted proinflammatory cytokine differences between patients with major depressive disorder and controls, including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), IL-1β, the soluble IL-2 receptor, the IL-1 receptor antagonist (IL-1ra), and C-reactive protein (CRP) (3–5). The stronger associations in clinical samples compared with community samples provide evidence of dose-response relationships (3). Supporting a causal pathway, higher IL-6 and CRP predicted the subsequent development of depressive symptoms (6). Relatedly, prospective studies also showed that depression predicted later IL-6 and CRP (7–10).
The pediatric literature also demonstrates bidirectional pathways between inflammation and depression (11). Data from two population-based prospective studies provided evidence for depression-inflammation relationships early in life. Children with higher IL-6 levels at age 9 were more likely to be depressed at age 18 compared with those with low IL-6 levels; importantly, IL-6 was measured prior to depression onset, thus suggesting that high IL-6 is indeed a risk factor (12). In another study with children who were 9, 11, or 13 years old at intake, depression predicted subsequent CRP level, with higher CRP levels following multiple depressive episodes (7).
However, depression is complex, and inflammation may contribute only in a subpopulation. Data from the National Health and Nutrition Examination Survey provide a rough estimate of the prevalence of heightened inflammation in depressed people; 47% of those whose depression inventory scores were above the clinical threshold had a CRP level ≥3.0 mg/L, and 29% had a CRP level ≥5.0 mg/L (13). Raison and Miller (14) suggest that inflammatory markers are noticeably higher in about a third of depressed patients compared to the majority of nondepressed comparison subjects. Thus, inflammation is neither necessary nor sufficient to induce or sustain depression (14, 15), but it clearly plays an important role in a substantial subpopulation (16). It follows that positive clinical responses to anti-inflammatory interventions may only occur in the subset with heightened inflammation (17, 18). Accordingly, we address mechanistic pathways between depression and inflammation, and then turn to questions of how (pathways) and for whom (predispositions) these links exist, with a focus on integrating newer research relevant to depression initiation, treatment response, and risk for recurrence (Figure 1).
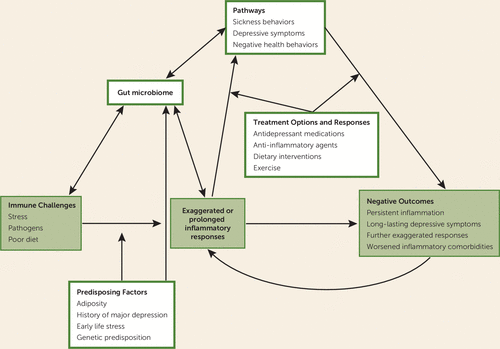
FIGURE 1. Factors That Increase Risk for Inflammatory Overresponsiveness, Along With Pathways Leading to Heightened Inflammation, Depression, and Health Risksa
a When combined with predisposing factors (moderators), immune challenges can lead to exaggerated or prolonged inflammatory responses. The resulting sickness behaviors (e.g., pain, fatigue, sleep disturbance), depressive symptoms, and negative health behaviors (e.g., poor diet) may act as mediating pathways that lead to further unrestrained inflammation. Depression, childhood adversity, stressors, and diet can all influence the gut microbiome and promote intestinal permeability, another pathway to enhanced inflammatory responses. Ultimately, this overresponsiveness could carry important physical and mental health risks and could amplify inflammatory responses to subsequent immune challenges. This pattern suggests novel treatment options that could halt both exaggerated inflammation and depressive symptoms, and may also help to pinpoint which patients might be expected to benefit from certain treatments.
Mechanistic Pathways
Cytokines induce depressive symptoms by influencing diverse mood-related processes. Elevated inflammatory signaling dysregulates neurotransmitter metabolism, impairs neuronal health, and alters neural activity in mood-relevant brain regions (2, 19).
Peripherally released cytokines send signals via molecular, cellular, and neural routes, which ultimately reach the brain and enhance CNS inflammation (2, 19, 20). Cytokines alter production, metabolism, and transport of neurotransmitters that synergistically affect mood, including dopamine, glutamate, and serotonin (21). For example, cytokines stimulate indoleamine 2,3-dioxygenase, an enzyme that affects tryptophan metabolism. This well-established pathway promotes depression by simultaneously slowing serotonin production and enhancing levels of kynurenine, a tryptophan metabolite (22).
Inflammation also affects neuronal growth and survival. Cytokines contribute to oxidative stress, which damages glial cells in mood-relevant brain regions, such as the prefrontal cortex and the amygdala (23). Cytokine-induced glutamate dysregulation can lead to excitotoxicity, thereby decreasing production of neurotrophic factors (e.g., brain-derived neurotrophic factor, BDNF) that typically support neuronal health, neuroplasticity, and neurogenesis (24). Notably, these neurotransmitter and cellular changes alter brain activity and neurocircuits underlying distress, motivation, and motor function (16, 19).
In addition to their effects on neural processes, cytokines promote dysregulated hypothalamic-pituitary-adrenal (HPA) axis functioning, a key characteristic of depression (25, 26). Abnormal glucocorticoid signaling can influence the maintenance and progression of depression (27). Briefly, glucocorticoids typically dampen inflammation via a negative feedback loop. However, inflammation can cause glucocorticoid resistance in immunocytes and their cellular targets by inducing MAP kinases c-jun N-terminal kinase (JNK) and p38 (1). In this way, cytokine signal transduction pathways (e.g., nuclear factor-kB, NF-kB) disrupt glucocorticoid receptor function and expression, leading to unrestrained inflammatory responses that could further fuel depressive symptoms (2, 26, 28). Cytokine-dependent glucocorticoid receptor resistance decreases inhibitory feedback on production of corticotropin-releasing hormone and cytokines, intensifying the stress-response system (29). The glucocorticoid receptor protein is abundantly expressed throughout the main neuronal subregions of the hippocampus. BDNF functions as a powerful modulator of structural plasticity in the hippocampus and mediates protective influences by enhancing neuronal survival (30). Sustained glucocorticoid exposure leads to dendritic atrophy in hippocampal subfields and decreases neuronal cell survival by evoking a decline in BDNF expression in hippocampal and cortical regions (31, 32).
Inflammation may affect people differently, depending on their individual physiology; some people have bodily systems that protect them from developing inflammation-based depression, while others do not. Mechanistically, even lower levels of inflammation could be depressogenic in vulnerable individuals; Raison and Miller (14) call this phenomenon “immune response element amplification.” These may include lower parasympathetic activity, poorer sensitivity to glucocorticoid inhibitory feedback, lower BDNF production, larger responses to social threat in the anterior cingulate cortex or amygdala, and smaller hippocampal volume. Indeed, these are all correlates of major depression that would influence sensitivity to the depressogenic consequences of inflammatory stimuli.
Traveling Companions: Inflammation and Depression
The bidirectional links between inflammation and depression have received considerable attention (1, 2, 16, 33–36). Heightened inflammation alerts the CNS to induce or intensify “sickness behaviors,” including negative mood, fatigue, anhedonia, increased pain sensitivity, loss of appetite, and cognitive deficits, a cluster of symptoms resembling human depression (29, 34, 37). For example, administration of cytokines, endotoxin, or vaccines has been found to worsen mood, fatigue, and pain sensitivity and to boost proinflammatory cytokine production in healthy volunteers (34).
Inflammatory mediators can also induce clinical depression, bolstering support for inflammation’s role in depression’s pathophysiology. These effects can be substantial; for example, cytokine therapies, used for treating some cancers and chronic viral infections, provoke the onset of major depression in up to 45% of patients (21, 38–40). Most people who receive interferon-alpha (IFN-α) treatment develop neurovegetative symptoms, including fatigue, sleep problems, anorexia, and psychomotor retardation; these symptoms persist throughout treatment (21). However, mood and cognitive symptoms develop primarily in vulnerable patients, including those with a mood disorder history or higher initial levels of depressive symptoms, chronic inflammatory exposure, higher baseline levels of inflammation, or genetic polymorphisms associated with risk for depression or inflammation (21, 38, 39).
Antidepressant medication responsiveness may be poorer in patients with major depression who have heightened plasma inflammatory markers, as well as those with polymorphisms in inflammation-related genes and proinflammatory gene expression profiles (17, 41–51). Recently, a provocative trial (17) assessed the efficacy of the monoclonal antibody infliximab, a TNF-α antagonist, in 60 patients with major depression that was at least moderately medication resistant. Despite the absence of any overall benefit for infliximab versus placebo, patients with high baseline CRP levels had substantially greater reductions in depressive symptoms than those with low CRP levels.
The Gut Microbiota, Inflammation, and Depression
The gut-brain axis involves bidirectional communication between the CNS and the gastrointestinal tract via neurocrine and endocrine signaling pathways (52). Physical and psychological stressors can alter the gut microbiota’s composition and metabolic activities, and signals produced by the gut microbiota can in turn affect the brain and emotional responses (52). Alterations in the gut microbiota shape physiology through contributions to inflammation, obesity, and mood, among other things (53). For example, both rodent and human studies provide causal evidence linking obesity and the gut microbiome (53).
Depression can promote intestinal permeability, that is, greater inflammation-inducing endotoxin translocation, described as a “leaky gut.” Indeed, depressed patients have been found to have higher antibody against gut bacteria than comparison subjects (54). In another study, patients with major depression had elevated expression of 16S rDNA, a marker of bacterial translocation, compared with nondepressed comparison subjects, and the magnitude was correlated with depressive symptom severity (55). Among alcohol-dependent patients, those with higher depression, anxiety, and craving symptom ratings also had greater gut permeability and gut-bacterial dysbiosis than those with normal gut permeability (56).
Targeting the gut-brain axis may offer novel treatment options with benefits mediated through the vagus nerve, spinal cord, or neuroendocrine system (57). Diet plays a key role in the gut’s microbiota composition and thus represents one potential therapeutic avenue, as do supplements (particularly probiotics and prebiotics) and medications, including antibiotics (52). In rats, probiotic pretreatment attenuated gut leakiness after a restraint stressor (58). Limited data from human subjects suggest that selected probiotics may reduce depressive symptoms as a result of their anti-inflammatory properties as well as their ability to reduce HPA axis activity (57).
Rodent studies show how the microbiota’s composition has potent effects on brain biochemistry and behavior early in development (53). Early-life maternal separation in mice can produce both long-lasting changes in HPA stress responses as well as persistent microbiome alterations (57), evidence for one pathway through which early adversity induces depression and inflammatory responses in adults.
Early Adversity
Adults who experienced abuse or neglect as children are more likely to develop psychiatric disorders (59). Indeed, childhood maltreatment is a particularly potent risk factor for depression in adults, especially when individuals encounter stressful life events (2, 59). Early adversity also predicts a greater risk for recurrent, treatment-resistant depressive episodes (59).
Convergent evidence shows that childhood adversity can have longer-term inflammatory consequences (60–64). Among adults with major depression, those with a history of early maltreatment had higher CRP levels than those without such a history (60). Additionally, early-life adversity was still associated with heightened IL-6 and TNF-α in an older adult sample with a mean age of 70 (61). Adult survivors of childhood abuse also have maladaptive alterations in the HPA axis and autonomic stress responses compared with similar individuals without an abuse history (65). For example, those with a history of early-life stress have lower heart rate variability, reflecting lower parasympathetic activity (62), which is linked to inflammation. Trauma survivors have enhanced glucocorticoid resistance and increased central corticotropin-releasing factor activity, further supporting neuroendocrine stress response sensitization in those with early adversity (65). Furthermore, inflammation-relevant epigenetic alterations associated with early adversity include alterations in glucocorticoid receptor expression (62).
Early adversity can enhance inflammatory responsiveness to stressors. IL-6 levels rose higher after a laboratory stressor in individuals who reported childhood trauma compared with those without a trauma history (66). These laboratory stress data parallel differences observed in response to daily stressors: IL-6 levels were 2.35 times greater in individuals with a childhood abuse history who experienced multiple stressors in the past 24 hours compared with participants with multiple daily stressors but no abuse history (67).
Sickness Behaviors: Paving the Way to Inflammation and Depression
Sickness behaviors serve an adaptive function by conserving energy during an acute illness (1). However, these symptoms can in turn fuel inflammation and depression, and thus it is not surprising that they can also predict treatment resistance and poorer treatment outcomes (68).
Pain generates an inflammatory response (69, 70), and amplified pain sensitivity serves as an additional inflammatory source that in turn provokes depressive symptoms (71, 72). The association appears to be reciprocal: greater pain is associated with a higher prevalence of depression, and improvements in depression are correlated with declines in pain (73). Pain increases the risk for recurrence of depression by worsening subthreshold depressive symptoms (74). Greater pain severity is associated with poorer treatment outcomes in depression, including poorer responses to antidepressant medications (73, 74).
Disturbed sleep, a cardinal symptom of depression, also has a contributory role, producing a twofold higher risk for depression (75). Sleep loss stimulates production of proinflammatory cytokines and cellular inflammatory signaling, thus facilitating depression (75). In turn, heightened inflammation disrupts sleep regulation (76, 77); pharmacological cytokine blockers can normalize sleep (75). In a longitudinal study, sleep disturbances increased the risk for systemic inflammation at the 5-year follow-up (78).
Thus, sleep and pain are additional, independent accelerators for depression and inflammation that also act in tandem, building on each other. Disturbed sleep exacerbates pain and fatigue (79). Conversely, pain clearly impairs sleep (79). Changes in appetite, another key symptom of both inflammation and depression, can be triggered by sleep loss and fatigue (76, 79). The poorer mood-related dietary choices that typically follow serve to promote inflammation and depression.
Diet as a Road to Depression and Inflammation
Observational studies have linked healthier diets with a lower risk for depression (80, 81). Prospective studies suggest that healthier diets offer some protection against the development of both depressive symptoms and depressive disorders (82, 83).
In addition to altering the risk for depression, diet quality also influences inflammation. Patients with metabolic syndrome (84) who were randomly assigned to a Mediterranean-style diet for 2 years had significant reductions in CRP and IL-6 levels. In a twin study (85), adherence to a Mediterranean diet was associated with lower IL-6 levels, and the results were not a function of shared environmental variance or genetic factors.
An innovative prospective study (86) addressed the question of whether a Mediterranean-style diet lowered the risk of increased inflammation over time in older adults with depressive symptoms at study entry. At the 6-year follow-up, the average increase in IL-6 levels was larger in depressed participants who had not followed a Mediterranean-style diet than in all other groups; in contrast, IL-6 levels did not change in those who were depressed but followed a Mediterranean-style diet, suggesting that the healthier diet buffered the impact of depression on inflammation (86).
To assess the question of whether inflammation serves as a mediator between diet and depression, researchers employed an empirically derived inflammatory dietary pattern score related to CRP, IL-6, and TNF-RII (87). Using food frequency questionnaire data collected six times over 18 years in the large Nurses’ Health Study, they found that the risk for depression increased with higher inflammatory scores in women who were not depressed at baseline (87).
Along with diet quality, quantity and timing matter. Caloric restriction produces powerful anti-inflammatory effects over periods of months to years (88). Intriguingly, caloric restriction is also strongly antidepressant in rodent depression models (89).
Even intermittent fasting or time-restricted feeding can reduce inflammation. Comparisons of IL-6 and CRP in observant Muslims 1 week before the month of Ramadan (no eating or drinking during daylight), during the final week, and 20 days after Ramadan showed that daytime fasting decreased IL-6 and CRP levels by about 50% compared with pre-Ramadan values, a dramatic reduction in the absence of weight change; a nonfasting group assessed at the same times showed no IL-6 or CRP changes (90). Time-restricted feeding also reduced inflammation in mice (91). Additionally, TNF-α and IL-1ra responses to endotoxin were attenuated in rats that fasted for 48 hours compared with nonfasted rats (92).
Short-term fasting can also benefit mood. Clinical observational studies have reported reductions in depressive symptoms that appear between days 2–7 of fasting (93). Accordingly, anorexia may serve an adaptive function in both clinical depression and inflammation-induced sickness behavior by reducing inflammation (94).
Thus, cross-sectional, prospective, and randomized-controlled-trial research demonstrates how diet quality, quantity, and timing influence both depression and inflammation. Diet-related inflammation can promote depression, and diet-linked depression in turn heightens inflammation. One dietary component, fish oil, has generated considerable interest.
Omega-3 Fatty Acids
Fish oil is the prime source for two key omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA). Patients with depression have, on average, lower plasma levels of omega-3 fatty acids than nondepressed comparison subjects. Furthermore, there are relationships within these populations between depressive symptom severity and omega-3 fatty acid plasma levels (95, 96).
Five meta-analyses of randomized controlled trials have reached different conclusions about the efficacy of omega-3 fatty acids for treatment of depression. The first concluded that omega-3 fatty acid supplementation benefited clinically depressed individuals, but not those with less severe depressed mood (97). In contrast, a second that focused only on major depression found that omega-3 fatty acids had a small, nonsignificant effect (98). A third determined that omega-3 fatty acid supplementation was effective in both patients with major depression and those with subclinical depressive symptoms (99). Two further meta-analyses suggested that EPA, not DHA, was the key omega-3 fatty acid related to efficacy in treating depression (100, 101), consistent with the evidence for EPA’s stronger anti-inflammatory properties compared with DHA (102, 103).
Epidemiological and observational studies have demonstrated that lower omega-3 fatty acid levels are associated with higher serum IL-6, TNF-α, and CRP (104–106). In contrast, most randomized controlled trials have not produced reliable serum cytokine changes (107); the strongest support for the anti-inflammatory properties of omega-3 fatty acids in vivo has come from studies with older, hypertriglyceridemic or diabetic individuals with elevated inflammatory markers (102), as well as a randomized controlled trial with sedentary, overweight middle-aged and older adults (108).
However, inflammatory challenge studies provide compelling evidence of protective effects. Omega-3 fatty acids have been shown to attenuate both endotoxin and IFN-α-induced inflammation and sickness behavior in rodents and humans (109–113). In a randomized controlled trial in which patients received EPA, DHA, or placebo for only 2 weeks before initiation of IFN-α treatment, EPA significantly reduced the incidence of IFN-α-induced depression, and both EPA and DHA substantially delayed onset of major depression (42). EPA was more effective than DHA, consistent with two meta-analyses (100, 101). Importantly, the study population was subjected to an inflammatory insult that carried a high risk for depression, providing a backdrop that highlighted the reduction in risk.
Both the results of inflammatory challenge studies and meta-analyses suggest that heightened pretreatment inflammation and/or clinical depression enhance the odds of demonstrating omega-3 fatty acid-related improvements. The IFN-α trial clearly identified important benefits of omega-3 fatty acid treatment (42).
Exercise
Considerable evidence supports the value of exercise in treating depression and preventing its onset (76, 114, 115). Physically active individuals have lower levels of inflammatory biomarkers than their sedentary counterparts (116); reductions in inflammation provide one potential explanation for the antidepressant benefits of exercise (117).
In the Treatment With Exercise Augmentation for Depression (TREAD) study, patients with major depression who did not achieve remission following an adequate trial of a single selective serotonin reuptake inhibitor (SSRI) were randomized to two exercise augmentation groups (118). The higher-dose exercise augmentation group had a 28.3% remission rate, compared with 15.5% for the lower-dose group, and effect sizes were the same or larger than those observed in pharmacological treatment augmentation studies (118). Although four cytokines did not change significantly during the 12-week intervention, higher preintervention TNF-α levels were associated with larger decreases in depressive symptoms, and changes in IL-1β were correlated with changes in depressive symptoms (119).
These TREAD study data are consistent with a paradox in the exercise literature. Despite the fact that observational studies reliably show that more active people have lower inflammation than their sedentary counterparts (117, 120), data from randomized controlled trials demonstrating that exercise training reduces inflammation are sparse and inconsistent (121, 122). In fact, two reviews of randomized controlled trials concluded that exercise produces little or no change in inflammatory markers in healthy people who do not lose weight (121, 122).
However, just as higher pretreatment inflammation predicted a better response to a TNF-α blocker (17), the TREAD study data suggest that exercise’s antidepressant effects may be greater in those who have higher pretreatment inflammation (119). Similarly, the IFN-α trial (42) demonstrated that treatment with omega-3 fatty acids was efficacious when individuals faced a major inflammatory challenge. In each case, the initial inflammatory profile made a difference.
Obesity
Depression promotes obesity, and obesity in turn promotes depression (1, 123). Depressed people have a 58% higher risk of becoming obese; the risk for developing depression over time is 55% for persons with obesity (123). Longitudinal studies suggest that depressive symptoms promote the development of the metabolic syndrome, which has central obesity as its cornerstone (124, 125).
Depression and obesity have key inflammatory mechanisms in common. Obesity has been characterized as a state of chronic inflammation due to elevated plasma IL-6, TNF-α, and CRP levels (1). What is more, the pathways are bidirectional; visceral adipose tissue’s secretion of proinflammatory cytokines can function as a stimulus for HPA axis activation, such that hypercortisolemia enhances adipocyte accumulation, and vice versa (124).
Adiposity appears to fuel inflammatory stress responses. Women with greater central adiposity produced larger inflammatory responses to a laboratory stress task than their leaner counterparts (126). Other authors reported that both higher BMI and greater body fat were associated with larger stress-induced IL-6 responses that did not habituate with repeated stress (127).
Interactive Influence of Obesity and Age in Mice
A recent mouse study highlighted the joint impact of obesity and age on inflammatory responsiveness. Because both the decrease in lean body mass and the increase in adiposity that occurs with advancing years play a role in the age-associated increases in inflammation (128), it was not surprising that immunotherapy induced a lethal cytokine storm in aged mice, but not young mice (129). However, in young obese mice, immunotherapy induced the same cytokine overresponsiveness, organ pathology, and mortality as seen in the aged mice (129).
Together, these convergent lines of evidence show how stress and depression can act synergistically with obesity to potentiate larger inflammatory responses that could in turn further fuel depression. In addition to higher baseline levels of inflammation, these data suggest that obesity also confers risk by generating larger inflammatory responses to stress or pathogens. Higher baseline inflammation provides an important substrate for subsequent exaggerated inflammatory responses to challenge.
Priming: Cross-Sensitization Among Cytokines, Stressors, and Depression
In addition to early life stress, other major life stressors function as proximal risk factors for major depression (2). Both currently and formerly depressed people experience more major and minor stressors than those who do not have a depression history, and current and past depression can also boost emotional reactivity to stressors (130–132). Furthermore, depression can damage close personal relationships, a key stress buffer; both current and formerly depressed men and women had poorer family functioning than those who had no depression history, even years after their depression had remitted (133). A history of depression may indicate a high-risk phenotype for stress responsiveness (134). Accordingly, a past or current mood disorder could act synergistically with stress to heighten inflammation.
Cross-Sensitization in Rodents and Monkeys
The close tie between depression and stress has implications for inflammation; cross-sensitization between stressors and cytokines has been well documented in rats (135, 136). For example, exposure to a novel environment, foot or tail shock, or even exposure to conditioned stimuli that were present during foot shock all served to enhance IL-6 production (70, 135). Furthermore, rats that had previously been stressed produced larger and more rapid proinflammatory responses to a bacterial endotoxin than did rats without a prior stress exposure (135).
The rats’ endotoxin exposure mimics the immune challenges that occur frequently in daily life. For example, high-fat meals can provoke mild postprandial endoxemia (137, 138) as well as alterations in gut microbiota and intestinal permeability (139).
These data are important because the physiological systems that respond to endotoxin also respond to behavioral challenges, and this shared responsivity is particularly detrimental when endotoxin exposure occurs in proximity to psychological stress (140). In rodents, when endotoxin was paired with stressors such as tail shock or restraint, it synergistically increased production of proinflammatory cytokines, exceeding the effect of endotoxin or the stressor alone (141). The situational context also substantially affected behavioral and immunological responses to low-dose endotoxin in rhesus monkeys; the potency of the stressor influenced the magnitude and nature of endotoxin responses (140).
Recent mouse studies provide provocative evidence that higher IL-6 production may influence behavioral responses to social stress (48). Both in vivo and in vitro IL-6 responses predicted subsequent behavioral responses to repeated social defeat. Mice with larger IL-6 responses to initial aggressor exposure later displayed a stress-susceptible behavioral phenotype (depressive-like behavior) and more persistent stress-related IL-6 elevations (48). Those with smaller initial IL-6 responses were more likely to display subsequent resilient or dominant behaviors. There were also preexisting immune differences in stimulated IL-6 production between mice that would later display stress-susceptible versus resilient behavioral profiles. In further studies, a pharmacological IL-6 blockade prevented the development of social avoidance behavior, highlighting its key role (48).
Accordingly, preexisting individual differences in IL-6 responsivity predict stress vulnerability (48). Importantly, because these differences occurred within an inbred, genetically similar strain, both epigenetic and environmental factors (e.g., parental transmission of stress sensitivity, differences in the stability of the home cage’s social hierarchy, or postnatal microbial exposures) likely played a prominent role in developing these profiles (142–144).
Depression Primes Inflammatory Responsiveness
In accord with the animal literature, human studies show that depression primes inflammatory responses, promoting larger cytokine increases in reaction to stressors and pathogens. For example, mild depressive symptoms were associated with amplified and prolonged inflammatory responses following influenza vaccination in older adults as well as pregnant women (145, 146). Among women who had just given birth, those who had a lifetime history of major depression showed greater increases in both serum IL-6 and the soluble IL-6 receptor after delivery than women without a depression history (147). Similarly, patients with major depression had larger increases in inflammatory markers than nondepressed controls in response to a laboratory stressor (148, 149). In another study, individuals with more depressive symptoms had larger stress-induced increases in IL-6 following laboratory stressors than those with fewer depressive symptoms (150).
Studies that have addressed the impact of repeated laboratory stressors on IL-6 production do not show evidence of habituation (127, 151). Thus, if both currently and formerly depressed people experience more stressors than those without a depression history (130–132), they would likely continue to experience repeated exaggerated inflammatory responses.
Just as individual differences in IL-6 responsivity predict stress vulnerability in mice, the IL-6 overresponsiveness in people with depression, childhood adversity, and obesity also reflects risk. Larger, more frequent, or more prolonged inflammatory responses have negative mental and physical health consequences.
Assessing Inflammation in Research and Clinical Practice
Inflammation signals responsiveness to certain therapies and provides a guide to potential targets for clinical symptom management. For example, a meta-analysis revealed that nonsteroidal anti-inflammatory drugs reduced depressive symptoms compared with placebo, particularly the selective COX-2 inhibitor celecoxib; patients with higher inflammation benefited most (152). Similarly, a clear theme across research with omega-3 fatty acids, exercise, and cytokine antagonists is that anti-inflammatory interventions have a substantially greater impact on mood in individuals with heightened inflammation. Higher CRP levels were associated with a better response to escitalopram than to nortriptyline (153). The antidepressant effects of anti-inflammatories may be magnified in patients with comorbid pain-related or inflammatory disorders—which cover a wide spectrum, from psoriasis to cardiovascular disease to obesity. Furthermore, individuals who have relevant inflammatory genetic polymorphisms or gene expression profiles may be more responsive to these treatments (38, 39, 46, 49, 109, 154). It follows that substantial inflammatory changes (and benefits) may not be observed in those with lower inflammation who undergo the same treatment.
Indeed, a finding that highlights the importance of heightened inflammation in the context of treatment choice is that patients with lower inflammation who received a placebo improved more than did patients assigned to active treatment in both infliximab and omega-3 fatty acid trials (17, 18). These findings led to the suggestion that anti-inflammatory therapy may be harmful for patients without inflammation-driven major depression (17, 18).
Identification of patients who can benefit from anti-inflammatory interventions is clearly important. Table 1 lists risk factors for heightened inflammation, an indirect way to evaluate a patient’s inflammatory phenotype.
| Risk Factor | Comments |
|---|---|
| Older age | Inflammation rises with age (155) |
| A large prospective study showed that older depressed adults gained visceral fat over 5 years, while nondepressed adults lost visceral fat (156) | |
| Among the genes up-regulated during late life, more than half regulate inflammation-related processes, one mechanism for exaggerated proinflammatory responses (157) | |
| Early-life stress | Adults who experienced abuse or neglect as children have a substantially heightened risk for inflammation as well as depression (60, 61) |
| Low childhood socioeconomic status confers enduring risk for depression and elevated inflammation, independent of concurrent risk factors such as abuse and neglect (158) | |
| Comorbidities | Both the number and the severity of comorbid inflammatory disorders or diseases influence inflammation and risk of depression; risk is further heightened by the pain and sleep disturbances that often occur in tandem with the comorbidities (159) |
| Atypical depression | Atypical major depressive disorder with features including hypersomnia, fatigue, increased appetite, and weight gain is associated with greater inflammatory dysregulation than melancholic depression (159) |
| Chronic or recurrent depression | A more chronic course of depression is associated with higher inflammation (7, 160) |
| Obesity | Adipocytes (fat cells) produce and secrete IL-6 and TNF-α, and abdominal fat is a major inflammatory source (129) |
| Central adiposity and greater body fat are associated with larger stress-induced inflammatory responses (126, 127) | |
| There is a medium-sized relationship between body mass index and CRP level in adults (r=0.36) (161) | |
| Poor sleep | Sleep loss stimulates production of proinflammatory cytokines and cellular inflammatory signaling (162) |
| Disturbed sleep accompanies many inflammation-associated comorbidities | |
| Both decreased sleep (<5 hours) and increased sleep (>9 hours) share a medium-sized relationship with CRP level (d=0.29 and d=0.34, respectively) (163) | |
| Unhealthy diet | “Western” diets (e.g., high in red and processed meats; sweets and desserts; French fries; and refined grains) have higher associated inflammation than healthier diets (e.g., Mediterranean diets) (164) |
| Adherence to a Mediterranean diet was associated with lower IL-6 levels (85) | |
| Sedentary lifestyle | Physically active individuals have lower inflammation than their sedentary counterparts (116) |
| Better cardiorespiratory fitness is associated with lower inflammation (165) | |
| Fatigue | Fatigue, pain, and depression function as a troublesome symptom cluster across multiple medical and community populations (166) |
| Like pain and depression, fatigue has strong ties with inflammation (167) | |
| Pain | Pain generates inflammatory responses (69, 70) |
| Amplified pain sensitivity serves as an additional inflammatory source that in turn provokes negative affect (69, 71, 72, 168) | |
| Smoking | Current smokers have higher values across multiple inflammatory markers than nonsmokers (169) |
| Some former smokers have persistently elevated inflammation compared with those who never smoked (169) | |
| Alcohol dependence | CRP and proinflammatory cytokines are higher in heavy drinkers and abstainers than in moderate drinkers (170) |
| Female sex | More women than men have elevated CRP levels (171) |
| IL-6 and TNF-α responses to low-dose endotoxin did not differ between men and women, but women’s reports of depressed mood and social disconnection increased more than those of men, suggesting that women may be more sensitive to heightened inflammation (172) | |
| Obesity and CRP are more strongly related in women than in men (161) | |
| Sleep loss stimulates longer-lasting elevations in inflammation in women compared to men (75) |
TABLE 1. Assessing the Likelihood of Heightened Inflammation: Risk Factors That Raise the Index of Suspicion
For objective confirmation, the optimal strategy would be to determine treatment based on a set of biomarkers, with IL-6, TNF-α, and CRP having the strongest relationships with depression (3, 4). Differences in inflammatory patterns are likely, given the heterogeneity of the major depression population (173), and thus multiple biomarkers could provide a clearer picture of inflammatory status than a single assay, facilitating identification of the best candidates for anti-inflammatory interventions (18). For example, in an omega-3 fatty acid trial, patients with high values on any one of five biomarkers were more responsive to EPA than to placebo, and the EPA-placebo differences were larger in those who had multiple heightened inflammatory markers (18). After identification of anti-inflammatory treatment candidates, additional inflammatory assessments can provide useful treatment response data.
For routine clinical use, a biomarker needs to provide accurate and reproducible data with well-validated norms (174). Hospital laboratories need standardized assays that provide replicable data across sites (174). CRP meets these criteria, but none of the cytokines do, limiting their utility for clinical practice at present, despite their obvious value (Table 2).
| Inflammatory Biomarker | Advantages | Disadvantages | Comments |
|---|---|---|---|
| CRP | Routinely assayed by local clinical labs | Less responsive to acute stress than cytokines | Synthesized in the liver, secreted in response to IL-6 |
| No diurnal variation | Less reliably linked to psychosocial stressors compared with other markers | Half-life is 15–19 hours | |
| >5 mg/L risk stratification value suggested by literature (17) | Well-established risk stratification values and clear clinical relevance for cardiovascular disease | ||
| IL-6 | Biomarker most strongly associated with depression (3) | Limited availability in local clinical labs | Can be ordered through national laboratories |
| Most widely used inflammatory biomarker in depression research because of its sensitivity | Values dependent on assay type and assay procedures within and between labs (4) | Half-life is <6 hours | |
| Responsive to both acute and chronic stressors | No risk stratification norms, values not comparable across labs | Rises within ∼30–45 minutes of lab stressors; catecholamines increase IL-6 levels | |
| Values can differ from threefold to upwards of 50-fold based on diurnal variation, recent sleep, and the duration and intensity of recent exercise (170) | Fasting morning blood draws reduce variability | ||
| TNF-α | Positively associated with depression in clinical and community samples (3) | Limited availability in local clinical labs | Can be ordered through national laboratories |
| Reliably elevated in major depression (4) | Values dependent on assay type and assay procedures within and between labs (4) | Fasting morning blood draws reduce variability | |
| No risk stratification norms, values not comparable across labs | Targeted by cytokine inhibitor treatments | ||
| IL–1ra | Positively associated with depression in clinical and community samples (3) | Limited availability in local clinical labs | Can be ordered through national laboratories |
| Values dependent on assay type and assay procedures within and between labs (4) | Fasting morning blood draws reduce variability | ||
| No risk stratification norms, values not comparable across labs | |||
| IL-1β | Positively associated with depression in a meta-analysis of clinical and community samples (3) | Not associated with major depression in a meta-analysis of major depression studies (4) | Can be ordered through national laboratories |
| Highly skewed distribution, many samples below limits of detection | Fasting morning blood draws reduce variability | ||
| Limited availability in local clinical labs | |||
| Values dependent on assay type and assay procedures within and between labs (4) | |||
| No risk stratification norms, values not comparable across labs |
TABLE 2. Inflammatory Biomarkers Commonly Used in Depression Researcha
Anti-inflammatory treatment trials should preselect patients with heightened inflammation, and routinely assess inflammatory change (175). Surprisingly, in anti-inflammatory depression trials to date, heightened inflammation has not been part of the inclusion criteria (175). Using baseline inflammation to predict treatment response has provided provocative data, substantially expanding our understanding of who may benefit (17, 18), a worthwhile approach for future studies. In addition, researchers should examine the extent of inflammatory change and relate it to changes in depressive symptoms to better understand how lowering inflammation influences depression.
Inflammation’s Implications for Treatment of Major Depression
Inflammation is not ubiquitous among people with depression, but when the two conditions co-occur, treating inflammation in tandem with depression can enhance recovery and reduce the risk of recurrence. Table 3 includes anti-inflammatory pharmacological treatments for depression. A meta-analysis of the effects of antidepressant medication on cytokines showed an average 50% reduction in depressive symptoms across antidepressants, but only SSRIs appeared to affect cytokine production (191).
| Intervention Type | Advantages | Disadvantages | Comments |
|---|---|---|---|
| Pharmacological interventions | |||
| Celecoxib and other NSAIDs | May be useful as a monotherapy, or in combination with antidepressant medication (152) | Risk for gastrointestinal bleeding with chronic use, especially for older adults or those who use alcohol | Patients with higher initial inflammation experienced greater benefit from celecoxib than those with lower inflammation (152) |
| Celecoxib is associated with better antidepressant efficacy (response and remission) than other NSAIDs (152) | Increased risk of cardiovascular events | Patients with comorbid pain- or inflammation-related disorders responded better to NSAID treatment than those without similar disorders (152) | |
| Affects other pathways in addition to inflammation (e.g., glucocorticoid receptors, adhesion molecules) | |||
| Cytokine inhibitors (e.g., infliximab) | Specifically targets individual inflammatory cytokines | Reduces the ability to fight infection; increases risk of death and reactivation of tuberculosis | Treatment-resistant major depression patients with high baseline CRP levels had substantially greater reductions in depressive symptoms than those with low CRP levels |
| Treatment response can be monitored by assessing change in the targeted cytokine | Not suitable for those with immunosuppressive conditions | Cytokine antagonists and anti-inflammatory cytokines have reversed or reduced cytokine-induced sickness behaviors in rodents (34) | |
| Can normalize sleep (75) | Patients with lower CRP responded better to placebo than to infliximab, suggesting that infliximab may not be appropriate for patients without inflammation-driven major depression (17) | ||
| Cytokine inhibitors have reduced depressive symptoms in people with psoriasis (176, 177), lessened fatigue during cancer treatment (178), and resolved major depression in Crohn’s disease patients (179) | |||
| Omega-3 fatty acids | Higher fish consumption is associated with a lower prevalence of depression | Less beneficial in individuals without an inflammatory profile (42, 102) | Omega-3 fatty acids attenuated both endotoxin- and IFN-α-induced inflammation and sickness behavior in rodents and humans (109–113) |
| Few side effects | EPA appears to be more beneficial than DHA (42, 100, 101) | ||
| Benefits extend to cardiovascular system (e.g., lowering triglyceride levels) | Patients with a lower inflammatory profile responded better to placebo than to EPA (42) | ||
| Prebiotics, probiotics, and antibiotics | Probiotics reduce gut leakiness and neuroinflammation in animal models | More information is needed on the particular microbiota alterations in depressed patients that need to be addressed | Most of the treatment data come from animal models |
| May also affect obesity (53) | |||
| Initial studies in humans suggest that probiotics improve mood in healthy adults and those with irritable bowel syndrome and chronic fatigue syndrome (57) | |||
| Lifestyle and behavioral interventions | |||
| Healthy diets (e.g., Mediterranean diet) | May confer additional benefits, such as reduced cardiovascular disease risk | Dietary change and adherence can be a substantial obstacle | A Mediterranean diet may reduce inflammation among individuals with health risks (84) |
| Healthier diets offer some protection against the development of both depressive symptoms and depressive disorders (82, 83) | Some people may have limited access to nutritious foods because of cost and availability | Can buffer the impact of depression on inflammation (86) | |
| Caloric restriction/time restricted eating | Caloric restriction can simultaneously attenuate production of proinflammatory cytokines while enhancing anti-inflammatory pathways (88) | Implementation can be challenging | Aged mice subjected to caloric restriction had the lower cytokine responses characteristic of young mice following immunotherapy, as well as lower mortality (129) |
| Low-cost intervention | Difficult to maintain long-term adherence | ||
| Diet must be nutrient-dense to compensate for caloric restriction | |||
| Weight loss | Reduces multiple obesity-related health risks, including depression | A minority of people maintain a substantially lower weight long-term after weight loss | Weight loss reduces inflammation; those with greater weight loss have greater CRP reductions (180) |
| Exercise | Can result in substantial long-term benefits for morbidity and mortality | Regular exercise is required for continued benefit | When assessed objectively by maximal exercise testing, fitness is inversely associated with inflammation, even after adjusting for confounders including age, smoking, medications, and visceral fat (165) |
| Integrative medicine interventions | Can be adapted for those with physical limitations | Regular practice required for efficacy | Benefits are proportional to the time invested (181, 182) |
| May reduce inflammatory overresponsiveness to stressors (181, 183, 184) | The specific active anti-inflammatory components of yoga, tai chi, meditation, and other integrative therapies are not known (e.g., the relative importance of breathing, meditation, movement, etc.) | ||
| Yoga, tai chi, and mindfulness-based meditation improve sleep, another path to reduced inflammation (182, 185, 186) | |||
| Family intervention | Benefits may be long-lasting and could extend to parents and other siblings | Significant parental investment | Family therapy buffered against elevated inflammation in at-risk youths (187) |
| Can be costly | |||
| Access may be limited (e.g., by lack of family support) | |||
| Cognitive-behavioral therapy (CBT) | Can address multiple behaviors leading to inflammation (e.g., depression, sleep habits, pain, negative health behaviors) | Requires the patient’s investment in change | CBT interventions addressing depression, sleep, and pain also lowered inflammation (55, 185, 188–190) |
| Long-lasting benefit possible | Significant time commitment | ||
| Poorer outcomes among those with comorbid conditions | |||
| Risk of relapse |
TABLE 3. Anti-Inflammatory Treatment Strategiesa
Other interventions can also be beneficial. Alcohol dependence and smoking are often comorbid with depression, and both have notable inflammatory consequences (169, 192); successful treatment of either can produce durable positive changes in both inflammation and depression. Lifestyle interventions (Table 3), including weight loss, dietary change, exercise, and some integrative medicine interventions, can provide significant positive long-term benefits for patients who make and maintain a commitment to them.
Early interventions may be particularly important as a prophylaxis for those with predispositions to depression and heightened inflammation. For example, in a randomized study, inflammation was lower in 17-year-olds who had been assigned to a brief family intervention 8 years earlier compared with controls (187).
The efficacy of cognitive-behavioral therapy (CBT) in depression treatment is well documented, and CBT may concurrently reduce inflammation (55, 188). In fact, one nonrandomized trial found posttreatment decreases in an indicator of intestinal bacterial translocation as well as other inflammatory markers (55).
Both pain and disturbed sleep boost inflammation (69, 70, 75). Improvements in pain and sleep can enhance treatment outcomes and reduce the risk for recurrent depression (73–75). CBT has well-documented efficacy in the long-term remission of insomnia, and the addition of CBT for insomnia to a standard antidepressant regimen can produce a more rapid and longer-lasting remission than antidepressant treatment by itself (193). Moreover, CBT for sleep disturbances can also reduce inflammation as well as depressive symptoms (185, 189). For example, CBT for insomnia produced greater positive change (improvements in sleep, daytime fatigue, depressive symptoms, and CRP level) than either tai chi or a sleep education control condition, and remission of insomnia was associated with lower CRP levels 16 months after treatment (185).
CBT for pain and pain-related problems has improved pain, physical disability, and mood across a range of chronic pain syndromes (194). In addition, a randomized study with rheumatoid arthritis patients found that decrements in IL-6 levels were larger with CBT than with mindfulness meditation or an education-only condition (190).
We have highlighted the impact of overresponsiveness to daily stressors as an important pathway deserving of greater attention. Indeed, exaggerated stress-induced inflammatory responses mark a number of conditions that increase depression risk—e.g., fatigue, loneliness, lower subjective social status, smoking history, and marital discord (195–199). Accordingly, exaggerated inflammatory responses could reflect a greater risk for depression. Inflammatory overresponsiveness may stem in part from decreased glucocorticoid stress responses (26, 154, 195), and blunted glucocorticoid signaling in concert with increased NF-κB signaling may provide one functional fingerprint for chronic stress (154). Consequently, interventions targeting overresponsivity may benefit mood and inflammation. For example, cognitive-behavioral treatments that mute affective overresponsiveness to stressors could have important protective effects. In addition, some evidence suggests that meditation and yoga may reduce inflammatory responsiveness (181, 183, 184).
We have focused on how depression and inflammation are intertwined, but the implications extend to other health outcomes. Depression and inflammation are both linked to a number of disorders and systemic diseases, and the processes we described clearly have an impact on those diseases as well. Depression has a substantial global disease burden, and excess depression-related mortality has been documented in multiple diseases (200). The bidirectional links between depression, inflammation, and disease make this research complex; they also suggest that effective depression treatments can have a far-reaching impact on mood, inflammation, and health.
1 : Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Prog Neurobiol 2010; 91:275–299Crossref, Medline, Google Scholar
2 : From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull 2014; 140:774–815Crossref, Medline, Google Scholar
3 : Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71:171–186Crossref, Medline, Google Scholar
4 : A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67:446–457Crossref, Medline, Google Scholar
5 : Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α), and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord 2012; 139:230–239Crossref, Medline, Google Scholar
6 : CRP, IL-6, and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord 2013; 150:736–744Crossref, Medline, Google Scholar
7 : Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry 2012; 71:15–21Crossref, Medline, Google Scholar
8 : Are there bi-directional associations between depressive symptoms and C-reactive protein in mid-life women? Brain Behav Immun 2010; 24:96–101Crossref, Medline, Google Scholar
9 : Depressive symptoms, race, and circulating C-reactive protein: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosom Med 2010; 72:734–741Crossref, Medline, Google Scholar
10 : Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: prospective findings from the heart and soul study. Am J Psychiatry 2011; 168:913–920Link, Google Scholar
11 : Inflammatory markers and the pathogenesis of pediatric depression and suicide: a systematic review of the literature. J Clin Psychiatry 2014; 75:1242–1253Crossref, Medline, Google Scholar
12 : Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry 2014; 71:1121–1128Crossref, Medline, Google Scholar
13 : Inflammation, obesity, and metabolic syndrome in depression: analysis of the 2009–2010 National Health and Nutrition Examination Survey (NHANES). J Clin Psychiatry 2014; 75:e1428–e1432Crossref, Medline, Google Scholar
14 : Is depression an inflammatory disorder? Curr Psychiatry Rep 2011; 13:467–475Crossref, Medline, Google Scholar
15 : Where there is depression, there is inflammation ... sometimes! Biol Psychiatry 2007; 62:280–281Crossref, Medline, Google Scholar
16 : Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012; 37:137–162Crossref, Medline, Google Scholar
17 : A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 2013; 70:31–41Crossref, Medline, Google Scholar
18 : Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry (Epub ahead of print, March 24, 2014)Medline, Google Scholar
19 : Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 2013; 30:297–306Crossref, Medline, Google Scholar
20 : Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol 2011; 11:625–632Crossref, Medline, Google Scholar
21 : Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 2011; 130:226–238Crossref, Medline, Google Scholar
22 : Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology 2011; 36:426–436Crossref, Medline, Google Scholar
23 : Mechanistic explanations how cell-mediated immune activation, inflammation, and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 2012; 36:764–785Crossref, Medline, Google Scholar
24 : Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology 2012; 37:1397–1416Crossref, Medline, Google Scholar
25 : Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 2011; 73:114–126Crossref, Medline, Google Scholar
26 : Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun 2007; 21:9–19Crossref, Medline, Google Scholar
27 : Molecular dynamics of ultradian glucocorticoid receptor action. Mol Cell Endocrinol 2012; 348:383–393Crossref, Medline, Google Scholar
28 : Glucocorticoids, cytokines, and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:722–729Crossref, Medline, Google Scholar
29 : From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9:46–56Crossref, Medline, Google Scholar
30 : Glucocorticoid regulation of brain-derived neurotrophic factor: relevance to hippocampal structural and functional plasticity. Neuroscience 2013; 239:196–213Crossref, Medline, Google Scholar
31 : The effects of chronic glucocorticoid exposure on dendritic length, synapse numbers, and glial volume in animal models: implications for hippocampal volume reductions in depression. Physiol Behav 2010; 99:186–193Crossref, Medline, Google Scholar
32 : Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology 2013; 38:872–883Crossref, Medline, Google Scholar
33 : Malaise, melancholia, and madness: the evolutionary legacy of an inflammatory bias. Brain Behav Immun 2013; 31:1–8Crossref, Medline, Google Scholar
34 : Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 2006; 27:24–31Crossref, Medline, Google Scholar
35 : Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65:732–741Crossref, Medline, Google Scholar
36 : So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 2013; 11:200Crossref, Medline, Google Scholar
37 : A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci Biobehav Rev 2010; 34:130–143Crossref, Medline, Google Scholar
38 : Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry 2009; 14:1095–1104Crossref, Medline, Google Scholar
39 : Serotonin and interleukin-6: the role of genetic polymorphisms in IFN-induced neuropsychiatric symptoms. Psychoneuroendocrinology 2013; 38:1803–1813Crossref, Medline, Google Scholar
40 : Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry 2012; 73:1128–1138Crossref, Medline, Google Scholar
41 : Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:445–450Crossref, Medline, Google Scholar
42 : Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biol Psychiatry 2014; 76:559–566Crossref, Medline, Google Scholar
43 : Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res 2007; 41:326–331Crossref, Medline, Google Scholar
44 : Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J Affect Disord 2013; 148:136–140Crossref, Medline, Google Scholar
45 : Higher plasma interleukin-6 (IL-6) level is associated with SSRI- or SNRI-refractory depression. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33:722–726Crossref, Medline, Google Scholar
46 : The interleukin 1 beta (IL1B) gene is associated with failure to achieve remission and impaired emotion processing in major depression. Biol Psychiatry 2010; 67:543–549Crossref, Medline, Google Scholar
47 : Late-life depression symptom profiles are differentially associated with immunometabolic functioning. Brain Behav Immun 2014; 41:109–115Crossref, Medline, Google Scholar
48 : Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci USA 2014; 111:16136–16141Crossref, Medline, Google Scholar
49 : Association study of the interleukin-1 beta (C-511T) genetic polymorphism with major depressive disorder, associated symptomatology, and antidepressant response. Neuropsychopharmacology 2003; 28:1182–1185Crossref, Medline, Google Scholar
50 : Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline “predictors” and longitudinal “targets”. Neuropsychopharmacology 2013; 38:377–385Crossref, Medline, Google Scholar
51 : Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol Psychiatry 2008; 13:800–812Crossref, Medline, Google Scholar
52 : Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci 2014; 34:15490–15496Crossref, Medline, Google Scholar
53 : Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012; 13:701–712Crossref, Medline, Google Scholar
54 : The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuroendocrinol Lett 2008; 29:117–124Medline, Google Scholar
55 : Expression of Toll-like receptors in peripheral blood mononuclear cells and response to cognitive-behavioral therapy in major depressive disorder. Brain Behav Immun 2014; 40:235–243Crossref, Medline, Google Scholar
56 : Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci USA 2014; 111:E4485–E4493Crossref, Medline, Google Scholar
57 : Psychobiotics: a novel class of psychotropic. Biol Psychiatry 2013; 74:720–726Crossref, Medline, Google Scholar
58 : Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012; 37:1885–1895Crossref, Medline, Google Scholar
59 : Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry 2012; 169:141–151Link, Google Scholar
60 : Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry 2008; 65:409–415Crossref, Medline, Google Scholar
61 : Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med 2011; 73:16–22Crossref, Medline, Google Scholar
62 : Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull 2011; 137:959–997Crossref, Medline, Google Scholar
63 : Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun 2013; 27:8–12Crossref, Medline, Google Scholar
64 : Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Transl Psychiatry 2014; 4:e413Crossref, Medline, Google Scholar
65 : The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 2008; 33:693–710Crossref, Medline, Google Scholar
66 : Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology 2010; 35:2617–2623Crossref, Medline, Google Scholar
67 : Childhood abuse and inflammatory responses to daily stressors. Ann Behav Med 2012; 44:287–292Crossref, Medline, Google Scholar
68 : Negative association of concomitant physical symptoms with the course of major depressive disorder: a systematic review. J Psychosom Res 2010; 68:511–519Crossref, Medline, Google Scholar
69 : Acute painful stress and inflammatory mediator production. Neuroimmunomodulation 2013; 20:127–133Crossref, Medline, Google Scholar
70 : Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology 1993; 133:2523–2530Crossref, Medline, Google Scholar
71 : Acute experimental endotoxemia induces visceral hypersensitivity and altered pain evaluation in healthy humans. Pain 2012; 153:794–799Crossref, Medline, Google Scholar
72 : Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med 2005; 257:139–155Crossref, Medline, Google Scholar
73 : Does pain interfere with antidepressant depression treatment response and remission in patients with depression and pain? An evidence-based structured review. Pain Med 2014; 15:1522–1539Crossref, Medline, Google Scholar
74 : Pain, not chronic disease, is associated with the recurrence of depressive and anxiety disorders. BMC Psychiatry 2014; 14:187Crossref, Medline, Google Scholar
75 : Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol 2015; 66:143–172Crossref, Medline, Google Scholar
76 : A review of lifestyle factors that contribute to important pathways associated with major depression: diet, sleep, and exercise. J Affect Disord 2013; 148:12–27Crossref, Medline, Google Scholar
77 : Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiatry 2010; 68:942–949Crossref, Medline, Google Scholar
78 : Sleep disturbance and longitudinal risk of inflammation: moderating influences of social integration and social isolation in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Brain Behav Immun 2015; 46:319–326Crossref, Medline, Google Scholar
79 : Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep 2012; 35:537–543Crossref, Medline, Google Scholar
80 : Dietary patterns and the risk of depression in adults: a systematic review of observational studies. Eur J Nutr 2014; 53:997–1013Crossref, Medline, Google Scholar
81 : A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr 2014; 99:181–197Crossref, Medline, Google Scholar
82 : Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry 2009; 66:1090–1098Crossref, Medline, Google Scholar
83 : Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: results from a large community-based prospective study. Eur J Clin Nutr 2013; 67:75–82Crossref, Medline, Google Scholar
84 : Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 2004; 292:1440–1446Crossref, Medline, Google Scholar
85 : Adherence to the Mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: a twin study. Circulation 2008; 117:169–175Crossref, Medline, Google Scholar
86 : Depressive symptoms and inflammation increase in a prospective study of older adults: a protective effect of a healthy (Mediterranean-style) diet. Mol Psychiatry 2011; 16:589–590Crossref, Medline, Google Scholar
87 : Inflammatory dietary pattern and risk of depression among women. Brain Behav Immun 2014; 36:46–53Crossref, Medline, Google Scholar
88 : Neuroendocrine factors in the regulation of inflammation: excessive adiposity and calorie restriction. Exp Gerontol 2009; 44:41–45Crossref, Medline, Google Scholar
89 : Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci 2008; 28:3071–3075Crossref, Medline, Google Scholar
90 : Interleukin-6, C-reactive protein, and biochemical parameters during prolonged intermittent fasting. Ann Nutr Metab 2007; 51:88–95Crossref, Medline, Google Scholar
91 : Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 2014; 20:991–1005Crossref, Medline, Google Scholar
92 : Immune-to-brain signaling and central prostaglandin E2 synthesis in fasted rats with altered lipopolysaccharide-induced fever. Am J Physiol Regul Integr Comp Physiol 2008; 295:R133–R143Crossref, Medline, Google Scholar
93 : Fasting in mood disorders: neurobiology and effectiveness: a review of the literature. Psychiatry Res 2013; 209:253–258Crossref, Medline, Google Scholar
94 : Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med 2012; 10:66Crossref, Medline, Google Scholar
95 : A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry 2010; 68:140–147Crossref, Medline, Google Scholar
96 : Omega-3 fatty acids influence mood in healthy and depressed individuals. Nutr Rev 2013; 71:727–741Crossref, Medline, Google Scholar
97 : Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr 2010; 91:757–770Crossref, Medline, Google Scholar
98 : Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatry 2012; 17:1272–1282Crossref, Medline, Google Scholar
99 : Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One 2014; 9:e96905Crossref, Medline, Google Scholar
100 : Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry 2011; 72:1577–1584Crossref, Medline, Google Scholar
101 : EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr 2009; 28:525–542Crossref, Medline, Google Scholar
102 : Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc Nutr Soc 2007; 66:237–259Crossref, Medline, Google Scholar
103 : Resolvins and protectins in the termination program of acute inflammation. Trends Immunol 2007; 28:176–183Crossref, Medline, Google Scholar
104 : Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab 2006; 91:439–446Crossref, Medline, Google Scholar
105 : Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: the Heart and Soul Study. Atherosclerosis 2009; 205:538–543Crossref, Medline, Google Scholar
106 : Unsaturated fatty acids are inversely associated and n-6/n-3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clin Chim Acta 2010; 411:584–591Crossref, Medline, Google Scholar
107 : Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr 2012; 107(Suppl 2):S159–S170Crossref, Medline, Google Scholar
108 : Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun 2012; 26:988–995Crossref, Medline, Google Scholar
109 : Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alpha-induced depression by regulating polyunsaturated fatty acids levels. Biol Psychiatry 2010; 67:550–557Crossref, Medline, Google Scholar
110 : Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol Psychiatry 2005; 57:343–350Crossref, Medline, Google Scholar
111 : Blunting the response to endotoxin in healthy subjects: effects of various doses of intravenous fish oil. Intensive Care Med 2010; 36:289–295Crossref, Medline, Google Scholar
112 : Intravenous fish oil blunts the physiological response to endotoxin in healthy subjects. Intensive Care Med 2007; 33:789–797Crossref, Medline, Google Scholar
113 : Effects of dietary n-3 or n-6 fatty acids on interleukin-1beta-induced anxiety, stress, and inflammatory responses in rats. J Lipid Res 2003; 44:1984–1991Crossref, Medline, Google Scholar
114 : Bidirectional association between physical activity and symptoms of anxiety and depression: the Whitehall II study. Eur J Epidemiol 2012; 27:537–546Crossref, Medline, Google Scholar
115 : Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med 2013; 45:649–657Crossref, Medline, Google Scholar
116 : The immunomodulating role of exercise in metabolic disease. Trends Immunol 2014; 35:262–269Crossref, Medline, Google Scholar
117 : The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011; 11:607–615Crossref, Medline, Google Scholar
118 : Exercise as an augmentation treatment for nonremitted major depressive disorder: a randomized, parallel dose comparison. J Clin Psychiatry 2011; 72:677–684Crossref, Medline, Google Scholar
119 : Pro-inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol Psychiatry 2013; 18:1119–1124Crossref, Medline, Google Scholar
120 : Exercise training as a treatment for chronic inflammation in the elderly. Exerc Sport Sci Rev 2009; 37:165–170Crossref, Medline, Google Scholar
121 : Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr 2011; 106(Suppl 3):S5–S78Crossref, Medline, Google Scholar
122 : Effect of exercise training on chronic inflammation. Clin Chim Acta 2010; 411:785–793Crossref, Medline, Google Scholar
123 : Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010; 67:220–229Crossref, Medline, Google Scholar
124 : Stress, visceral obesity, and metabolic complications. Ann N Y Acad Sci 2006; 1083:77–110Crossref, Medline, Google Scholar
125 : Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: a comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care 2007; 30:872–877Crossref, Medline, Google Scholar
126 : Stress-induced cytokine responses and central adiposity in young women. Int J Obes 2008; 32:443–450Crossref, Google Scholar
127 : Measures of adiposity predict interleukin-6 responses to repeated psychosocial stress. Brain Behav Immun 2014; 42:33–40Crossref, Medline, Google Scholar
128 : Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000; 908:244–254Crossref, Medline, Google Scholar
129 : Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J Exp Med 2014; 211:2373–2383Crossref, Medline, Google Scholar
130 : Generation of stress in the course of unipolar depression. J Abnorm Psychol 1991; 100:555–561Crossref, Medline, Google Scholar
131 : Past depression and gender interact to influence emotional reactivity to daily life stress. Cognit Ther Res 2009; 33:264–271Crossref, Google Scholar
132 : Depression history, depression vulnerability, and the experience of everyday negative events. J Soc Clin Psychol 2010; 29:949–974Crossref, Medline, Google Scholar
133 : Current and past depression as predictors of family functioning: a comparison of men and women in a community sample. J Fam Psychol 2007; 21:694–702Crossref, Medline, Google Scholar
134 : Marital discord, past depression, and metabolic responses to high-fat meals: interpersonal pathways to obesity. Psychoneuroendocrinology 2015; 52:239–250Crossref, Medline, Google Scholar
135 : Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun 2002; 16:461–476Crossref, Medline, Google Scholar
136 : Cytokines and depression: fortuitous or causative association? Mol Psychiatry 1999; 4:328–332Crossref, Medline, Google Scholar
137 : The capacity of foodstuffs to induce innate immune activation of human monocytes in vitro is dependent on food content of stimulants of Toll-like receptors 2 and 4. Br J Nutr 2011; 105:15–23Crossref, Medline, Google Scholar
138 : High-fat meal induced postprandial inflammation. Mol Nutr Food Res 2014; 58:136–146Crossref, Medline, Google Scholar
139 : Influence of a high-fat diet on gut microbiota, intestinal permeability, and metabolic endotoxaemia. Br J Nutr 2012; 108:801–809Crossref, Medline, Google Scholar
140 : Environmental context differentially affects behavioral, leukocyte, cortisol, and interleukin-6 responses to low doses of endotoxin in the rhesus monkey. Brain Behav Immun 2007; 21:807–815Crossref, Medline, Google Scholar
141 : Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des 2005; 11:963–972Crossref, Medline, Google Scholar
142 : Paternal transmission of stress-induced pathologies. Biol Psychiatry 2011; 70:408–414Crossref, Medline, Google Scholar
143 : The maternal-neonatal neuro-immune interface: are there long-term implications for inflammatory or stress-related disease? J Clin Invest 2001; 108:1567–1573Crossref, Medline, Google Scholar
144 : Do environments in infancy moderate the association between stress and inflammation in adulthood? Initial evidence from a birth cohort in the Philippines. Brain Behav Immun 2013; 31:23–30Crossref, Medline, Google Scholar
145 : Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry 2003; 60:1009–1014Crossref, Medline, Google Scholar
146 : Depressive symptoms predict exaggerated inflammatory responses to an in vivo immune challenge among pregnant women. Brain Behav Immun 2010; 24:49–53Crossref, Medline, Google Scholar
147 : The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J Affect Disord 2001; 63:85–92Crossref, Medline, Google Scholar
148 : Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry 2006; 163:1630–1633Link, Google Scholar
149 : Neurohormonal and inflammatory hyper-responsiveness to acute mental stress in depression. Biol Psychol 2010; 84:228–234Crossref, Medline, Google Scholar
150 : Depressive symptoms enhance stress-induced inflammatory responses. Brain Behav Immun 2013; 31:172–176Crossref, Medline, Google Scholar
151 : Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behav Immun 2006; 20:40–48Crossref, Medline, Google Scholar
152 : Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 2014; 71:1381–1391Crossref, Medline, Google Scholar
153 : An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry 2014; 171:1278–1286Link, Google Scholar
154 : A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry 2008; 64:266–272Crossref, Medline, Google Scholar
155 : Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev 2007; 65:S173–S176Crossref, Medline, Google Scholar
156 : Depressive symptoms and change in abdominal obesity in older persons. Arch Gen Psychiatry 2008; 65:1386–1393Crossref, Medline, Google Scholar
157 : The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry 2011; 26:1109–1118Medline, Google Scholar
158 : Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med 2009; 163:1135–1143Crossref, Medline, Google Scholar
159 : Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med 2013; 11:129Crossref, Medline, Google Scholar
160 : Inflammatory and metabolic dysregulation and the 2-year course of depressive disorders in antidepressant users. Neuropsychopharmacology 2014; 39:1624–1634Crossref, Medline, Google Scholar
161 : Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev 2013; 14:232–244Crossref, Medline, Google Scholar
162 : Sleep loss activates cellular inflammatory signaling. Biol Psychiatry 2008; 64:538–540Crossref, Medline, Google Scholar
163 : Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep 2013; 36:769–779ECrossref, Medline, Google Scholar
164 : Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2004; 80:1029–1035Crossref, Medline, Google Scholar
165 : The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol 2005; 45:1563–1569Crossref, Medline, Google Scholar
166 : Loneliness predicts pain, depression, and fatigue: understanding the role of immune dysregulation. Psychoneuroendocrinology 2013; 38:1310–1317Crossref, Medline, Google Scholar
167 : The associations of adiposity, physical activity, and inflammation with fatigue in older adults. Brain Behav Immun 2011; 25:1482–1490Crossref, Medline, Google Scholar
168 : Inflammation-induced hyperalgesia: effects of timing, dosage, and negative affect on somatic pain sensitivity in human experimental endotoxemia. Brain Behav Immun 2014; 41:46–54Crossref, Medline, Google Scholar
169 : Relation of smoking status to a panel of inflammatory markers: the framingham offspring. Atherosclerosis 2008; 201:217–224Crossref, Medline, Google Scholar
170 : To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun 2009; 23:887–897Crossref, Medline, Google Scholar
171 : Sex differences in age trajectories of physiological dysregulation: inflammation, metabolic syndrome, and allostatic load. J Gerontol A Biol Sci Med Sci 2011; 66:493–500Crossref, Medline, Google Scholar
172 : Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology 2015; 40:1709–1716Crossref, Medline, Google Scholar
173 : Evidence for a differential role of HPA-axis function, inflammation, and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry 2013; 18:692–699Crossref, Medline, Google Scholar
174 : Inflammatory cytokines as biomarkers in heart failure. Clin Chim Acta 2015; 443:71–77Crossref, Medline, Google Scholar
175 : Are anti-inflammatory therapies viable treatments for psychiatric disorders? Where the rubber meets the road. JAMA Psychiatry 2015; 72:527–528Crossref, Medline, Google Scholar
176 : Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 2006; 367:29–35Crossref, Medline, Google Scholar
177 : Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase III trial. J Am Acad Dermatol 2010; 63:457–465Crossref, Medline, Google Scholar
178 : Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol 2006; 24:1852–1859Crossref, Medline, Google Scholar
179 : The impact of major depressive disorder on the short- and long-term outcome of Crohn’s disease treatment with infliximab. Aliment Pharmacol Ther 2005; 22:101–110Crossref, Medline, Google Scholar
180 : The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med 2007; 167:31–39Crossref, Medline, Google Scholar
181 : Stress, inflammation, and yoga practice. Psychosom Med 2010; 72:113–121Crossref, Medline, Google Scholar
182 : Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol 2014; 32:1040–1049Crossref, Medline, Google Scholar
183 : Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc Natl Acad Sci USA 2014; 111:7379–7384Crossref, Medline, Google Scholar
184 : Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology 2014; 43:20–29Crossref, Medline, Google Scholar
185 : Cognitive behavioral therapy vs tai chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep 2014; 37:1543–1552Crossref, Medline, Google Scholar
186 : Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain 2014; 155:1547–1554Crossref, Medline, Google Scholar
187 : A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proc Natl Acad Sci USA 2014; 111:11287–11292Crossref, Medline, Google Scholar
188 : The impact of cognitive behavioral therapy on IL-6 levels in unmedicated women experiencing the first episode of depression: a pilot study. Psychiatry Res 2013; 209:742–745Crossref, Medline, Google Scholar
189 : Cognitive-behavioral therapy for sleep disturbance decreases inflammatory cytokines and oxidative stress in hemodialysis patients. Kidney Int 2011; 80:415–422Crossref, Medline, Google Scholar
190 : Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. J Consult Clin Psychol 2008; 76:408–421Crossref, Medline, Google Scholar
191 : The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology 2011; 36:2452–2459Crossref, Medline, Google Scholar
192 : Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol Psychiatry 2014; 76:725–733Crossref, Medline, Google Scholar
193 : Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep 2008; 31:489–495Crossref, Medline, Google Scholar
194 : Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol 2014; 69:153–166Crossref, Medline, Google Scholar
195 : Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain Behav Immun 2007; 21:251–258Crossref, Medline, Google Scholar
196 : Loneliness promotes inflammation during acute stress. Psychol Sci 2013; 24:1089–1097Crossref, Medline, Google Scholar
197 : Lower subjective social status exaggerates interleukin-6 responses to a laboratory stressor. Psychoneuroendocrinology 2013; 38:2676–2685Crossref, Medline, Google Scholar
198 : Long lasting effects of smoking: breast cancer survivors’ inflammatory responses to acute stress differ by smoking history. Psychoneuroendocrinology 2013; 38:179–187Crossref, Medline, Google Scholar
199 : Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry 2005; 62:1377–1384Crossref, Medline, Google Scholar
200 : Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry 2014; 171:453–462Link, Google Scholar



