Frontostriatal Connectivity and Its Role in Cognitive Control in Parent-Child Dyads With ADHD
Abstract
Objective: Many studies have linked the structure and function of frontostriatal circuitry to cognitive control deficits in attention deficit hyperactivity disorder (ADHD). Few studies have examined the role of white matter tracts between these structures or the extent to which white matter tract myelination and regularity correlate in family members with the disorder. Method: Functional imaging maps from a go/nogo task were used to identify portions of the ventral prefrontal cortex and striatum involved in suppressing an inappropriate action (i.e., cognitive control) in 30 parent-child dyads (N=60), including 20 dyads (N=40) with ADHD and 10 dyads (N=20) without ADHD. An automated fiber-tracking algorithm was used to delineate white matter fibers adjacent to these functionally defined regions based on diffusion tensor images. Fractional anisotropy, an index of white matter tract myelination and regularity derived from diffusion tensor images, was calculated to characterize the associations between white matter tracts and function. Results: Fractional anisotropy in right prefrontal fiber tracts correlated with both functional activity in the inferior frontal gyrus and caudate nucleus and performance of a go/nogo task in parent-child dyads with ADHD, even after controlling for age. Prefrontal fiber tract measures were tightly associated between ADHD parents and their children. Conclusions: Collectively, these findings support previous studies suggesting heritability of frontostriatal structures among individuals with ADHD and suggest disruption in frontostriatal white matter tracts as one possible pathway to the disorder.
Theoretical accounts of the core symptoms in attention deficit hyperactivity disorder (ADHD) (e.g., 1 , 2) almost all include some form of cognitive control. Cognitive control refers to the ability to suppress inappropriate thoughts and actions in favor of more appropriate ones and is measured by neuropsychological tasks, including stop signal, go/nogo, and Stroop paradigms. Empirical studies of cognitive control deficits in ADHD suggest that problems in this ability may be particularly important in relation to impairing symptoms of inattention-disorganization, rather than hyperactivity-impulsivity per se(2 , 3) . Neural circuits, linking regions of the prefrontal cortex and the striatum, have been associated with this ability (3 , 4) . Development of frontostriatal circuitry, as measured histologically by synaptic pruning and myelination of prefrontal fibers (5 , 6) and indexed by imaging methods (7 – 10) , proceeds slowly throughout late childhood and adolescence. Concomitantly, capacity for cognitive control develops at a protracted rate, with younger children being more susceptible to interference on a variety of tasks in this domain (3 , 11 – 13) . Less efficient cognitive control in ADHD has been shown to result from some form of abnormality in the development of frontostriatal brain circuitry, as evidenced by pediatric structural and functional imaging studies on the disorder (14 – 22) . How development and refinement of projections within these regions may contribute to enhanced control remains an important question. In the present study, we used diffusion tensor imaging to examine individual differences in frontostriatal white matter tracts and their contribution to performance of a cognitive control task in parent-child dyads with ADHD relative to dyads without ADHD.
Diffusion tensor imaging is an imaging technique that can detect changes in white matter microstructure based on properties of diffusion (23 , 24) . Diffusion of water molecules in white matter tracts is affected by myelin and the orientation and regularity of fibers. Water diffuses more readily parallel rather than perpendicular to a tract, a property termed anisotropic diffusion. Magnetic resonance images can be sensitized to water diffusion to yield a solution to the diffusion tensor, from which variables describing the magnitude and anisotropy of diffusion can be derived (23) . These variables can be used as a measure of myelination and white matter microstructure in vivo (25) and to investigate prefrontal changes during normal maturation (8 , 26 , 27) or with atypical (28 – 30) or premature development (31) . Such measures go beyond simple gray and whiter matter volumetric measures by providing specificity in the directionality of fiber tracts. Diffusion tensor image-based fiber tracking algorithms can be applied to delineate these white matter tracts automatically and reliably (32 , 33) .
In this study, we used diffusion tensor imaging to assess how variation in frontostriatal white matter tracts may contribute to individual differences in performance of a go/nogo task in individuals with and without ADHD. Subjects responded with a button press to repeated presentations of a visual stimulus (“go” trials) but inhibited this response when presented with a second, distinctive, and infrequent stimulus (“nogo” trials). Accuracies to nogo trials (false alarms) are the conventional means of indexing cognitive control in this paradigm, but a measure that takes into account both hits and false alarms was used: d-prime. d-prime provides a measure of sensitivity in the discrimination and ultimate detection of target stimuli relative to nontarget stimuli (34) , a key component of cognitive control (2) (i.e., how well the subject can discriminate and appropriately respond to targets and nontargets).
In a previous functional magnetic resonance imaging (fMRI) study, with a subset of the current sample (35) , we showed less frontostriatal activity in ADHD individuals relative to non-ADHD comparison subjects during performance of a go/nogo task. Performance and frontostriatal activity were enhanced with stimulant medication, confirming previous work by Vaidya et al. (15) . In the present study, we sought to relate frontostriatal white matter tracts, as indexed by diffusion tensor imaging, to this hypometabolic activity and behavioral performance. Similar to another previous study (27) , in which we showed that efficient recruitment of cognitive control in healthy volunteers is supported by the development of white matter tracts in frontostriatal regions, we measured fractional anisotropy in these regions in the present study.
Method
Subjects
Twenty youth-parent dyads with ADHD (N=40) and 10 youth-parent dyads without ADHD (N=20) were recruited from the Multimodal Treatment Study of Children with ADHD study. (One ADHD parent-child dyad included in the current sample from the Columbia site was not part of the original Multimodal Treatment Study of Children with ADHD study but met a similar set of criteria as those used for that study.) All ADHD youths were recruited from three of the seven geographical recruiting sites for the Multimodal Treatment Study of Children with ADHD study (i.e., Duke University Medical Center; University of California, Berkeley; and New York State Psychiatric Institute) and had received a diagnosis of ADHD, combined type, at 7–9 years of age. At the time of entry into the study, 5–9 years following the completion of randomly assigned treatments, the Diagnostic Interview Schedule for Children, Parent Report, was readministered (36) . Youths were required to meet DSM-IV ADHD diagnostic criteria for any ADHD subtype in order to be included in the study. Biological parents were interviewed to determine whether they met DSM-IV ADHD criteria using the Conners Adult ADHD Diagnostic Interview for DSM-IV. Only dyads in which a youth met DSM-IV ADHD criteria and a biological parent met DSM-IV ADHD criteria were included. Sixteen of the 20 ADHD youth were men. Fifteen of the 20 ADHD parents were women. Demographic information on this sample is presented in Table 1 .
All 20 ADHD youths and one ADHD parent had a history of receiving stimulant medications for ADHD. Of these, three youths and no parents were receiving stimulant medications for ADHD at the time of recruitment for this study. Before participating in this study, a washout period (5 multiplied by the medication’s half-life) was required. In addition, participants had to be free of neuroleptic medications for 6 months prior to the study.
The 10 healthy comparison dyads (N=20) were recruited from a local normative comparison group that was part of the Multimodal Treatment Study of Children with ADHD study. For the purposes of this study, children in the comparison group were required to have fewer than three ADHD symptoms within each DSM-IV ADHD symptom domain as assessed by the Diagnostic Interview Schedule for Children, Parent Report. In addition, parents in the comparison group completed the Conners Adult ADHD Diagnostic Interview for DSM-IV and were required to have fewer than three symptoms in each DSM-IV ADHD symptom domain in order to be included. The distribution of gender for youth and parents was similar to the ADHD sample ( Table 1 ). After complete description of the study to the subjects, written informed consent was obtained.
Image Acquisition and Analysis
Subjects were scanned with General Electric 1.5 Tesla fMRI scanners (General Electric Medical Systems, Milwaukee) at facilities at Duke University Medical Center, Stanford University, and Weill Medical College of Cornell University. In order to ensure comparability across sites, all sites used identical scanners and software for imaging. Prior to image acquisition, the same individuals were scanned at all sites in order to ensure similar signal to noise and contrast to noise across sites. Further, all sites scanned identical phantoms on a monthly basis to check and guard against any drift during the course of the study. Last, all data were normalized during preprocessing to correct for any small signal-to-noise variations across sites. This cross-site methodology was developed and based on a similar multisite functional imaging study (37) . Diffusion tensor imaging scans were obtained using a multislice, spin-echo, diffusion tensor pulse sequence (33 slices, 3.8 mm thick with 0.4 mm skip, TR=12200 msec, echo time=minimum, field of view=24) covering the whole brain and weighted to diffusion in six directions. A whole brain, high-resolution, T1-weighted anatomic scan (256×256 in-plane resolution, field of view=240 mm; 124 slices at 1.5 mm per slice) was acquired for each subject for transformation and localization of functional data into Talairach space. Functional data were collected with a spiral in-and-out sequence (TR=2500 msec, echo time=40 msec, flip angle=90°, field of view=240 mm, 64×64 matrix) (38) . Each volume contained 33 oblique slices (3.2 mm thick with 1 mm skip), with an in-plane resolution of 3.125×3.125 mm covering the entire brain. A set of matching T2 images (field of view=240 mm, 256×256 resolution) was acquired with the same prescription as the functional images.
Functional image processing and analysis were performed using the BrainVoyager QX software package (Brain Innovations, Maastricht, the Netherlands). Preprocessing of the functional data involved three-dimensional motion detection and correction (spatial alignment of all volumes to the first volume by rigid transformation) and linear tendency removal. In order to obtain registration, functional data were coregistered to the anatomic volume by alignment of corresponding points and manual adjustments and then transformed into Talairach space with standard landmarks and interpolated to a resolution of 1 mm.
Signal values in each time course were normalized to z scores representing a change from the mean signal for each run. The signal values for the correct nogo trials were considered effects of interest and modeled with a convolution of an ideal boxcar response (assuming a value of 1 for the volume of the nogo task presentation and a value of 0 for the remaining time points) with a linear model of the hemodynamic response (39) . These predictors were used to build a design matrix for each time course in the experiment. Only correct trials were included in the matrices and subsequent analyses. Hence, correct nogo trials were contrasted with correct go trials to identify activation patterns, which were compared between groups. Three-dimensional statistical maps were generated by assigning an F value to each voxel corresponding to the correct nogo trials and calculated on the basis of the least mean squares solution of the general linear model. Contrast analyses were then performed based on t score differences between the beta weights of this predictor relative to the mean beta weights for each subject, with a random-effects analysis and p value of 0.05, corrected with a contiguity threshold of five acquisition-based voxels to correct for multiple comparisons (40) . In regions of interest where between-group differences were present, mean beta weights for these regions were then correlated with the behavioral measures to examine relations between functional brain activation and performance. Subsequent analysis focused on those regions that correlated with behavioral performance (35) .
Diffusion image reconstruction and analysis were performed using Diffusion Tensor Imaging Studio (version 2.4, H. Jiang and S. Mori, Department of Radiology, Johns Hopkins University, Baltimore). For each participant, all available series were averaged to produce one mean image for each direction. Six apparent diffusion-weighted coefficients were calculated, from which the six independent elements of the diffusion tensor were determined for each voxel. Eigenvalues and eigenvectors of the tensor were calculated using a Jacobi transformation. Fractional anisotropy was calculated from the eigenvalues as described by Basser and Pierpaoli (33) . The eigenvector corresponding to the largest eigenvalue was interpreted as the primary fiber direction within the voxel. The calculation of fractional anisotropy for each region and subject was performed while still blind to diagnosis.
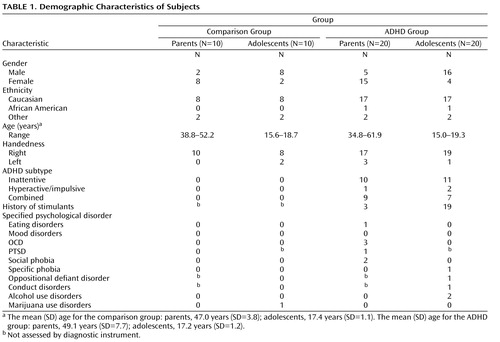
Figure 1 depicts the functional map on which the diffusion tensor imaging analyses were based. The axial plane shows the only two functional regions that correlated with task performance (inferior frontal gyrus and caudate nucleus) from our previous study (35) . Figure 1 also shows the fibers that were identified by using an automated tractography algorithm in this same axial plane. The fiber tracts were automatically generated by planting seeds bilaterally and at the anterior border of the caudate nucleus, excluding fibers crossing the hemispheres in the genu of the corpus callosum. A segment of the corticospinal tract, which was not expected to have any direct relation to cognitive control performance per se , was delineated as described by Liston et al. (27) . This tract extended from the posterior limb of the internal capsule superiorly into the centrum semiovale. For each of these regions, average fractional anisotropy values were calculated for each subject for each analysis, with an alpha of 0.006 to control for multiple comparisons (0.05/8 with four groups and two tracts).
a A) Depiction of the functional map on which the diffusion tensor imaging analyses were based. The axial plane shows the two functional regions that correlated with task performance (inferior frontal gyrus and caudate nucleus [35]). B) Representative illustration of fibers that were identified using an automated tractography algorithm in the same axial plane as the functional map. C) Activity in the inferior frontal gyrus correlated with fractional anisotropy in the prefrontal cortex in youths with ADHD (r=0.76, p<0.007) but not in comparison youths (r=–0.24, p<0.51). D) Activity in the left caudate correlated with fractional anisotropy in the left prefrontal cortex in both ADHD youths (r=0.83, p<0.005) and comparison youths (r=0.73, p<0.02) but not in comparison parents (p>0.73).
Given that the present study focused on parent-child dyads, associations between parent and child prefrontal white matter tracts were analyzed by correlating fractional anisotropy values for these regions between parents and children in each diagnostic group. To ensure that any significant correlations between parent and child fractional anisotropy measures were not driven by chance or by defining subject groups by behavioral phenotype (i.e., diagnosis of ADHD), 200 permutations of random pairings of parent and child were made for each of these regions, and each was reanalyzed.
Results
Behavioral Results
A two- (age group) by-two (diagnostic group) between-subjects analysis of variance (ANOVA) for the behavioral measure of d-prime showed a main effect of age group (F=6.7, df=1, 54, p<0.02) but not diagnostic group (F=2.9, df=1, 54, p<0.09). There was no interaction of age-by-diagnostic group (F=0.61, df=1, 54, p<0.38). Post hoc t tests showed that d-prime values increased from adolescence to adulthood (2.9 to 3.4, p<0.05). A similar pattern of results was shown for false alarm rate on nogo trials (F=5.9, df=1, 54, p<0.02) and mean reaction time for go trials (F=8.2, df=1, 54, p<0.01), with youths making more false alarms and faster responses than their parents, but was not distinguishing between diagnostic groups.
Imaging Results
To examine the extent to which functional activity associated with go/nogo task performance was correlated with prefrontal white matter measure, functional activity in the inferior frontal gyrus and the caudate nucleus identified from the Epstein et al. (35) study was correlated with fractional anisotropy measures in the prefrontal cortex. These functionally defined regions were selected because they were the only two regions to correlate with performance in the fMRI study of ADHD parent-child dyads. Activity in the inferior frontal gyrus correlated with fractional anisotropy in the prefrontal cortex in youths with ADHD (r=0.76, p<0.007) but not in comparison youths (r=–0.24, p<0.51) or either parent group (p<0.13 [ Figure 1 ]). Activity in the left caudate correlated with fractional anisotropy in the left prefrontal cortex in both ADHD parents (r=0.70, p<0.04) and youths (r=0.83, p<0.005) and in comparison youths (r=0.73, p<0.02 [ Figure 1 ]) but not in comparison parents (p>0.73).
To investigate the relation between the frontostriatal white matter tracts and enhanced performance, fractional anisotropy values for individual subjects were correlated with d- prime scores for each group separately. To determine the reliability of the diffusion tensor images-based measures of fractional anisotropy, we used three different analytic methods (voxel-based, region of interest-based, and algorithm-based fiber tracking). The findings from the algorithm-based fiber tracking method are reported, but similar associations were observed between our d- prime measure of performance and fractional anisotropy for the region of interest-based and uncorrected voxel-based analyses. Fractional anisotropy in the right prefrontal region was correlated with d- prime scores for the ADHD group (r=0.40 p<0.01 [ Figure 2 ]), driven largely by the correlation for the ADHD parents (r=0.65, p<0.003 [ Figure 2 ]), since youths showed only a tendency for this association (r= 0.38, p<0.09). d- prime scores decreased in ADHD adults as prefrontal anisotropy values decreased in ADHD adults (see Figure 2 ). Specifically, a subset of ADHD parents who performed poorly on the task had lower fractional anisotropy vaules than ADHD parents who performed as well as comparison parents. Since there was a main effect of age (F=3.79, df=1, 58, p<0.001) across all regions, partial correlations were performed controlling for the subject variable of age. The correlations above remained significant after controlling for this variable (all ADHD subjects: r=0.57, p<0.001; ADHD parents: r=0.70, p<0.001, respectively). Less significant correlations were shown between fractional anisotropy in fibers in the left prefrontal region and d- prime scores in ADHD youth (r=0.49, p<0.03) and parents (r=0.48, p<0.04), which remained significant after controlling for age (r=0.53, p<0.03; r=51, p<0.03, respectively). There were no correlations between fractional anisotropy and d-prime for the comparison group, presumably because of less variance in behavioral performance, and there was no diagnostic group-by-region interaction (F=1.85, df=3, 171, p<0.14).
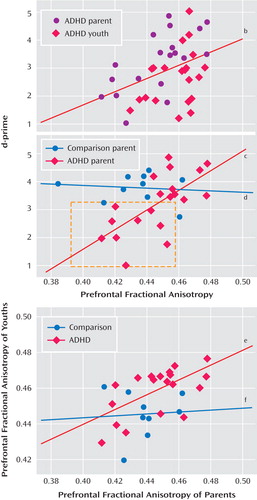
a Fractional anisotropy in the right prefrontal region was positively correlated with d-prime scores for the ADHD group, even after controlling for age (r=0.57, p<0.001). This association was driven largely by the correlation for the ADHD parents, approximately one-half (N=9) of whom performed poorly on the task and had lower fractional anisotropy than those who performed as well as the comparison parents (N=11). The red boxes indicate the subset of individuals showing this pattern. Correlations between prefrontal fiber tracts in parents and their children showed family resemblance in the ADHD dyads.
b r=0.40, p<0.01.
c r=0.65, p<0.003.
d r=–0.12, p<0.74.
e r=0.61, p<0.005.
f r=0.06, p<0.88.
A segment of the corticospinal tract that was not expected to have any direct relation to go/nogo task performance was delineated as a comparison region (28) . Fractional anisotropy in this region did not differentiate the ADHD and comparison groups, nor did it correlate with behavioral performance (for left and right corticospinal tracts: r=–0.23, p<0.16; r=–0.05, p<0.73, respectively). Further, there was no age-by-region interaction (F=1.44, df=3, 171, p<0.23), suggesting no significant differences between the fiber tracts of interest and these comparison tracts as a function of age for this sample.
Given the correlation between fractional anisotropy in the right frontostriatal tract and performance, we examined family resemblance in this measure for the parent-child dyads. Correlations between prefrontal fiber tracts in parents and their children showed family resemblance in the ADHD dyads (see Figure 2 ). There was a positive association between the ADHD parent and child in fiber tracking-based right and left prefrontal fractional anisotropy (r=0.61, p<0.005; r=0.66, p<0.001, respectively), and to a lesser extent, in corticospinal tract (right and left regions: r=0.54, p<0.02; r=0.49, p<0.03, respectively). These associations were not present in the comparison parent-child dyads. To ensure that any significant correlations between prefrontal fiber-tract measures in ADHD parents and their children were not driven by chance or variability attributable to the disorder alone, 200 permutations of random pairings of the parent and child were conducted for each of these regions, and each was reanalyzed. Of these random pairings, two reached significance at the corrected alpha level of 0.005 for the left prefrontal regions, and none reached significance for the right prefrontal regions, except for the analysis using the actual parent-child pairing, suggesting a tight association between right prefrontal white matter fiber tracts in parents and their children with ADHD.
Discussion
In this study, we examined the contribution of frontostriatal white matter tracts to cognitive control in parent-child dyads with ADHD. A diffusion tensor imaging-based measure of regularity and myelination (i.e., fractional anisotropy) of frontostriatal fiber tracts was shown to be correlated with performance and with fMRI beta values of the blood-oxygen-level dependent response in frontostriatal regions (i.e., prefrontal cortex and caudate nucleus) during performance of the go/nogo task. Specifically, less activity in these regions was associated with lower fractional anisotropy in adjacent white matter and with poorer cognitive performance, even after controlling for the effects of age. Further, there was an association between ADHD children and their parents in fractional anisotropy in right frontostriatal fiber tracts. The comparison parent-child dyads did not show this association, presumably because of the lack of variability in measures and power given the smaller sample of comparison dyads. Collectively, these data support previous studies indicating heritability of right frontostriatal brain structure among individuals with ADHD and suggest that atypical development of frontostriatal tracts could lead to cognitive deficits in this disorder.
Fractional anisotropy has been used extensively as a measure of white matter microstructure (8 , 26 , 28 , 29 , 31) . Combined with previous studies indicating that prefrontal white matter matures slowly during childhood and adolescence (6 – 9) , it is likely that increases in cognitive control ability may correspond with ongoing myelination of frontostriatal tracts as shown in our earlier study (27) and in the study by Klingberg et al. (8) . The hypothesis that atypical development and regularity of fiber tracts may explain our current findings and is based on the observation that divergence between diagnostic groups in the association between performance and prefrontal fractional anisotropy was not observed until adulthood. This pattern only emerged for those ADHD adults with poor cognitive performance.
Specifically, approximately one-half of the ADHD parents performed poorly on the task and all had lower fractional anisotropy than those who performed as well as comparison parents. The importance of white matter tract development in ADHD is supported by the tight association between measures of the right prefrontal fiber tracts of the ADHD parent and child. Previous familial studies of ADHD have suggested an important role of prefrontal regions in this disorder. For example, Durston et al. (19) showed that children with ADHD and their unaffected siblings activate the prefrontal cortex less during performance of cognitive control tasks (e.g., go/nogo task) relative to comparison subjects. Taken together, these findings suggest that prefrontal function and structure may be suitable candidate endophenotypes for studies investigating gene effects in ADHD.
Although automated fiber tractography has been used elsewhere for delineation of anatomic white matter tracts (23 , 24) , use of this methodology for selecting a region of interest has limitations. A selection bias may arise if path geometries are significantly affected by group differences in diffusion properties. However, an analysis of fiber-tract volumes revealed no group differences, and tract volumes were not correlated with fractional anisotropy or age. These findings suggest that a potential selection bias did not significantly confound the results presented in the present study.
Collectively, these findings suggest that variability in the myelination and regularity of right prefrontal fibers may contribute to cognitive deficits in ADHD. Family resemblance in prefrontal tracts in the patients underscores the importance of considering irregularities in prefrontal fiber tracts as an important factor in this disorder. Overall, the findings add to a growing body of evidence (16) , suggesting that genetic studies of disorders such as ADHD, which traditionally have focused on dopaminergic and neuroadrenergic neurotransmission, may benefit from examination of the regulation of myelination and axon migration in the prefrontal cortex.
1. Barkley RA: Advancing age, declining ADHD. Am J Psychiatry 1997; 154:1323–1325Google Scholar
2. Nigg JT, Casey BJ: An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol 2005; 17:785–806Google Scholar
3. Casey BJ, Nigg J, Durston S: New potential leads in the biology and treatment of attention deficit-hyperactivity disorder. Curr Opin Neurol 2006; 20:119–124Google Scholar
4. Koechlin E, Ody C, Kouneiher F: The architecture of cognitive control in the human prefrontal cortex. Science 2003; 302:1181–1185Google Scholar
5. Huttenlocher PR: Synaptic density in human frontal cortex: developmental changes and effects of aging. Brain Res 1979; 163:195–205Google Scholar
6. Yakovlev PI, Lecours AR: The myelogenetic cycles of regional maturation of the brain, in Regional Development of the Brain in Early Life. Edited by Minkowski A. Oxford, UK, Blackwell Scientific, 1967, pp 3–70Google Scholar
7. Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW: In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci 1999; 2:859–861Google Scholar
8. Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M: Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport 1999; 10:2817–2821Google Scholar
9. Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A: Maturation of white matter in the brain: a review of magnetic resonance studies. Brain Res Bull 2001; 54:255–266Google Scholar
10. Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM: Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 2004; 101:8174–8179Google Scholar
11. Brophy M, Taylor E, Hughes C: To go or not to go: inhibitory control in hard to manage children. Infant Child Dev 2002; 11:125–140Google Scholar
12. Munakata Y, Yerys BE: All together now: when dissociations between knowledge and action disappear. Psychol Sci 2001; 12:335–337Google Scholar
13. Diamond A: Normal development of prefrontal cortex from birth to young adulthood: cognitive functions, anatomy, and biochemistry, in Principles of Frontal Lobe Function. Edited by Stuss DT, Knight RT. Oxford, UK, Oxford University Press, 2002, pp 466–503Google Scholar
14. Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL: Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1996; 53:607–616Google Scholar
15. Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD: Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Nat Acad Sci U S A 1998; 95:14494–14499Google Scholar
16. Durston S, Mulder M, Casey BJ, Ziermans T, van Engeland H: Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for ADHD. Biol Psychiatry 2006; 60:1062–1107Google Scholar
17. Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E: Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry 2005; 162:1067–1075Google Scholar
18. Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Ulug AM, Casey BJ: Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry 2003; 53:871–878Google Scholar
19. Durston S, Fossella JA, Casey BJ, Hulshoff Pol HE, Galvan A, Schnack HG, Steenhuis MP, Minderaa RB, Buitelaar JK, Kahn RS, van Engeland H: Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Mol Psychiatry 2005; 10:678–685Google Scholar
20. Pliszka SR, Glahn DC, Semrud-Clikeman M, Franklin C, Perez R, Xiong J, Liotti M: Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. Am J Psychiatry 2006; 163:1052–1060Google Scholar
21. Smith AB, Taylor E, Brammer M, Toone B, Rubia K: Task specfic hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and tasking switching in medication-naive children and adolescents with attention deficit/hyperactivity disorder. Am J Psychiatry 2006; 163:1044–1051Google Scholar
22. Scheres A, Oosterlaan J, Swanson J, Morein-Zamir S, Meiran N, Schut H, Vlasveld L, Sergeant JA: The effect of methylphenidate on three forms of response inhibition in boys with AD/HD. J Abnorm Child Psychol 2003; 31:105–120Google Scholar
23. Pierpaoli C, Jezzard P, Basser PJ, Barnett A, DiChiro G: Diffusion tensor MR imaging of the human brain. Radiology 1996; 201:637–648Google Scholar
24. Le Bihan D: Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 2003; 4:469–480Google Scholar
25. Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH: Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003; 20:1714–1722Google Scholar
26. Nagy Z, Westerberg H, Klingberg T: Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci 2004; 16:1227–1233Google Scholar
27. Liston C, Watts R, Tottenham N, Davidson M, Niogi M, Ulug A, Casey BJ: Frontostriatal microstructure predicts individual differences in cognitive control. Cereb Cortex 2006; 16:553–560Google Scholar
28. Lim KO, Hedehus M, Moseley M, deCrespigny A, Sullivan EV, Pfefferbaum A: Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry 1999; 56:367–374Google Scholar
29. Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA: Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron 2000; 25:493–500Google Scholar
30. Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, Rhinewine J, Kane JM, Adesman A, Milanaik R, Maytal J, Diamond A, Szeszko P, Ardekani BA: Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry 2005; 57:448–455Google Scholar
31. Deipolyi AR, Mukherjee P, Gill K, Henry RG, Partridge SC, Veeraraghavan S, Jin H, Lu Y, Miller SP, Ferriero DM, Vigneron DB, Barkovich AJ: Comparing microstructural and macrostructural development of the cerebral cortex in premature newborns: diffusion tensor imaging versus cortical gyration. Neuroimage 2005; 27:579–586Google Scholar
32. Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME: Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A 1999; 96:10422–10427Google Scholar
33. Basser P, Pierpaoli C: Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996; 111:209–219Google Scholar
34. Wickens TD: Elementary Signal Detection Theory. New York, Oxford University Press, 2002Google Scholar
35. Epstein JN, Casey BJ, Tonev ST, Davidson M, Reiss AL, Garrett A, Hinshaw SP, Greenhill LL, Glover G, Shafritz KM, Vitolo A, Kotler LA, Jarrett MA, Spicer J: ADHD- and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. J Child Psychol Psychiatry 2007; 48:899–913Google Scholar
36. Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME: NIMH Diagnostic Interview Schedule for Children-Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 2000; 39:28–38Google Scholar
37. Casey BJ, Cohen JD, O’Craven K, Davidson RJ, Irwin W, Nelson CA, Noll DC, Hu X, Lowe MJ, Rosen BR, Truwitt CL, Turski PA: Reproducibility of fMRI results across four institutions using a spatial working memory task. Neuroimage 1998; 8:249–261Google Scholar
38. Glover GH, Law CS: Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med 2001; 46:515–522Google Scholar
39. Boynton GM, Engel SA, Glover GH, Heeger DJ: Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci 1996; 16:4207–4221Google Scholar
40. Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC: Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995; 33:636–647Google Scholar