Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia
Abstract
Objective: The study of human anxiety disorders has benefited greatly from functional neuroimaging approaches. Individual studies, however, vary greatly in their findings. The authors searched for common and disorder-specific functional neurobiological deficits in several anxiety disorders. The authors also compared these deficits to the neural systems engaged during anticipatory anxiety in healthy subjects. Method: Functional magnetic resonance imaging and positron emission tomography studies of posttraumatic stress disorder (PTSD), social anxiety disorder, specific phobia, and fear conditioning in healthy individuals were compared by quantitative meta-analysis. Included studies compared negative emotional processing to baseline, neutral, or positive emotion conditions. Results: Patients with any of the three disorders consistently showed greater activity than matched comparison subjects in the amygdala and insula, structures linked to negative emotional responses. A similar pattern was observed during fear conditioning in healthy subjects. Hyperactivation in the amygdala and insula were, of interest, more frequently observed in social anxiety disorder and specific phobia than in PTSD. By contrast, only patients with PTSD showed hypoactivation in the dorsal and rostral anterior cingulate cortices and the ventromedial prefrontal cortex—structures linked to the experience and regulation of emotion. Conclusions: This meta-analysis allowed us to synthesize often disparate findings from individual studies and thereby provide neuroimaging evidence for common brain mechanisms in anxiety disorders and normal fear. Effects unique to PTSD furthermore suggested a mechanism for the emotional dysregulation symptoms in PTSD that extend beyond an exaggerated fear response. Therefore, these findings help refine our understanding of anxiety disorders and their interrelationships.
Fear and avoidance of trigger cues are common to many anxiety disorders (1) and resemble the arousal and avoidance responses shown by normal subjects to conditioned fear cues (2) . Thus, a common element of anxiety disorders may be an abnormally elevated fear response. Based on animal models of fear learning (3 , 4) , this hypothesis leads to the prediction that amygdalar dysfunction is common to a variety of anxiety disorders. Indeed, amygdalar hyperactivity has been observed during symptom provocation or negative emotional processing in patients with posttraumatic stress disorder (PTSD) (5 – 8) , social anxiety disorder (9 – 14) , specific phobia (15 – 18) , panic disorder (19) , and obsessive-compulsive disorder (OCD) (19 , 20) . However, because of the low statistical power of individual studies and heterogeneity in task design, patient characteristics, imaging modality, and analytic approach, results across these studies have often been inconsistent, and analyses of replicability across studies are needed.
In PTSD, for example, there have been reports of patients having decreased, rather than increased, amygdalar activity (21 , 22) , as well as reports of no differences between patients and comparison subjects (23 – 32) . Similarly, although many studies have reported increases in amygdalar activity in specific phobia, several studies have reported no patient/comparison subject differences (33 – 35) , and one reported decreased amygdala activation in patients (36) . Finally, in panic disorder and OCD, amygdalar hyperactivity appears to be the exception, rather than the rule (37 , 38) . These inconsistencies have led various authors to argue that the amygdala may play a role in only some fear states, i.e., that it may not play a critical role in symptoms of PTSD (23 , 24 , 29) , specific phobia (34 , 37) , panic disorder (39) , or OCD (37) . The roles of several other brain regions are also in controversy, particularly those with a less extensive animal literature (40) .
Despite some shared key features, anxiety disorders also differ in a number of fundamental ways. Symptoms of hypervigilance and hyperarousal, dissociation, emotional numbing, and reexperiencing phenomena (nightmares and flashbacks) are particularly characteristic of PTSD (1) . These symptoms are not observed during normal fear conditioning, suggesting that PTSD involves either different or more profound emotional dysregulation than other anxiety disorders. Direct comparisons of functional abnormalities between disorders within the context of a single study are, however, rare. Quantitative meta-analysis can help resolve these ambiguities by permitting formal assessment of the evidence for regional brain dysfunction and quantitative comparisons across disorders.
In this article, we used a voxelwise meta-analysis to compare activation patterns from positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies across three of the most well-studied anxiety disorders—PTSD, social anxiety disorder, and specific phobia. First, we tested for evidence that the three disorders involve common neural alterations, which reflect abnormally elevated fear. To do so, we compared neuroimaging results from each disorder to results from studies of fear conditioning in healthy subjects. Second, we identified regions in which disorder-specific abnormalities may be related to disorder-specific symptoms. Finally, we performed novel tests of coactivation across regions to test whether, across individual studies, limbic dysregulation is reliably associated with medial prefrontal dysfunction (41 , 42) .
This meta-analysis offers a unique window onto the neural mechanisms of clinical anxiety that may guide further hypothesis-driven investigations into the neural basis of psychiatric disorders. Comparing brain activity between disorders in this way also holds promise for providing insight into the relationships between clinical conditions and may inform current efforts to classify psychiatric disorders (43) .
Method
Study Selection
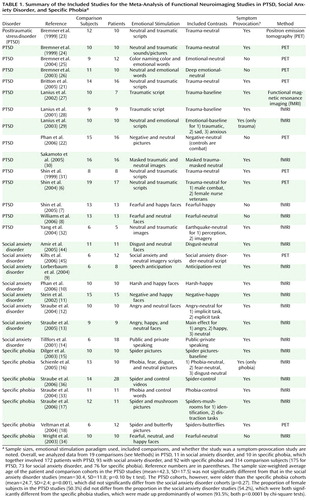
Studies were selected by searching PubMed and reference lists for PET or fMRI studies of anxiety disorders (PTSD, social anxiety disorder, specific phobia, OCD, generalized anxiety disorder, and panic disorder) or fear conditioning in healthy volunteers available until September 2006. To be eligible, studies must have reported coordinates of peak activations for comparisons between patient and matched comparison groups across multiple brain regions. We included the studies that contrasted a negative emotional condition with neutral or positive emotional conditions or a resting baseline. Only PTSD (6 – 8 , 21 – 32) , social anxiety disorder (9 – 14 , 44 , 45) , specific phobia (15 – 18 , 33 , 36) , and fear conditioning (46 – 55) had the required 10 comparisons meeting inclusion criteria to allow for a viable meta-analysis. Examples of negative stimuli include trauma-related stimuli or reminder scripts, emotional words, or faces in PTSD; emotional faces and public speaking or its anticipation in social anxiety disorder; and feared objects (i.e., spiders) and emotional faces in specific phobia. Fear-conditioning studies compared brain responses to a conditioned stimulus presented alone (without an associated unconditioned aversive stimulus) with responses to nonconditioned neutral stimuli. This contrast avoids confounding fear responses related to a conditioned stimulus with those to unconditioned aversive stimuli.
Data Analysis
As in previous meta-analytic work, we analyzed the locations of reported peak activation coordinates across the three-dimensional space of the brain (56 – 61) . Studies reported a set of coordinates for each discrete negative versus comparison relation within each study, separately for the patients > comparison subjects (hyperactivation) and comparison subjects > patients (hypoactivation) directions. We note that hyperactivations may occur either because patients activated a region more than comparison subjects or deactivated it less than comparison subjects (relative to a third, arbitrary, baseline condition) and vice versa for hypoactivations. Nonetheless, in both circumstances, hyperactivation in patients reflects greater overall regional activity in patients (patients > comparison subjects) and the inverse for hypoactivation. As shown in Table 1 , studies reported coordinates from between one and three comparisons.
We constructed indicator maps (I, with values of 1 or 0) of whether each comparison resulted in activation coordinates within a 10-mm sphere surrounding each voxel in a 2×2×2 mm standard brain (Montreal, Que., Canada, Neurologic Institute avg152t1.img, SPM2 version; http://www.fil.ion.ucl.ac.uk/spm/spm2.html). Talairach coordinates were converted to Montreal Neurologic Institute coordinates by using Matthew Brett’s tal2mni.m script, implemented in Matlab (http://imaging. mrccbu.cam.ac.uk/imaging/MniTalairach) (62) . The meta-analysis statistic at each voxel was the proportion of comparisons that activated within 10 mm of that voxel (Pˆ v ), weighted by the square root of the sample size for each study. These weights allowed the larger, and thus more reliable, studies to carry more weight in the meta-analysis. Weights were normalized by the sum across comparisons so that for each voxel v in the brain, where wn is the weight for the nth of N comparison maps. To make statistical analysis tractable with the information available from published studies, we treated each comparison map as independent. However, we contacted investigators to determine the extent of nonindependence in subject cohorts (personal communications from Shin LM, Phan KL, Bremner JD, and Straube T). Of the studies for which this information was available, two studies of PTSD had complete overlap in subject cohorts (21 , 22) , and two pairs of studies had partial overlap (6 , 7 , 28 , 29) . In social anxiety disorder, two had partial overlap (12 , 13) , and in specific phobia, two had partial overlap (17 , 36) . To address the issue of partial nonindependence, we performed supplementary leave-one-study-out jackknife analyses, described below, that increased confidence that subject overlap did not qualitatively influence our results.
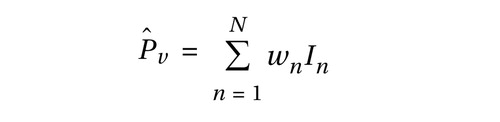
The approach described above has several advantages (63) : 1) it treats comparison maps within studies as random effects, so that no single comparison map can contribute disproportionately, even if many peak coordinates are reported; 2) sample size weighting increases accuracy without the complexity of z-score-based weighting for studies with different types of analyses; and 3) the proportion of comparisons metric (P) is straightforward to interpret, unlike methods based on Gaussian or other kernels. Furthermore, unlike other methods that rely on z-score weighting, our meta-analysis approach is better suited to assess the replicability of activation across studies (63) .
For each disorder, we created an activation mask consisting of voxels in which activation proportions Pˆ v exceeded the null-hypothesis density P0 , established through Monte Carlo simulation. Probability values were corrected for multiple comparisons using false-discovery-rate control (64) at q<0.05, corrected. An additional threshold of at least two studies was imposed to ensure that one study could not create a significant meta-analytic result. The null hypothesis was a uniform random distribution of peaks within each comparison in a gray matter (plus 8-mm border) mask in the standard brain (SPM2 segmented avg152t1.img with 8-mm Gaussian smoothing). We then compared Pˆ v for hyperactivations (patients > comparison subjects) versus hypoactivations (comparison subjects > patients) at each voxel within the activation mask using nonparametric chi-square tests (63) . We used a statistical threshold of p<0.005 and a 10-voxel spatial extent.
To directly compare the disorders, we constructed regions-of-interest using the WFU PickAtlas (65) , including those in the amygdala, insula, and thalamus. A ventromedial prefrontal region of interest consisted of medial frontal voxels below z=0. A rostral anterior cingulate cortex region of interest was defined as areas 24 and 32 between z=–4 and z=+12, and a dorsal anterior cingulate cortex region of interest corresponded to the anterior cingulate cortex dorsal to z=+12 and anterior to y=+16. We computed Pˆ for hyper- and hypoactivation within each region of interest and compared them across disorders using nonparametric chi-square tests.
To assess whether any single study had a substantial impact on the meta-analytic results from the chi-square analyses, we calculated jackknife chi-square statistics and corresponding p values on each of the regions of interest (66) . To implement the jackknife chi-square on the 40 comparisons within each region of interest, we recomputed the chi-square test 40 times, each time leaving out one of the 40 studies. We summarized the results in terms of the percentage of jackknife tests that were significant at a p<0.05 and the maximum p value with one study removed. A value of 100% at p<0.05 indicates that the p value was less than 0.05 for every test, and there was no single study whose omission changed the result at that alpha level.
Coactivation Analysis
We subjected data derived from a priori regions of interest to multivariate analysis to test whether coactivation patterns (the meta-analytic equivalent of functional connectivity) across comparison maps differed across disorders. An indicator matrix I (of size studies by regions) was constructed that encoded whether each comparison reported an activation coordinate within the region of interest. Patients > comparison subjects and comparison subjects > patients differences were integrated by coding them with values of 1 and –1. Associations between all pairs of regions were assessed using Kendall’s tau b (t) (67 , 68) , a nonparametric measure of association. Positive t values for a pair of regions indicate that across studies, observing activation in one region increases the likelihood of observing activation in the other (i.e., either hyper- or hypoactivation tends to co-occur). Negative t values indicate that activation in one region predicts less frequent activation in another (i.e., hypoactivation in one region is associated with hyperactivation in another). These analyses, which exploit across-study variance in the directions of region-of-interest activation, can be distinguished from common forms of connectivity analyses in individual neuroimaging studies, which exploit within-study sources of variance (either across time or across subjects).
To visualize the relationships among all regions of interest, in Figure 1 , we display regions and lines showing significant bivariate t on a “flattened” map of the connectivity space determined by nonmetric multidimensional scaling (69 – 71) . We first converted the interregion t matrix into a dissimilarity matrix (D) using the formula Dij = (1–t ij )/2. Nonmetric multidimensional scaling was used to find latent components without assuming that distances are euclidean with the s-stress criterion, as implemented in mdscale.m in Matlab 7.3 (Mathworks, Natick, Mass.). A three-dimensional space accurately reproduced the data (s-stress<0.05).
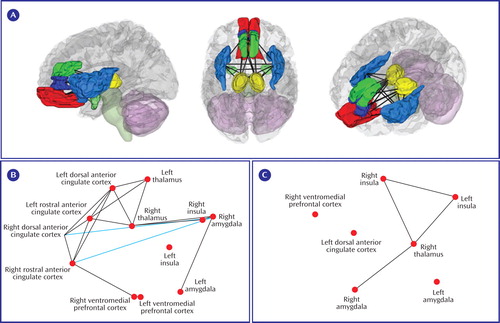
a (A) Three-dimensional rendering of the regions of interest and lines indicating significant coactivation correlations across the entire meta-analysis data set. (B) Patterns of coactivation correlations in either the positive (black lines) or inverse (blue lines) direction for the PTSD comparisons. (C) The same as for B, but for the combination of the social anxiety disorder and specific phobia data sets. For B and C, regions of interest are plotted along dimensions of the first two principal components on the x and y axes, respectively (axes not shown). Coactivation lines represent p<0.05, uncorrected.
Results
Common Mechanisms for Anxiety Disorders and Normal Fear
Studies and patient-comparison subject comparisons included in the meta-analysis, as well as overall differences in age and gender ratios, are summarized in Table 1 . In patients with PTSD, we observed areas of both hyper- and hypoactivity. By contrast, in patients with social anxiety disorder and specific phobia, we only observed areas of hyperactivity. We first focused on hyperactivation clusters (patients > comparison subjects) to identify common mechanisms across anxiety disorders.
Patients with PTSD showed hyperactivity during emotional processing in the amygdalae, parahippocampal gyrus, insula, inferior parietal lobule, mid-cingulate, and precuneus (see data supplement Table 1 available at http://ajp.psychiatryonline.org). Patients with social anxiety disorder showed hyperactivity in the amygdalae, parahippocampal gyrus, fusiform gyrus, globus pallidus, insula, inferior frontal gyrus, and superior temporal gyrus (see data supplement Table 2). Finally, in patients with specific phobia, hyperactivity was seen in the amygdalae, fusiform gyrus, substantia nigra, insula, and mid-cingulate (see data supplement Table 3). Thus, hyperactivity was observed in all three disorders in only two structures—the amygdala (see Figure 2 A) and the insula (see Figure 2 B).
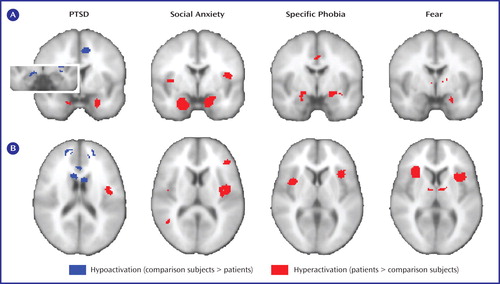
a Results are shown for the amygdalae (A) and insular cortices (B). Note that within the left amygdala there were two distinct clusters for PTSD, a ventral anterior hyperactivation cluster and a dorsal posterior hypoactivation cluster. The right side of the image corresponds to the right side of the brain.
We next examined activity during fear conditioning in healthy individuals, which involved 10 comparisons with 117 subjects (see data supplement Table 4). As shown in Figure 2 and Figure 3 , fear conditioning also increased activity in the amygdala and bilateral insula (other regions are listed in data supplement Table 5).

Differences Between Anxiety Disorders
Hypoactivations (comparison subjects > patients) were seen only in PTSD, specifically in the inferior occipital gyrus, ventromedial prefrontal cortex, rostral anterior cingulate cortex, parahippocampal gyrus, lingual gyrus, dorsal amygdala and anterior hippocampus, orbitofrontal cortex, putamen, middle occipital gyrus, dorsomedial prefrontal cortex, dorsal anterior cingulate cortex, and mid-cingulate (see Figures 2A and 3 and data supplement Table 1). Of importance, five comparisons also reported correlations between PTSD symptom severity and brain activity (6 – 8 , 21) , and all five noted negative correlations in the medial prefrontal cortex, signifying that hypoactivity is associated with greater symptom severity.
We next directly compared regional frequencies of hyper- or hypoactivation between disorders within regions of interest for the ventromedial prefrontal cortex, rostral anterior cingulate cortex, dorsal anterior cingulate cortex, amygdala, and insula, as well as the thalamus because this region was highlighted in recent reviews of PTSD neuroimaging studies (40 , 72) .
Hyperactivation in the amygdalae and insular cortices of patients was found more frequently in social anxiety disorder and specific phobia than in PTSD (see Figure 4 , left). No difference in frequencies of hypoactivation were found in these regions (p>0.15, data not shown). By contrast, rostral anterior cingulate cortex, dorsal anterior cingulate cortex, and ventromedial prefrontal cortex hypoactivity was seen more frequently in PTSD than in either social anxiety disorder or specific phobia (see Figure 4 , right). Finally, for the thalamus, hypoactivity was observed more frequently than hyperactivity in PTSD patients in relation to matched comparison subjects (left thalamus: p=0.004; right thalamus: p=0.06; data not shown). Thalamic hypoactivity was also more commonly seen in PTSD than either social anxiety disorder or specific phobia (see Figure 4 , right).
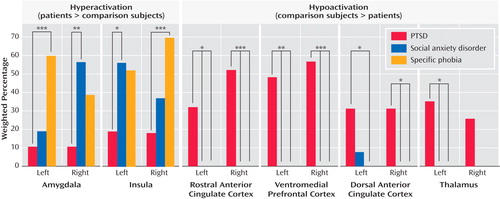
a (Left) Hyperactivation in patients, relative to comparison subjects, was observed more frequently in the amygdala and insula of patients with either social anxiety disorder or specific phobia than in patients with PTSD. (Right) Hypoactivation in the ventromedial prefrontal cortex, rostral and dorsal anterior cingulate cortices, and thalamus was specifically observed in patients with PTSD in relation to matched comparison subjects and not in patients with social anxiety disorder or specific phobia.
*p<0.05. **p<0.01. ***p<0.005.
Potential Confounds for Between-Disorder Differences
We explored several potential confounds for between-disorder regional differences, including the use of symptom-provocation designs, medication status, PET versus fMRI imaging, and whether effects were driven by single outlying studies. First, we tested whether the lower proportion of PTSD studies with symptom-provocation designs affected the results ( Table 1 ). Restricting our meta-analysis to symptom-provocation studies, however, largely confirmed our previous findings: hypoactivation was more frequent in PTSD than in social anxiety disorder or specific phobia in the ventromedial prefrontal cortex, rostral anterior cingulate cortex, and thalamus (data not shown). Similarly, hyperactivation was more frequently found in specific phobia than PTSD in the amygdala (the PTSD versus social anxiety disorder comparison was nearly significant) and in the insular cortices for both social anxiety disorder and specific phobia versus PTSD. Second, we examined whether medication status was a confounder. Most studies reported this information, and in only one (44) were some subjects taking medication at the time of the study. Thus, current medication status did not confound our findings.
Third, we assessed whether the greater proportion of PET studies in the PTSD data set may have affected our results in the amygdala region-of-interest analyses because each method offers different advantages and disadvantages for detecting amygdalar activity (59) . Therefore, we restricted our amygdala region-of-interest analyses to fMRI studies. These results confirmed our original findings, with amygdala hyperactivation observed more frequently in social anxiety disorder and specific phobia than in PTSD (data not shown).
Finally, we assessed whether any individual studies included in the meta-analysis had a disproportionate effect on the results. This was done through a jackknife analysis, in which we iteratively left each study out of the chi-square test for each region of interest with significant effects. Doing so provided strong evidence for the robustness of our findings because the reported between-disorder differences in the frequencies of hyperactivation or hypoactivation remained significant at p<0.05 for 100% of the leave-one-out analyses for all regions of interest except for the left dorsal anterior cingulate cortex (35% of leave-one-out tests significant at p<0.05, maximum leave-one-out p=0.12), left insula (95% significant at p<0.05, maximum p<0.07), and right amygdala (92% significant at p<0.05, maximum p<0.08).
Consistency of Relationships Between Regions of Interest
Individual studies in our analysis frequently reported sets of activated regions as “networks,” even in the absence of evidence that the regions are intercorrelated. We quantified interregional relationships using a novel coactivation analysis on the region-of-interest data (see Methods and Figure 4 A). Because our results suggest a similarity between social anxiety disorder and specific phobia, we pooled the data for these disorders, thus preserving statistical power. One study in the social anxiety disorder/specific phobia data set (44) , however, was a clear outlier because it was the only such study to activate a large number of frontal regions. Therefore, we report results excluding this study and for completeness publish results including this study in supplemental data, although doing so did not alter our overall findings (see data supplement Figure 1 ).
Notable in the PTSD coactivation map is the consistency of coactivation—in this case, cohypoactivation—among diverse medial frontal regions of interest (see Figure 1 B). Also, we observed significant negative coactivation between the dorsal or rostral anterior cingulate cortex and the amygdala or insula, indicating that hypoactivation of frontal regions was associated with hyperactivation in limbic and perilimbic regions (see Figure 1 B). Direct comparison of frontal-limbic coactivation in PTSD with that in social anxiety disorder/specific phobia was not possible because frontal hyper- or hypoactivity was not observed in social anxiety disorder or specific phobia, and there was thus no variance in frontal regions to correlate with limbic activation. Thus, limbic hyperactivity associated with decreased frontal inhibition appears to be a distinguishing feature of PTSD.
Discussion
The Amygdala, the Insula, and Fear Responses in Anxiety Disorders
It has been repeatedly suggested that there is exaggerated amygdala activity in clinical anxiety (4 , 37 , 39 , 40) . Empirical support for this hypothesis, however, has been inconsistent across neuroimaging studies and between anxiety disorders. Our quantitative meta-analysis revealed consistent amygdalar hyperactivity in all three disorders. Because we also observed consistent amygdala activation during fear conditioning in healthy subjects, we conclude that amygdalar hyperactivation in PTSD, social anxiety disorder, and specific phobia reflects a common exaggerated engagement of fear circuitry, which results in shared symptoms among the disorders.
The role of the amygdala in PTSD, however, appears to be more complicated than previously thought. We found a ventral anterior hyperactive cluster and a dorsal posterior hypoactive cluster. Although the exact relevance of these two clusters is uncertain, we note that the amygdala is composed of multiple subregions. The basal and lateral nuclei (together the basolateral complex) lie ventral to the central nucleus and extended amygdala. The basolateral complex is the primary site of sensory input into the amygdala, whereas the central nucleus contains efferent subnuclei mediating autonomic, endocrine, and behavioral responses to threat (4 , 73) . We speculate that the ventral hyperactive cluster relates to the basolateral amygdala and may be relevant to acquired fear responses in PTSD, given the role of this region in forming emotional memories (3) . The dorsal hypoactive cluster lies at the border between the dorsal amygdala and anterior hippocampus. Hypoactivation of the dorsal amygdala, containing the central nucleus, may be relevant for the autonomic blunting associated with emotional numbing or dissociation in PTSD (74) . Hypoactivation of the anterior hippocampus may be important for both declarative memory (75) and regulation of the hypothalamic-pituitary-adrenal axis (76 , 77) , both of which are perturbed in PTSD (78 , 79) . The rodent homologue of the anterior hippocampus in humans has also been shown to play a role in endogenous anxiety (80 , 81) . These interesting subregional distinctions might serve as a guide for more directed investigations of PTSD with high-resolution fMRI methods.
Methodological factors may also contribute to amygdalar hyperactivity versus hypoactivity. Specifically, amygdala activity strongly habituates to repeated presentation of emotional stimuli (48 , 82 , 83) , which may result in below-baseline levels of activity (48 , 82) . Although such habituation favors elimination of patient-comparison subject differences rather than hypoactivity (84) , it suggests that greater attention should be paid to the time course of amygdala effects.
Unlike for the amygdala, a role for the insular cortex in anxiety disorders has not been frequently highlighted. The insula is heavily interconnected with the amygdala, hypothalamus, and periaqueductal gray matter (73) , regulates the autonomic nervous system (85) , and is activated during the processing of a variety of negative emotions (86) . Thus, it is notable that insular hyperactivity was consistently observed in PTSD, social anxiety disorder, and specific phobia, as well as during normal fear conditioning. Insular hyperactivity therefore likely also reflects increased activation of a network responsible for generating fear responses to symptom-provoking stimuli.
Of interest, amygdalar and insular hyperactivity was more common in social anxiety disorder and specific phobia than in PTSD, a finding not previously suggested in the literature. An intriguing explanation, however, may be that while each of the three disorders involves an excessive fear component, PTSD is a more complex disorder in which the fear part per se is only one element. Thus, PTSD symptoms may be more attributable to dysfunctional emotion regulation systems, whereas the symptoms of social anxiety disorder and specific phobia may be more readily described as intense states of fear.
A Final Common Pathway for Anxiety?
Biological commonalities may be sought among different psychiatric disorders at many levels, ranging from genetic vulnerabilities to alterations in widespread neuronal networks (87) . Our data argue that amygdala and insula hyperactivation may be key components of a common neurobiological pathway for at least the three anxiety disorders studied, which may reflect overactivation of a core fear system (88) . Whether “fear” or other descriptors best explain the common hyperactivations, the activation of similar areas in neuroimaging studies is one kind of evidence for shared mechanisms (89 – 91) , and these results may provide an anatomical basis in which to explore other molecular, cellular, and circuit-level commonalities and differences among anxiety disorders.
Additional evidence for our argument comes from a recent study of “anxiety-prone” individuals, which noted both amygdalar and insular hyperactivity (92) . Furthermore, administration of the anxiolytic drug lorazepam decreases activity in these regions in a dose-dependent fashion during an emotional processing task (93) . Thus, identification of a neural signature common to anxiety disorders may be useful in terms of both diagnosis and nosology (43 , 87) , as well as for the development of novel therapeutics (94) . The presence of amygdalar and insular hyperactivity may eventually become a useful aspect of disorder categorization in DSM-V. A focus on the shared role of both of these regions in fear may also yield new molecular targets for novel therapeutics.
Emotion Regulation, the Medial Prefrontal Cortex, and Anxiety Generalization
Conceptual models of PTSD have not distinguished between the functional relevance of abnormalities in the dorsomedial prefrontal cortex/dorsal anterior cingulate cortex and those in the rostral anterior cingulate cortex/ventromedial prefrontal cortex, which represent anatomically dissociable subregions of the medial prefrontal cortex. Therefore, we propose a distinction between these regions based on their roles in emotion generation and regulation, respectively. Moreover, we distinguish between the type of emotion regulation subserved by the medial prefrontal cortex and that subserved by the lateral prefrontal cortex, a region not implicated in previous models of PTSD.
Emotion generation versus regulation
Emotion regulation has been investigated using tasks that instruct subjects to deliberately decrease their emotional responses using distraction, reappraisal, suppression, or detachment strategies (95 – 100) . These studies consistently point to involvement of the lateral prefrontal cortex, a locus of executive control for nonemotional stimuli (99 , 101) . A different picture emerges when one considers “reflexive” forms of emotion regulation, in which the generation and/or modulation of emotion is based on an individual’s expectations about stimuli but without any self-reflective focus on the emotions themselves or the explicit goal of regulating them.
Etkin et al. (102) recently found that monitoring of emotional conflict was associated with dorsomedial prefrontal activation, whereas resolution of emotional conflict was associated with rostral anterior cingulate cortex increases and amygdala decreases, consistent with its top-down inhibition. Extinction of conditioned fear also involves increased activity in the rostral anterior cingulate cortex and ventromedial prefrontal cortex and decreased activity in the amygdala (54) . Likewise, rostral anterior cingulate cortex activation and amygdala decreases have been observed during placebo anxiolysis (103) , and placebo-induced increases in mu-opioid activity have been found in the rostral anterior cingulate cortex, the ventromedial prefrontal cortex, and the amygdala while the subject was experiencing pain (104) . Based on these data, we have previously proposed that emotional control processes mediated by the rostral anterior cingulate cortex/ventromedial prefrontal cortex may reflect an individual’s emotional coping or resilience mechanisms (102) , which normally function in absence of explicit task instructions to regulate emotion.
Our meta-analysis demonstrated robust hypoactivations in PTSD in the rostral anterior cingulate cortex and ventromedial prefrontal cortex but failed to show alterations in the lateral prefrontal cortex. Thus, we propose that hypoactivation of the rostral anterior cingulate cortex and ventromedial prefrontal cortex in patients with PTSD reflects a deficit in reflexive emotion regulation processes occurring in the absence of self-reflection about emotion or deliberate attempts at emotional control and is reflected clinically in emotional dysregulation symptoms and anxiety generalization. A reflexive emotion regulation deficit may thus encompass and extend beyond a fear extinction deficit in PTSD, as has been proposed previously (42 , 105) . The work on the rostral anterior cingulate cortex and the ventromedial prefrontal cortex in humans parallels animal data showing that ventromedial prefrontal cortex lesions in rats impair the ability of these animals to extinguish learned fear (106) . Electrical stimulation of this region, which has direct inhibitory projections to the amygdala (107 , 108) , facilitates fear extinction (109) . Although the ventromedial prefrontal cortex in rodents is not an exact homologue of the human rostral anterior cingulate cortex and the ventromedial prefrontal cortex, the shared roles of these areas in fear extinction suggest some degree of analogous function (105) and support a translational approach to anxiety.
Unlike the regulatory roles proposed for the rostral anterior cingulate cortex and ventromedial prefrontal cortex, activity in the dorsomedial prefrontal cortex and adjacent dorsal anterior cingulate cortex seems to relate to emotional experience (59) or awareness (110) . These regions are activated by emotional conflict (102) , track levels of emotional arousal (111) , correlate with autonomic activity (112 , 113) , and respond in anticipation of an aversive event (96) , among related functions. Thus, inasmuch as the dorsomedial prefrontal cortex and dorsal anterior cingulate cortex function in the experience of negative emotion, hypoactivation of these regions in PTSD may be related to a decrease in the experience or impact of negative emotion. Because these regions may help recruit rostral anterior cingulate cortex emotion regulation mechanisms (102) , dysfunction of the dorsomedial prefrontal cortex and dorsal anterior cingulate cortex may indirectly further contribute to emotional dysregulation in PTSD. Likewise, hypoactivation of the thalamus may relate to decreased processing of sensory information and thereby decreased experience of negative emotion, as suggested previously (40 , 72) , although the thalamus also plays diverse roles in cortical-cortical interactions.
Therapeutic implications
Studies of the rodent ventromedial prefrontal cortex not only shed light on the consequence of rostral anterior cingulate cortex/ventromedial prefrontal cortex dysfunction in PTSD but also suggest avenues for novel therapeutics. Animals who have been exposed to inescapable stress display subsequent potentiation of fear and anxiety in unrelated tasks (114 – 116) , whereas control over the stressor promotes resilience (114 – 116) , an effect that depends on the ventromedial prefrontal cortex (114) . In humans, perceived controllability during a trauma is related to the severity of subsequent PTSD symptoms (117) , an idea that is incorporated into the diagnostic criteria in DSM-IV (1) . New treatments, both psychotherapeutic and psychopharmacologic, aimed at bolstering medial prefrontal emotion regulation systems may therefore allow PTSD patients to exert greater control over subsequently encountered fear- and anxiety-producing stimuli or thoughts. Work in monkeys suggests that early life exposure to mild stress can have an inoculating effect against fear and anxiety in unrelated situations later in the animal’s life (118) . Although the neural substrates of stress inoculation have not yet been described, the rodent data mentioned above on control over stress suggest that the medial prefrontal cortex may play an important role. As such, stress inoculation may inform approaches at enhancing medial prefrontal emotion regulation systems.
Limitations and Future Directions
Our study has several limitations. First, despite the large number of subjects studied, these numbers still represent a relatively limited population size given significant across-study variation in subject characteristics and study methodologies. This may have compromised our ability to detect more subtle, but highly informative, changes in neural activity. Second, there is some overlap in the published subject cohorts across studies, producing a partial nonindependence between studies. This partial nonindependence is an aspect of neuroimaging data that may be addressed by further refinements of the statistical techniques. The jackknife analyses, however, mitigate in part against results driven by pairs of studies with overlapping cohorts by eliminating one member of any pair. To our knowledge, subjects were not shared across more than two studies.
Finally, we noted age and gender ratio differences between subjects in studies of the three anxiety disorders. These differences did not affect within-study results because patient and control groups were well matched. It is possible, however, that age or gender differences between disorders may affect the sensitivity for detecting differences between patients and comparison subjects—that is, age-by-disorder or gender ratio-by-disorder interactions—a possibility not generally addressed in the psychiatric neuroimaging literature. Although possible, this is unlikely, given that the limbic structures in question are among those particularly spared during aging (119) and that none of our cohorts were elderly. Moreover, in our neuroimaging data, the social anxiety disorder and specific phobia data sets showed similar effects (both of which differed from the PTSD results), despite being different in both age and gender. Thus, neither of these potential confounds produced clear brain differences between social anxiety disorder and specific phobia.
Conclusion
This meta-analysis of functional neuroimaging studies compared the neural correlates of emotional processing in PTSD, social anxiety disorder, and specific phobia. Meta-analyses provided a unique opportunity to assess replicability of regional activations across individual studies and the overlap in affected brain systems across disorders. This approach yielded several key findings. First, patients with all three disorders demonstrated hyperactivity (patients > comparison subjects) in the amygdala and insula. Second, this pattern of activation was also noted for healthy subjects experiencing anticipatory anxiety during fear conditioning. In combination, these data support the hypothesis that shared symptoms—an exaggerated fear response—might be reflected in shared neurobiology. Of interest, amygdala and insula hyperactivity was more commonly observed in social anxiety disorder and specific phobia than in PTSD. Third, we also proposed that PTSD-specific alterations would be related to the emotional dysregulation symptoms characteristic of this disorder. Only PTSD featured prominent hypoactivations (comparison subjects > patients), which were seen in the ventromedial prefrontal cortex, rostral and dorsal anterior cingulate cortex, and thalamus, regions associated with the experience or regulation of emotion. Extension of these findings to other anxiety disorders on which there is currently less information, as well as affective disorders, may lead to advances in the understanding of their psychopathological mechanisms and provide further insight into the relationships between these disorders.
1. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Press, 2000Google Scholar
2. Grillon C: Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry 2002; 52:958–975Google Scholar
3. LeDoux JE: Emotion circuits in the brain. Annu Rev Neurosci 2000; 23:155–184Google Scholar
4. Davis M, Whalen PJ: The amygdala: vigilance and emotion. Mol Psychiatry 2001; 6:13–34Google Scholar
5. Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK: Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 2000; 47:769–776Google Scholar
6. Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK: Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 2004; 61:168–176Google Scholar
7. Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL: A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 2005; 62:273–281Google Scholar
8. Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, Gordon E, Bryant RA: Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage 2006; 29:347–357Google Scholar
9. Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB, Ballenger JC, Lydiard RB, Brodrick PS, Bohning DE, George MS: Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport 2004; 15:2701–2705Google Scholar
10. Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME: Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry 2006; 59:424–429Google Scholar
11. Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG: Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 2002; 59:1027–1034Google Scholar
12. Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH: Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol Psychiatry 2004; 56:921–930Google Scholar
13. Straube T, Mentzel HJ, Miltner WH: Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology 2005; 52:163–168Google Scholar
14. Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Långström B, Fredrikson M: Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry 2001; 158:1220–1226Google Scholar
15. Dilger S, Straube T, Mentzel HJ, Fitzek C, Reichenbach JR, Hecht H, Krieschel S, Gutberlet I, Miltner WH: Brain activation to phobia-related pictures in spider phobic humans: an event-related functional magnetic resonance imaging study. Neurosci Lett 2003; 348:29–32Google Scholar
16. Schienle A, Schafer A, Walter B, Stark R, Vaitl D: Brain activation of spider phobics towards disorder-relevant, generally disgust- and fear-inducing pictures. Neurosci Lett 2005; 388:1–6Google Scholar
17. Straube T, Mentzel HJ, Miltner WH: Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry 2006; 59:162–170Google Scholar
18. Veltman DJ, Tuinebreijer WE, Winkelman D, Lammertsma AA, Witter MP, Dolan RJ, Emmelkamp PM: Neurophysiological correlates of habituation during exposure in spider phobia. Psychiatry Res 2004; 132:149–158Google Scholar
19. van den Heuvel OA, Veltman DJ, Groenewegen HJ, Witter MP, Merkelbach J, Cath DC, van Balkom AJ, van Oppen P, van Dyck R: Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Arch Gen Psychiatry 2005; 62:922–933Google Scholar
20. van den Heuvel OA, Veltman DJ, Groenewegen HJ, Dolan RJ, Cath DC, Boellaard R, Mesina CT, van Balkom AJ, van Oppen P, Witter MP, Lammertsma AA, van Dyck R: Amygdala activity in obsessive-compulsive disorder with contamination fear: a study with oxygen-15 water positron emission tomography. Psychiatry Res 2004; 132:225–237Google Scholar
21. Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I: Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry 2005; 57:832–840Google Scholar
22. Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I: Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry 2006; 63:184–192Google Scholar
23. Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS: Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry 1999; 156:1787–1795Google Scholar
24. Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS: Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry 1999; 45:806–816Google Scholar
25. Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney DS: Neural correlates of the classic color and emotional Stroop in women with abuse-related posttraumatic stress disorder. Biol Psychiatry 2004; 55:612–620Google Scholar
26. Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib LH, Soufer R, Charney DS: Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol Psychiatry 2003; 53:879–889Google Scholar
27. Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, Gati JS, Menon RS: Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry 2002; 52:305–311Google Scholar
28. Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS: Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry 2001; 158:1920–1922Google Scholar
29. Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS: Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry 2003; 53:204–210Google Scholar
30. Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, Shirouzu I, Yamasue H, Akiyama T, Kato N: Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. Neuroimage 2005; 26:813–821Google Scholar
31. Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK: Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am J Psychiatry 1999; 156:575–584Google Scholar
32. Yang P, Wu MT, Hsu CC, Ker JH: Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neurosci Lett 2004; 370:13–18Google Scholar
33. Straube T, Mentzel HJ, Glauer M, Miltner WH: Brain activation to phobia-related words in phobic subjects. Neurosci Lett 2004; 372:204–208Google Scholar
34. Wright CI, Martis B, McMullin K, Shin LM, Rauch SL: Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biol Psychiatry 2003; 54:1067–1076Google Scholar
35. Larson CL, Schaefer HS, Siegle GJ, Jackson CA, Anderle MJ, Davidson RJ: Fear is fast in phobic individuals: amygdala activation in response to fear-relevant stimuli. Biol Psychiatry 2006; 60:410–417Google Scholar
36. Straube T, Glauer M, Dilger S, Mentzel HJ, Miltner WH: Effects of cognitive-behavioral therapy on brain activation in specific phobia. Neuroimage 2006; 29:125–135Google Scholar
37. Rauch SL, Shin LM, Wright CI: Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci 2003; 985:389–410Google Scholar
38. Cannistraro PA, Wright CI, Wedig MM, Martis B, Shin LM, Wilhelm S, Rauch SL: Amygdala responses to human faces in obsessive-compulsive disorder. Biol Psychiatry 2004; 56:916–920Google Scholar
39. Kent JM, Rauch SL: Neurocircuitry of anxiety disorders. Curr Psychiatry Rep 2003; 5:266–273Google Scholar
40. Bremner JD: Brain imaging in anxiety disorders. Expert Rev Neurother 2004; 4:275–284Google Scholar
41. Quirk GJ, Beer JS: Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol 2006; 16:723–727Google Scholar
42. Rauch SL, Shin LM, Phelps EA: Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry 2006; 60:376–382Google Scholar
43. Watson D: Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. J Abnorm Psychol 2005; 114:522–536Google Scholar
44. Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS: Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry 2005; 57:975–981Google Scholar
45. Kilts CD, Kelsey JE, Knight B, Ely TD, Bowman FD, Gross RE, Selvig A, Gordon A, Newport DJ, Nemeroff CB: The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology 2006; 31:2243–2253Google Scholar
46. Armony JL, Dolan RJ: Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsychologia 2002; 40:817–826Google Scholar
47. Buchel C, Dolan RJ, Armony JL, Friston KJ: Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J Neurosci 1999; 19:10869–10876Google Scholar
48. Buchel C, Morris J, Dolan RJ, Friston KJ: Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron 1998; 20:947–957Google Scholar
49. Critchley HD, Mathias CJ, Dolan RJ: Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 2002; 33:653–663Google Scholar
50. Gottfried JA, Dolan RJ: Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci 2004; 7:1144–1152Google Scholar
51. Gottfried JA, O’Doherty J, Dolan RJ: Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci 2002; 22:10829–10837Google Scholar
52. Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S: Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron 2003; 40:1251–1257Google Scholar
53. Knight DC, Nguyen HT, Bandettini PA: The role of the human amygdala in the production of conditioned fear responses. Neuroimage 2005; 26:1193–1200Google Scholar
54. Phelps EA, Delgado MR, Nearing KI, LeDoux JE: Extinction learning in humans: role of the amygdala and vmPFC. Neuron 2004; 43:897–905Google Scholar
55. Yaguez L, Coen S, Gregory LJ, Amaro E Jr, Altman C, Brammer MJ, Bullmore ET, Williams SC, Aziz Q: Brain response to visceral aversive conditioning: a functional magnetic resonance imaging study. Gastroenterology 2005; 128:1819–1829Google Scholar
56. Chein JM, Fiez JA: Dissociation of verbal working memory system components using a delayed serial recall task. Cereb Cortex 2001; 11:1003–1014Google Scholar
57. Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT: ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 2005; 25:155–164Google Scholar
58. Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA: Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 2002; 16(part 1):765–780Google Scholar
59. Wager TD, Barrett LF, Bliss-Moreau E, Lindquist K, Duncan S, Kober H, Joseph J, Davidson M, Mize J: The neuroimaging of emotion, in Handbook of Emotions, 3rd ed. Edited by Lewis M, Haviland-Jones JM, Barrett LF. New York, Guilford (in press)Google Scholar
60. Wager TD, Jonides J, Reading S: Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage 2004; 22:1679–1693Google Scholar
61. Wager TD, Phan KL, Liberzon I, Taylor SF: Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage 2003; 19:513–531Google Scholar
62. Brett M, Christoff K, Cusack R, Lancaster J: Using the Talairach atlas with the MNI template. Neuroimage 2001; 13(suppl 1):85Google Scholar
63. Wager TD, Lindquist M, Kaplan L: Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci 2007; 2:150–158Google Scholar
64. Genovese CR, Lazar NA, Nichols T: Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002; 15:870–878Google Scholar
65. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH: An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19:1233–1239Google Scholar
66. Good PI: Resampling Methods. Boston, Birkhäuser, 2006Google Scholar
67. Gibbons JGD, Chakraborti S: Nonparametric Statistical Inference. New York, Marcel Dekker, 2003Google Scholar
68. Gibbons JD: Nonparametric Measures of Association. Newbury Park, Calif, Sage Publications, 1993Google Scholar
69. Kruskal JB: Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 1964; 29:1–27Google Scholar
70. Shepard RN: The analysis of proximities: multidimensional scaling with an unknown distance function, I. Psychometrika 1962; 27:125–140Google Scholar
71. Shepard RN: Multidimensional Scaling, Tree-Fitting, and Clustering Science 1980; 210:390–398Google Scholar
72. Lanius RA, Bluhm R, Lanius U, Pain C: A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J Psychiatr Res 2006; 40:709–729Google Scholar
73. Paxinos G: Human Nervous System. San Diego, Academic Press, 2003Google Scholar
74. Griffin MG, Resick PA, Mechanic MB: Objective assessment of peritraumatic dissociation: psychophysiological indicators. Am J Psychiatry 1997; 154:1081–1088Google Scholar
75. Scoville WB, Milner WB: Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 1957; 20:11–21Google Scholar
76. Fendler K, Karmos G, Telegdy M: The effect of hippocampal lesion on pituitary-adrenal function. Acta Physiol Scand 1961; 20:293–301Google Scholar
77. Jacobson L, Sapolsky R: The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 1991; 12:118–134Google Scholar
78. Buckley TC, Blanchard EB, Neill WT: Information processing and PTSD: a review of the empirical literature. Clin Psychol Rev 2000; 20:1041–1065Google Scholar
79. de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG: Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res 2006; 40:550–567Google Scholar
80. Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J: Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev 2004; 28:273–283Google Scholar
81. Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB: Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A 2002; 99:10825–10830Google Scholar
82. Petrovic P, Carlsson K, Petersson KM, Hansson P, Ingvar M: Context-dependent deactivation of the amygdala during pain. J Cogn Neurosci 2004; 16:1289–1301Google Scholar
83. Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL: Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport 2001; 12:379–383Google Scholar
84. Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, LeDoux J, Silbersweig D, Stern E: Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry 2005; 57:464–473Google Scholar
85. Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC: Cardiovascular effects of human insular cortex stimulation. Neurology 1992; 42:1727–1732Google Scholar
86. Phan KL, Wager T, Taylor SF, Liberzon I: Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 2002; 16:331–348Google Scholar
87. Pittenger C, Etkin A: Are there biological commonalities among different psychiatric disorders? in Psychiatry, 3rd ed. Edited by Tasman A, Kay J, Lieberman JA, First MB, Maj M. Chichester, UK, John Wiley & Sons (in press)Google Scholar
88. Barrett LF, Wager TD: The structure of emotion: evidence from neuroimaging studies. Curr Dir Psych Sci 2006; 15:79–83Google Scholar
89. Duncan J, Owen AM: Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 2000; 23:475–483Google Scholar
90. Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S: The role of the medial frontal cortex in cognitive control. Science 2004; 306:443–447Google Scholar
91. Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J: Common and unique components of response inhibition revealed by fMRI. Neuroimage 2005; 27:323–340Google Scholar
92. Stein MB, Simmons AN, Feinstein JS, Paulus MP: Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry 2007; 164:318–327Google Scholar
93. Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB: Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry 2005; 62:282–288Google Scholar
94. Paulus MP, Stein MB: Role of functional magnetic resonance imaging in drug discovery. Neuropsychol Rev 2007; 17:179–188Google Scholar
95. Beauregard M, Levesque J, Bourgouin P: Neural correlates of conscious self-regulation of emotion. J Neurosci 2001; 21:RC165Google Scholar
96. Kalisch R, Wiech K, Critchley HD, Seymour B, O’Doherty JP, Oakley DA, Allen P, Dolan RJ: Anxiety reduction through detachment: subjective, physiological, and neural effects. J Cogn Neurosci 2005; 17:874–883Google Scholar
97. Kalisch R, Wiech K, Herrmann K, Dolan RJ: Neural correlates of self-distraction from anxiety and a process model of cognitive emotion regulation. J Cogn Neurosci 2006; 18:1266–1276Google Scholar
98. Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M: Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry 2003; 53:502–510Google Scholar
99. Ochsner KN, Gross JJ: The cognitive control of emotion. Trends Cogn Sci 2005; 9:242–249Google Scholar
100. Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ: For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage 2004; 23:483–499Google Scholar
101. Miller EK, Cohen JD: An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001; 24:167–202Google Scholar
102. Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J: Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 2006; 51:871–882Google Scholar
103. Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M: Placebo in emotional processing-induced expectations of anxiety relief activate a generalized modulatory network. Neuron 2005; 46:957–969Google Scholar
104. Wager TD, Scott DJ, Zubieta JK: Placebo effects on human m-opioid activity during pain. Proc Natl Acad Sci U S A 2007; 104:11056–11061Google Scholar
105. Milad MR, Rauch SL, Pitman RK, Quirk GJ: Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol 2006; 73:61–71Google Scholar
106. Morgan MA, Romanski LM, LeDoux JE: Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett 1993; 163:109–113Google Scholar
107. Quirk GJ, Likhtik E, Pelletier JG, Pare D: Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 2003; 23:8800–8807Google Scholar
108. Rosenkranz JA, Grace AA: Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci 2002; 22:324–337Google Scholar
109. Milad MR, Quirk GJ: Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 2002; 420:70–74Google Scholar
110. Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE: Neural correlates of levels of emotional awareness. evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci 1998; 10:525–535Google Scholar
111. Taylor SF, Phan KL, Decker LR, Liberzon I: Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage 2003; 18:650–659Google Scholar
112. Critchley HD: Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 2005; 493:154–166Google Scholar
113. Williams LM, Phillips ML, Brammer MJ, Skerrett D, Lagopoulos J, Rennie C, Bahramali H, Olivieri G, David AS, Peduto A, Gordon E: Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage 2001; 14:1070–1079Google Scholar
114. Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF: Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci 2005; 8:365–371Google Scholar
115. Maier SF, Watkins LR: Stressor controllability, anxiety, and serotonin. Cognit Ther Res 1998; 22:595–613Google Scholar
116. Seligman ME, Maier SF: Failure to escape traumatic shock. J Exp Psychol 1967; 74:1–9Google Scholar
117. Kushner MG, Riggs DS, Foa EB, Miller SM: Perceived controllability and the development of posttraumatic stress disorder (PTSD) in crime victims. Behav Res Ther 1993; 31:105–110Google Scholar
118. Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM: Prospective investigation of stress inoculation in young monkeys. Arch Gen Psychiatry 2004; 61:933–941Google Scholar
119. Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E: Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp 2005; 25:391–401Google Scholar