Co-Occurring Mental and Substance Use Disorders: The Neurobiological Effects of Chronic Stress
Abstract
The high rate of co-occurrence of substance use disorders and other psychiatric disorders is well established. The population of people with co-occurring disorders is heterogeneous, and the prevalence of comorbidity differs by diagnostic group. One of the overarching issues in the area of comorbidity is the nature of the connection between psychiatric disorders and substance use disorders. The rapid development of technical advances in the neurosciences has led to a better understanding of the molecular biology, neurotransmitter systems, and neural circuitry involved in mental illness and substance use disorders. The authors discuss the neurobiological interface between substance use disorders and other psychiatric disorders with an emphasis on emerging data concerning four psychiatric disorders that commonly co-occur with substance use disorders: depression/mood disorders, posttraumatic stress disorder, attention deficit hyperactivity disorder, and schizophrenia. Better understanding of the connection between substance use disorders and psychiatric disorders could have a profound effect on prevention and treatment.
The high rate of co-occurrence of substance use disorders and other psychiatric disorders is well established (1, 2). The implications of comorbidity are far-reaching and raise important questions that are unlikely to have simple answers. One of the overarching issues is the question of why substance use and other mental disorders so often co-occur. Are there genetic mediators and/or neurobiological connections between these disorders that drive the comorbidity? Do different psychiatric disorders have differing relationships with various substances of abuse? Better understanding of the connection between substance use disorder and mental illness could have a profound effect on both prevention and treatment.
In this article, we focus on four psychiatric disorders—depression/mood disorders, posttraumatic stress disorder (PTSD), attention deficit hyperactivity disorder (ADHD), and schizophrenia—because research concerning the neurobiological and mechanistic connections between these disorders and substance use disorders is particularly active. With the rapid development of technical advances in the neurosciences, the amount of information concerning the molecular biology, neurotransmitter systems, and neural circuitry involved in mental illness and substance use disorders has increased dramatically. In this article, we conceptualize chronic distress as a central construct underlying the association of each of these four psychiatric disorders with substance use disorders and examine emerging neurobiological findings within this framework.
Prevalence: Epidemiological and Clinical Perspectives
Epidemiological surveys in the 1990s emphasized the prevalence of comorbid psychiatric and substance use disorders in community samples of adults (1, 3, 4). In the Epidemiologic Catchment Area study (3), an estimated 45% of individuals with alcohol use disorders and 72% of individuals with drug use disorders had at least one co-occurring psychiatric disorder. In the National Comorbidity Study (1), approximately 78% of alcohol-dependent men and 86% of alcohol-dependent women met the criteria for a lifetime diagnosis of another psychiatric disorder, including drug dependence. The risk relationship appears to be reciprocal, with psychiatric disorder predicting increased risk of later substance use and vice versa. A study involving a subset of National Comorbidity Study subjects found that active psychiatric disorders predicted an increased risk for the first onset of daily smoking and progression to nicotine dependence (5). Comorbidity is greater in individuals who are dependent on illicit drugs, compared to alcohol-dependent individuals, and individuals with multiple dependencies experience the highest rates of psychiatric comorbidity (6). Because acute intoxication and withdrawal from drugs of abuse can mimic symptoms of psychiatric disorders, the overlap of symptoms can be problematic in making an accurate diagnosis of a psychiatric disorder in an individual with a substance use disorder. This difficulty may account for some of the high comorbidity rates reported in epidemiological studies, which are not generally designed to tease apart substance-related and independent psychiatric symptoms. Despite this caveat, even conservative estimates suggest a high rate of comorbidity between psychiatric disorders and substance use disorders.
Etiological Relationships: Theoretical Perspective
Although convincing data support a strong association between a variety of psychiatric disorders and substance use disorders, the nature of the relationship is complex and may vary depending on the disorder in question and substance that is used. Several theories have been proposed to explain the high co-occurrence. Certain psychiatric disorders may be risk factors for development of substance use disorders or may modify the course of substance use disorders. One of the more overarching theories of addiction is that drugs and their specific psychotropic effects are used to cope with emotional distress (7). Psychiatric disorders have been conceptualized as chronic distress states associated with neurobiological alterations in brain stress circuits (8–10). On the other hand, chronic drug use is associated with neuroadaptations in brain reward pathways that produce secondary psychiatric symptoms during acute and protracted withdrawal states (10). With increasing severity of addiction, neuroadaptations in stress and reward circuits occur, and these changes may underlie the increasing emotional distress often associated with substance use disorders (11, 12).
A growing body of evidence from basic science and translational studies implicates common neurobiological pathways and abnormalities involved in addiction and a number of psychiatric disorders. Within a neurobiological framework, at least two hypotheses can be postulated to explain comorbidity: 1) addiction and other psychiatric disorders are different symptomatic expressions of similar preexisting neurobiological abnormalities, and 2) repeated drug administration, through neuroadaptation, leads to biological changes that have common elements with the abnormalities mediating certain psychiatric disorders (13).
One of the bridging constructs between psychiatric and substance use disorders is the role of stress in the development and relapse of substance use disorders and other psychiatric disorders. Figure 1 provides a heuristic model of the relationship between chronic distress states, substance use disorders, and psychiatric comorbidity. Although the model conceptualizes chronic distress as the bridging construct, various genetic and environmental vulnerability factors contribute to the development of the distress states, as noted in Figure 1. Additional research on these factors will contribute to a more specific understanding of the mechanisms underlying the associations between psychiatric and substance use disorders.
Corticotropin-releasing factor (CRF), one of the key hormones involved in the stress response, has been implicated in the pathophysiology of anxiety, affective, and addictive disorders (8, 14). Preclinical evidence suggests that CRF and noradrenergic pathways are involved in stress-induced reinstatement of drug-seeking behavior in drug-dependent laboratory animals (15). Stress stimuli that activate CRF circuits are also known to potentiate mesolimbic dopaminergic reward pathways in laboratory animals (16). Similarly, human laboratory studies have shown that emotional stress and negative affect states increase drug craving in drug-dependent individuals (17, 18). Evidence of an association between severity of depressive symptoms in patients with major depression and the subjective reinforcing effect of an acute dose of dextroamphetamine (19) suggests dysregulation of reward systems with increasing levels of distress in major depression. In animal models, early life stress and chronic stress result in long-term changes in stress responses (20). Such changes can alter the sensitivity of the dopamine system to stress and can increase susceptibility to self-administration of substances of abuse (16, 21, 22).
Corticolimbic dopamine and noradrenergic pathways modulate prefrontal cortical function under conditions of increasing cognitive or emotional demand, including persistent distress states, tasks involving high levels of cognitive challenge, and working memory tasks (23, 24). Glutamatergic and γ-aminobutyric acid (GABA)-ergic pathways are also important in modulating prefrontal cortical function (25).
It is important to note that different substances of abuse have widely varying effects on neurobiological systems. Cocaine and amphetamines have a stimulating effect on catecholaminergic systems. Opioid analgesic drugs act through a complex system of opioid receptors, and nicotine acts through specific nicotinic receptors distributed throughout the central and peripheral nervous systems. GABA-ergic and glutamatergic systems are particularly important in acute intoxication and withdrawal from alcohol and benzodiazepines. Clearly, the effects of acute intoxication and withdrawal differ for each of these drugs, and the effect on psychiatric disorders also differs by drug. It is interesting to note, however, that there appear to be common neurobiological pathways operating across substances of abuse. Dopamine activity in the nucleus accumbens has been implicated in the mechanism of reinforcement for almost all drugs of abuse (26). Furthermore, drugs of abuse activate the CRF/hypothalamic-pituitary-adrenal (HPA) axis during use/abuse, and alterations in the CRF/HPA and noradrenergic systems during acute withdrawal/abstinence are also well documented (11, 12, 26). Some animal models of reinstatement (i.e., stress-induced reinstatement, cue-induced reinstatement) operate across substances of abuse, also arguing for some common mechanisms.
In the following sections, we review emerging data that shed light on the neurobiological connections between various substance use disorders and the four psychiatric disorders considered here (depression/mood disorders, PTSD, ADHD, and schizophrenia). Figure 2 summarizes the neurobiological evidence cited in the following sections and identifies the overlapping neurotransmitter systems and associated brain regions.
Depression and Substance Use Disorders
Epidemiological studies reported rates of comorbidity of major depression with nicotine, alcohol, and illicit drug abuse ranging from 32% to 54% (1, 27, 28). Individuals with major depression are more likely to develop substance use disorders, and individuals with substance use disorders are at greater risk for the development of major depression, compared to the general population (27–29). Clinical similarities exist between major depression and substance use disorders. Depressive symptoms are commonly reported during acute and chronic withdrawal from drugs of abuse. Irritability, sleep difficulties, anxiety, and trouble with attention/concentration are associated with both protracted withdrawal states and major depression.
Neurobiological similarities between major depression and substance use disorders likely contribute to both symptom overlap and high rates of comorbidity (13). Substantial data indicate that extrahypothalamic CRF and HPA axis abnormalities (8) and alterations in catecholamine, serotonin, GABA, and glutamate systems are associated with major depression (30, 31). Neuroadaptations associated with chronic drug abuse are associated with alterations in these neurotransmitter systems, especially during acute withdrawal states (13). CRF/HPA response during acute drug withdrawal has a positive association with withdrawal-related distress and with depressive symptoms (32, 33). In addition, a growing amount of evidence indicates that the neurobiological alterations associated with acute withdrawal last for varying time periods and contribute to drug craving and relapse in substance use disorders (12). In a recent study (34), individuals with substance use disorders, both with and without depressive symptoms, were found to have significantly lower ACTH and cortisol response to CRF stimulation, compared to healthy subjects. These findings are consistent with studies of abstinent smokers, alcoholic subjects, and subjects with polysubstance dependence in which a blunted cortisol response to standard psychological stressors was demonstrated (12). Blunted cortisol and prolactin responses to d-fenfluramine challenge in abstinent heroin-dependent individuals with and without depression have also been reported (35). Blunted peripheral stress hormone responses may be a marker for increased HPA axis activity (36, 37).
Evidence of altered neuroendocrine response to stress challenges in substance use disorders is consistent with clinical observations that individuals with substance use disorder have difficulty managing stressful situations and emotional distress states and often relapse in the face of stressful situations (12, 38). In laboratory studies, stress and negative affect states increase drug craving and emotional distress in abstinent substance-dependent individuals (17, 39–41). These changes are accompanied by physiological arousal (18), and this finding suggests that drug-craving states that are marked by increased levels of anxiety and distress are accompanied by biological stress responses. Increased distress-related drug craving is associated with vulnerability to continued drug use and relapse (12, 42), and this association suggests a mechanistic connection between depressive symptoms and substance use disorders.
In other studies, specific associations between monoamine oxidase (MAO) activity in smoking and major depression have been examined. MAO (with A and B subtypes), an enzyme involved in oxidizing serotonin, norepinephrine, and dopamine in the brain, has long been associated with negative mood and depression. For example, MAO inhibitors are known to have antidepressant properties. It is interesting to note that smokers show reduced MAO-A and MAO-B levels in the brain, compared to nonsmokers and former smokers (43, 44). These findings provide some support for the notion that smoking may have antidepressant effects through inhibition of MAO-A and MAO-B activity and suggest a pharmacological explanation for the high rates of smoking reported among individuals with major depression.
Recent findings from neuroimaging studies implicate similar alterations in frontal-limbic brain circuitry in substance use disorders and major depression. Reduced frontal metabolism and hypoactivity of the anterior cingulate have been reported in individuals with substance use disorders (45, 46). Significant reduction in dopamine D2 receptors, particularly in frontal-striatal regions, has been noted in cocaine- and alcohol-dependent individuals, compared to healthy subjects (45). Reduced frontal-limbic metabolism has also been found in subjects with major depression, relative to healthy subjects (47). Such findings are consistent with postmortem studies showing reduced cell density and gray matter volume in individuals with a diagnosis of major depression (48). Furthermore, amygdala hyperactivity and anterior cingulate hypoactivity are associated with major depression (47), and studies of individuals with substance use disorders indicate activation in the amygdala associated with cue-induced drug craving (49, 50). Under conditions of distress, cocaine-dependent subjects exhibited decreased activity in frontal regions such as the medial prefrontal cortex and the anterior cingulate, similar to that seen with negative mood in subjects with major depression (51, 52). Similarly, a recent study (53) reported lower levels of glucose metabolism in the anterior cingulate and insula, but higher levels in the orbitofrontal region, amygdala, middle and posterior cingulate, and ventral striatum in methamphetamine abusers with severe mood and anxiety symptoms, compared to healthy subjects.
In conclusion, neuroendocrine and neuroimaging studies indicate dysregulation in frontal-limbic systems associated with stress and reward pathways in both major depression and substance use disorders. This common dysregulation is likely to contribute to the high rate of comorbidity of these illnesses. Evidence concerning negative affect and stress-related drug seeking/craving provides additional insight into emotional distress states and drug use in drug-experienced individuals. A better understanding of these connections will contribute to the development of new treatments for major depression, substance use disorders, and the comorbidity of these disorders.
PTSD and Substance Use Disorders
The high prevalence of the comorbidity of substance use disorders and PTSD has been reported in a number of studies. Initial reports focused on veterans with PTSD, of whom 64%–84% met the criteria for a lifetime alcohol use disorder and 40%–44% met the criteria for a lifetime drug use disorder, including nicotine dependence (54, 55). In civilian populations with PTSD, estimates of the lifetime prevalence of substance use disorders range from 22% to 43% (56, 57), far higher than the estimates for substance use disorders in the general population.
As in other comorbidities, PTSD and substance use disorders have a number of connecting pathways. Substance intoxication may heighten the likelihood of exposure to trauma, hence the likelihood of developing PTSD. Furthermore, chronic substance use and withdrawal may increase anxiety/arousal states, making it more likely that individuals with substance use disorders will develop PTSD after trauma exposure. On the other hand, PTSD could increase the risk of developing a substance use disorder, because individuals may abuse substances in an attempt to relieve symptoms of PTSD. Substance use could also exacerbate symptoms and/or prolong the course of PTSD by preventing habituation to traumatic memories. These pathways are not mutually exclusive, and new evidence is emerging concerning the neurobiological underpinnings of potential causal pathways. In one recent study (58), individuals who had experienced any trauma and developed PTSD had an increased risk for the development of drug dependence, particularly nicotine dependence, but not alcohol dependence. This finding suggests specificity between substance of abuse and psychopathology.
The HPA axis, extrahypothalamic CRF, and the noradrenergic system are all intimately involved in the stress response, PTSD, and the pathophysiology of substance use disorders. Evidence is accumulating to support a role for CRF in mediating the effects of stress in increasing self-administration of drugs. Studies in rats have also demonstrated that withdrawal from chronic cocaine (59) or alcohol administration (60) in rats is associated with increases in CRF in the hypothalamus, amygdala, and basal forebrain. Elevated CSF CRF has been found in humans during alcohol withdrawal (61). Two studies examining CSF concentrations of CRF have demonstrated higher levels in individuals with PTSD, compared to healthy subjects (62, 63). This finding is of particular interest because elevated brain CRF levels, especially in the amygdala, potentiate fear-related behavioral responses (64). As such, elevated levels of CRF may mediate both the symptoms of hyperarousal and the increased risk for substance use disorders in PTSD. Increased CRF may enhance the reinforcing properties of some drugs, worsen the severity of withdrawal symptoms, and exacerbate symptoms of PTSD.
Evidence implicating abnormalities in noradrenergic systems has been found for both PTSD and substance use disorders. Individuals with PTSD have elevated urinary excretion of both norepinephrine and epinephrine and elevated plasma levels of norepinephrine (65). Markers of noradrenergic activity are increased in both alcohol and opioid withdrawal (66–68). Brain CRF and noradrenergic systems modulate each other in a number of ways. Stress increases CRF in the locus ceruleus (69), and intraventricular administration of CRF increases norepinephrine turnover in the hypothalamus, hippocampus, and prefrontal cortex (70). In the amygdala, norepinephrine stimulates the release of CRF (71). Koob and colleagues (72, 73) hypothesized that interactions between CRF and the noradrenergic systems can function as a “feed-forward” system, with progressive augmentation of the stress response with repeated stress exposure. Specifically, substance use or withdrawal or other stress may stimulate CRF release in the locus ceruleus, leading to the release of norepinephrine in the cortex, which would, in turn, stimulate the release of CRF in the hypothalamus and amygdala. This interaction could help to explain the attempt to self-medicate PTSD symptoms with substances of abuse, the worsening of PTSD symptoms during substance withdrawal, and the increase in vulnerability to the development of PTSD in traumatized individuals with substance use disorders.
Neuroimaging studies have shed light on the connection between PTSD, other anxiety disorders, and substance use disorders. Amygdala activation occurs during symptom provocation in PTSD, panic disorder, and social phobia (74). As mentioned earlier, increased amygdalar blood flow is also seen in cocaine-dependent individuals presented with cocaine-related cues (50, 75).
ADHD Spectrum and Substance Use Disorders
Substantial evidence suggests that ADHD, conduct disorder, and oppositional defiant disorder co-occur at high rates among children and adolescents. This group of disorders, conceptualized as externalizing disorders, is associated with shared genetic and environmental risk factors (76–78). Externalizing disorders are commonly comorbid with substance use disorders in adolescents, with prevalence estimates ranging from 30%–50% (79). Adolescents with comorbid substance use disorder and ADHD, conduct disorder, and/or oppositional defiant disorder have an earlier age at onset and a more severe course of substance use disorder (80–82). Research has identified genetic, neurobiological, and psychosocial risk factors that contribute to the core pathophysiology in the development of comorbid ADHD and substance use disorders.
Externalizing disorders are characterized by behavioral disinhibition and personality traits such as aggression, high levels of impulsivity, and poor self-control (77, 78, 83). Substantial evidence suggests that externalizing disorders are associated with problems in higher-order “executive” (frontal) cognitive function. In fact, ADHD has often been characterized as a disorder of frontal and prefrontal cortex dysfunction (23, 71). Children with ADHD, conduct disorder, oppositional defiant disorder, and early-onset substance use disorder showed poor performance on neuropsychological tests of abilities involving the prefrontal cortex, including planning, attention, cognitive flexibility, working memory, self-monitoring, and behavioral and motor control (71).
In a large twin study that examined P3 amplitude—a robust electrophysiological marker with a strong genetic basis—lower P3 amplitude was associated with presence of ADHD, conduct disorder, oppositional defiant disorder, and substance use disorder in adolescent boys (78). Lower P3 amplitude at age 17 years predicted development of substance use disorder at age 20 years. Although genetic factors contribute to the development of comorbid ADHD, conduct disorder, and oppositional defiant disorder, a single shared environmental factor, identified as parent-child conflict (i.e., negative social interactions between parents and children), accounts for an even larger proportion of the variance (84). Negative parent-child interactions, high levels of negative affect, and emotional distress are also known to increase the risk of substance use disorder in adolescents (85). These data suggest that coping with high levels of family conflict may play an important role in the development of both ADHD and substance use disorders.
Preclinical research has demonstrated that dopamine and norepinephrine modulate prefrontal cortical function (23, 86) and that stress impairs prefrontal cortical function (86, 87). Evidence from brain imaging studies indicates that the prefrontal cortex and anterior cingulate cortex play important roles in cognitive conflict monitoring (88, 89) and self-regulation processing (90, 91). Difficulties in response inhibition and self-regulation are core symptoms of externalizing disorders (78, 83). Compared to healthy subjects, boys with comorbid ADHD, conduct disorder, and oppositional defiant disorder show greater behavioral aggression, heart rate reactivity, and higher levels of anger in a laboratory-induced provocation paradigm (92). Furthermore, in boys with externalizing symptoms, lower cortisol levels and the personality traits of low levels of self-control and harm avoidance are associated with the development of substance use disorders (93, 94). Thus, consistent with preclinical evidence indicating that stress impairs prefrontal cortical function, human studies suggest that individuals with ADHD and early-onset substance use disorders have poor stress-related coping and poor self-regulation.
The prefrontal cortex and anterior cingulate cortex are also important in regulating behavior related to future rewards. Primate studies have shown that the prefrontal cortex and anterior cingulate cortex are involved in assessing reward expectancy (95) and motor responses based on future reward (96). These data are consistent with the critical role of the prefrontal cortex in drug self-administration and in the reinforcement and reinstatement of drug use (97, 98). Children with ADHD and conduct disorder show disinhibited physiological and behavioral responses during reward-related cognitive tasks (99, 100). These findings are consistent with decreased prefrontal cortical and striatal activity and increased activity in posterior and sensory cortices in ADHD (101, 102) that is normalized by chronic methylphenidate treatment (103). Preliminary findings indicated that decreasing catecholamine input to the prefrontal cortex by means of α2-adrenergic agonists such as guanfacine, which inhibit norepinephrine centrally, enhances prefrontal cortical function and decreases ADHD symptoms (104). It is interesting to note that other α2-adrenergic agonists, such as clonidine and lofexidine, attenuate stress-induced reinstatement of drug-seeking behavior in laboratory models (105, 106). To the extent that stress and reward dysfunction contribute to prefrontal cortical deficits in ADHD and substance use disorders, α2-adrenergic agonists may be beneficial in addressing this comorbidity.
Schizophrenia and Substance Use Disorders
Recent studies have demonstrated that up to 50% of individuals with schizophrenia have either alcohol or illicit drug dependence and more than 70% are nicotine dependent (2, 107, 108). In addition to having the expected adverse medical consequences, substance use in schizophrenic patients is associated with poor social function, symptom exacerbation, frequent hospitalization, medication noncompliance, and poor treatment response (109, 110). Schizophrenia and substance use are connected by multiple potential links, including genetic vulnerability, medication side effects, negative symptoms, and psychosocial factors. Self-medication has been commonly invoked to explain the high comorbidity. Specifically, self-medication of negative symptoms, such as social withdrawal and apathy, and drug use in the attempt to decrease discomfort from the side effects of typical antipsychotic medications have been suggested as explanations for the high prevalence of substance use disorders in individuals with schizophrenia. Although these factors may play some role, advances in neurobiology suggest that the neuropathology of schizophrenia affects the neural circuitry mediating drug reward, leading to an increased vulnerability to addiction. Specifically, Chambers and colleagues (111) hypothesized that abnormalities in hippocampal-cortical function in schizophrenia impair the inhibitory hippocampal projections to the nucleus accumbens, resulting in reduced inhibitory control over dopamine-mediated functional hyperresponsivity to dopamine release. In this model, dysregulated neural integration of dopamine and glutamate in the nucleus accumbens resulting from frontal and hippocampal dysfunction could lead, in subjects without prior drug exposure, to neural and motivational changes similar to those in long-term substance use. Thus, the predilection of schizophrenic patients to substance use disorders may be a primary disease symptom.
Recent studies focused on the neurobiological interface between schizophrenia substance use disorders support this hypothesis. In one study (112), magnetic resonance images in groups of subjects with schizophrenia, schizophrenia plus alcohol dependence, and alcohol dependence only were compared with those from a matched control group. Gray matter deficits were found in all three patient groups, but were greatest in the group with comorbidity. The most prominent deficits were in the prefrontal and anterior superior temporal regions, indicating that comorbidity compounded the prominent prefrontal cortical deficits that are present independently in schizophrenia and alcohol dependence. Lifetime alcohol consumption in subjects with comorbidity was approximately five times less than that in the alcohol-dependent subjects, yet the subjects with comorbidity exhibited the full detrimental effects of alcohol, which suggests an interactive effect.
One area of particular interest is nicotine dependence and schizophrenia. It has been estimated that 70%–90% of individuals with chronic schizophrenia are nicotine dependent (113). Nicotine interacts with many of the same central pathways involved in schizophrenia, including the dopaminergic and glutamatergic pathways in the mesolimbic areas. Several abnormalities associated with schizophrenia are improved with nicotine administration, including deficits in the inhibitory gating of the P-50 evoked response to repeated auditory stimuli (114) and deficits in smooth pursuit eye movement dysfunction (115). George and colleagues (116) found deficits in visuospatial working memory in schizophrenic and nonschizophrenic individuals with nicotine dependence. With increasing periods of abstinence, the nonschizophrenic smokers had improvements in visuospatial working memory, whereas the schizophrenic smokers experienced further impairment in visuospatial working memory. The authors postulated that the high rates of cigarette smoking in schizophrenic patients may be related to the effects of smoking in alleviating some of the cognitive dysfunction associated with the presumed hypofunctionality of cortical dopamine systems in schizophrenia. In a recent study of more than 14,000 adolescents followed over a 4–16-year period, adolescents who smoked more than 10 cigarettes/day at the initial evaluation were significantly more likely to be hospitalized for schizophrenia during the follow-up period (117). These findings suggest that smoking might constitute self-medication of premorbid symptoms, might reflect an intrinsic, disease-related disorder of nicotinic transmission, or might play a causative role in the development of schizophrenia through chronic activation of mesolimbic dopaminergic neurotransmission in vulnerable individuals. The development of novel approaches based on nicotinic receptor mechanisms may have implications for both prevention and treatment of schizophrenia.
Data from small, largely uncontrolled studies suggest that treatment with clozapine and other atypical antipsychotics may be associated with decreases in substance abuse in schizophrenic patients. Although the data are limited, this favorable response to atypical agents is consistent with the theory that dysfunction of the brain reward system leads to an increased vulnerability to addictions in schizophrenia (118). Typical antipsychotic agents are potent antagonists of D2 receptors. Although this blockade may initially decrease the reinforcing properties of some substances of abuse, with chronic use there may be enhancement of the substances’ reinforcing properties. Studies in rodents demonstrated that chronic treatment with haloperidol increased the reinforcing properties of cocaine, presumably through up-regulation of the postsynaptic dopamine receptor secondary to chronic blockade (119). In contrast, atypical agents, such as clozapine, have varied actions on a number of neurotransmitter systems and are much weaker D2 antagonists. It is possible that these agents have a normalizing effect on the signal detection capabilities of the mesocorticolimbic reward circuitry, and this action may explain the association with decreased substance use.
Conclusions
Although the nature of the relationship between psychiatric disorders and substance use disorders is complex and multifaceted, there are likely to be unifying constructs. Neuroadaptations in brain stress and reward pathways associated with chronic stress may predispose or unmask a vulnerability to psychiatric disorders, substance use disorders, or both. Dysfunction in the prefrontal cortex and frontal cortex associated with deficits in self-monitoring and behavioral control are evident in ADHD, other externalizing disorders, and substance use disorders. Emerging evidence suggests that abnormalities of glutamatergic function in schizophrenia and other psychiatric disorders may mediate vulnerability to the development of substance use disorders.
Although the focus of this article has been on neurobiological connections between psychiatric and substance use disorders, it is important to note that these connections constitute just one facet of a complex issue. Further exploration of overlapping neural circuitry and mechanistic relationships will be essential in guiding treatment and prevention efforts. However, improvement in our understanding of co-occurring disorders will be useful only if there is a treatment system in place to implement these findings. Clearly, change at public policy levels will be necessary to maximize the benefits derived from the findings of neurobiological explorations in order to improve the lives of individuals with comorbidity.
Received Oct. 8, 2004; revision received Jan. 12, 2005; accepted Jan. 27, 2005. From the Clinical Neuroscience Division, Institute of Psychiatry and Behavioral Sciences, Medical University of South Carolina; and Connecticut Mental Health Center, Yale University School of Medicine, New Haven, Conn. Address correspondence and reprint requests to Dr. Brady, Clinical Neuroscience Division, Institute of Psychiatry and Behavioral Sciences, Medical University of South Carolina, 69 President St., Charleston, SC 29425; [email protected] (e-mail). Supported by grant 1 P50 AR-049551 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, grants 5 M01 RR-01070-26 and M01 RR-00125 from the National Center for Research Resources, and grants K24 DA-00435-04, 1 P50 DA-016556, and K02-DA-017232 from the National Institute on Drug Abuse.
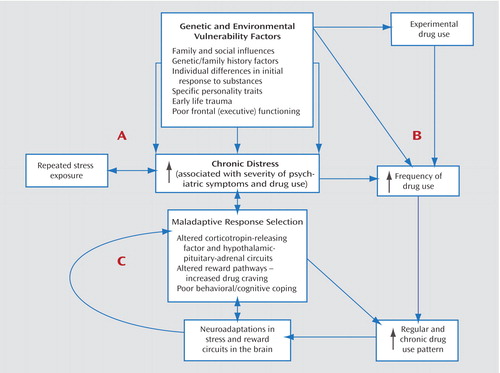
Figure 1. Schematic Model of Chronic Distress and Perpetuation of Psychiatric Symptoms and Drug Use in Individuals With Comorbid Disordersa
aVarious genetic and environmental vulnerability factors contribute to the development of psychiatric/emotional distress (A) and to drug abuse (B). Chronic distress is a common construct underlying both the disabling psychiatric symptoms associated with specific psychiatric disorders and the increasing distress associated with severity of substance use disorder. Chronic distress states are associated with selection of maladaptive responses, such as drug use, in order to attain desired goals or homeostasis. Maladaptive response selection mechanisms are associated with alterations in various neurotransmitter systems (including corticotropin-releasing factor and hypothalamic-pituitary-adrenal circuits), increased levels of stress-induced drug craving, and poor adaptive coping, representing neuroadaptations in the stress and reward circuits (C). This mechanism contributes to the escalation of drug use to the chronic levels characteristic of dependence, supporting a feed-forward loop that leads to greater alterations in stress and reward systems. These alterations perpetuate chronic distress and susceptibility to repeated stress exposures, thereby promoting a cycle of distress and drug use in individuals with comorbid disorders (adapted from Sinha [12]).
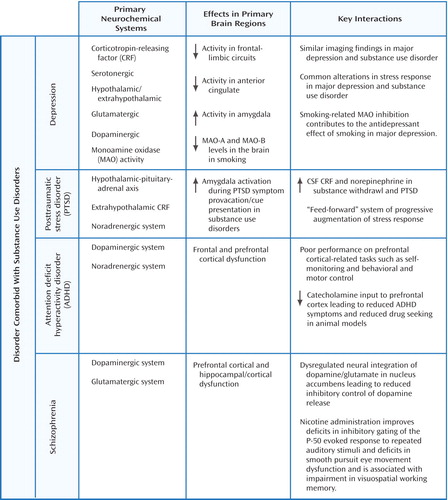
Figure 2. Central Systems/Pathways Involved in the Comorbidity of Psychiatric and Substance Use Disorders
1. Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H-U, Kendler KS: Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51:8–19Crossref, Medline, Google Scholar
2. Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LJ, Goodwin FK: Comorbidity of mental disorders with alcohol and other drug abuse. JAMA 1990; 264:2511–2518Crossref, Medline, Google Scholar
3. Regier DA, Narrow WE, Rae DS, Manderscheid RW, Locke BZ, Goodwin FK: The de facto US mental and addictive disorders service system: Epidemiologic Catchment Area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry 1993; 50:85–94Crossref, Medline, Google Scholar
4. Grant BF: Prevalence and correlates of drug use and DSM-IV drug dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse 1996; 8:195–210Crossref, Medline, Google Scholar
5. Breslau N, Novak SP, Kessler RC: Psychiatric disorders and stages of smoking. Biol Psychiatry 2004; 55:69–76Crossref, Medline, Google Scholar
6. Kandel DB, Huang FY, Davies M: Comorbidity between patterns of substance use dependence and psychiatric syndromes. Drug Alcohol Depend 2001; 64:233–241Crossref, Medline, Google Scholar
7. Khantzian EJ: The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry 1985; 142:1259–1264Link, Google Scholar
8. Nemeroff CB: The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry 1996; 1:336–342Medline, Google Scholar
9. Sapolsky RM, Alberts SC, Altmann J: Hypercortisolism associated with social subordinance or social isolation among wild baboons. Arch Gen Psychiatry 1997; 54:1137–1143Crossref, Medline, Google Scholar
10. McEwen B: Allostatsis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology 2000; 22:108–124Crossref, Medline, Google Scholar
11. Koob GF, Le Moal M: Drug abuse: hedonic homeostatic dysregulation. Science 1997; 278:52–58Crossref, Medline, Google Scholar
12. Sinha R: How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001; 158:343–359Crossref, Medline, Google Scholar
13. Markou A, Kosten TR, Koob GF: Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 1998; 18:135–174Crossref, Medline, Google Scholar
14. Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S: Compulsive drug-seeking behavior and relapse: neuroadaptation, stress, and conditioning factors. Ann NY Acad Sci 2001; 937:1–26Crossref, Medline, Google Scholar
15. Shalev U, Grimm JW, Shaham Y: Neurobiology of relapse to heroin and cocaine: a review. Pharmacol Rev 2002; 54:1–42Crossref, Medline, Google Scholar
16. Piazza PV, Le Moal M: The role of stress in drug self-administration. Trends Pharmacol Sci 1998; 19:67–74Crossref, Medline, Google Scholar
17. Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L: Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol 1997; 106:243–250Crossref, Medline, Google Scholar
18. Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ: Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003; 170:62–72Crossref, Medline, Google Scholar
19. Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, Busto UE: Probing brain reward system function in major depressive disorder: altered response to dextroamphetamine. Arch Gen Psychiatry 2002; 59:409–416Crossref, Medline, Google Scholar
20. Meaney MJ, Bhatnagar S, Larocque S, McCormick C, Shanks N, Sharma S, Smythe J, Viau V, Plotsky PM: Individual differences in the hypothalamic-pituitary-adrenal stress response and the hypothalamic CRF system. Ann NY Acad Sci 1993; 697:70–85Crossref, Medline, Google Scholar
21. Meaney MJ, Brake W, Gratton A: Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology 2002; 27:127–138Crossref, Medline, Google Scholar
22. Higley JD, Thompson WW, Champoux M, Goldman D, Hasert MF, Kraemer GW, Scanlan JM, Suomi SJ, Linnoila M: Paternal and maternal genetic and environmental contributions to cerebrospinal fluid monoamine metabolites in rhesus monkeys (Macaca mulatta). Arch Gen Psychiatry 1993; 50:615–623Crossref, Medline, Google Scholar
23. Arnsten AF, Steere JC, Hunt RD: The contribution of alpha 2-noradrenergic mechanisms of prefrontal cortical cognitive function: potential significance for attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1996; 53:448–455Crossref, Medline, Google Scholar
24. Goldman-Rakic P: Circuitry of primate prefrontal cortex and regulation of behavior by representational memory, in Handbook of Physiology, Section 1, The Nervous System, vol 5: Higher Functions of the Brain, Part 1. Edited by Mountcastle VB, Plum F. Bethesda, Md, American Physiological Society, 1987, pp 373–417Google Scholar
25. Moghaddam B: Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry 2002; 51:775–787Crossref, Medline, Google Scholar
26. Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP: Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev 2004; 27:739–749Crossref, Medline, Google Scholar
27. Rao U, Ryan ND, Dahl RE, Birmaher B, Rao R, Williamson DE, Perel JM: Factors associated with the development of substance use disorder in depressed adolescents. J Am Acad Child Adolesc Psychiatry 1999; 38:1109–1117Crossref, Medline, Google Scholar
28. Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J: Smoking, smoking cessation, and major depression. JAMA 1990; 264:1546–1549Crossref, Medline, Google Scholar
29. Brook DW, Brook JS, Zhang C, Cohen JD, Whiteman M: Drug use and the risk of major depressive disorder, alcohol dependence, and substance use disorders. Arch Gen Psychiatry 2002; 59:1039–1044Crossref, Medline, Google Scholar
30. Gold PW, Chrousos GP: Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry 2002; 7:254–275Crossref, Medline, Google Scholar
31. Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF: Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 2004; 61:705–713Crossref, Medline, Google Scholar
32. Frederick SL, Reus VI, Ginsberg D, Hall SM, Munoz RF, Ellman G: Cortisol and response to dexamethasone as predictors of withdrawal distress and abstinence success in smokers. Biol Psychiatry 1998; 43:525–530Crossref, Medline, Google Scholar
33. Elman I, Breiter HC, Gollub RL, Krause S, Kantor HL, Baumgartner WA, Gastfriend DR, Rosen BR: Depressive symptomatology and cocaine-induced pituitary-adrenal axis activation in individuals with cocaine dependence. Drug Alcohol Depend 1999; 56:39–45Crossref, Medline, Google Scholar
34. Contoreggi C, Herning RI, Na P, Gold PW, Chrousos G, Negro PJ, Better W, Cadet JL: Stress hormone responses to corticotropin-releasing hormone in substance abusers without severe comorbid psychiatric disease. Biol Psychiatry 2003; 54:873–878Crossref, Medline, Google Scholar
35. Gerra G, Zaimovic A, Zambelli U, Delsignore R, Baroni MC, Laviola G, Macchia T, Brambilla F: Neuroendocrine correlates of depression in abstinent heroin-dependent subjects. Psychiatry Res 2000; 96:221–234Crossref, Medline, Google Scholar
36. Meier CA, Biller BM: Clinical and biochemical evaluation of Cushing’s syndrome. Endocrinol Metab Clin North Am 1997; 26:741–762Crossref, Medline, Google Scholar
37. Newell-Price J, Trainer P, Besser M, Grossman A: The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev 1998; 19:647–672Medline, Google Scholar
38. O’Brien CP, Childress AR, Ehrman R, Robbins SJ: Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 1998; 12:15–22Crossref, Medline, Google Scholar
39. Drobes DJ, Tiffany ST: Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. J Abnorm Psychol 1997; 106:15–25Crossref, Medline, Google Scholar
40. Sinha R, Catapano D, O’Malley S: Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999; 142:343–351Crossref, Medline, Google Scholar
41. Sinha R, Fuse T, Aubin LR, O’Malley SS: Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000; 152:140–148Crossref, Medline, Google Scholar
42. Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC: Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev 2004; 111:33–51Crossref, Medline, Google Scholar
43. Fowler JS, Volkow ND, Wang G-J, Pappas N, Logan J, Shea C, Alexoff D, MacGregor RR, Schlyer DJ, Zezulkova I, Wolf AP: Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci USA 1996; 93:14065–14069Crossref, Medline, Google Scholar
44. Fowler JS, Volkow ND, Wang G-J, Pappas N, Logan J, MacGregor R, Alexoff D, Shea C, Schyler D, Wolf AP, Warner D, Zezulkova I, Cilento R: Inhibition of monoamine oxidase B in the brains of smokers. Nature 1996; 379:733–736Crossref, Medline, Google Scholar
45. Volkow ND, Fowler JS: Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 2000; 10:318–325Crossref, Medline, Google Scholar
46. Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR: Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry 2002; 51:134–142Crossref, Medline, Google Scholar
47. Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, Drevets WC, Farah MJ, Kagan J, McClelland JL, Nolen-Hoeksema S, Peterson BS: Neural and behavioral substrates of mood and mood regulation. Biol Psychiatry 2002; 52:478–502Crossref, Medline, Google Scholar
48. Cotter D, Mackay D, Landau S, Kerwin R, Everall I: Reduced glial cell density and neuronal size in the anterior cingulated cortex in major depressive disorder. Arch Gen Psychiatry 2001; 58:545–553Crossref, Medline, Google Scholar
49. Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP: Limbic activation during cue-induced cocaine craving. Am J Psychiatry 1999; 156:11–18Link, Google Scholar
50. Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP: Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 2001; 58:334–341Crossref, Medline, Google Scholar
51. Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Kosten TR, Rounsaville BJ, Wexler BE: Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (in press)Google Scholar
52. George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM: Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry 1995; 152:341–351Link, Google Scholar
53. London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W: Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry 2004; 61:73–84Crossref, Medline, Google Scholar
54. Keane T, Kaloupek D: Comorbid psychiatric disorders in PTSD. Ann NY Acad Sci 1998; 24–32Google Scholar
55. Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, Weiss DS: National Vietnam Veterans Readjustment Study (NVVRS): Description, Current Status, and Initial PTSD Prevalence Estimates. Washington, DC, Veterans Administration, 1988Google Scholar
56. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB: Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52:1048–1060Crossref, Medline, Google Scholar
57. Breslau N, Davis GC, Andreski P, Peterson E: Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry 1991; 48:216–222Crossref, Medline, Google Scholar
58. Breslau N, Davis GC, Schultz, LR: Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry 2003; 60:289–294Crossref, Medline, Google Scholar
59. Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G: Brain corticotropin-releasing factor mediates “anxiety-like” behavior induced by cocaine withdrawal in rats. Brain Res 1995; 675:89–97Crossref, Medline, Google Scholar
60. Merlo-Pich EM, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F: Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci 1995; 15:5439–5447Crossref, Medline, Google Scholar
61. Adinoff B, Martin PR, Bone GH, Eckardt MJ, Roehrich L, George DT, Moss HB, Eskay R, Linnoila M, Gold PW: Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Arch Gen Psychiatry 1990; 47:325–330Crossref, Medline, Google Scholar
62. Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS: Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 1997; 154:624–629Link, Google Scholar
63. Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD Jr: Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1999; 156:585–588; correction, 156:986Abstract, Google Scholar
64. Swerdlow NR, Britton KT, Koob GF: Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by alpha-helical CRF (9–41). Neuropsychopharmacology 1989; 2:285–292Crossref, Medline, Google Scholar
65. Southwick SM, Bremner JD, Rasmusson A, Morgan CA III, Arnsten A, Charney DS: Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry 1999; 46:1192–1204Crossref, Medline, Google Scholar
66. Smith AJ, Brent PJ, Henry DA, Foy A: Plasma noradrenaline, platelet alpha 2-adrenoceptors, and functional scores during ethanol withdrawal. Alcohol Clin Exp Res 1990; 14:497–502Crossref, Medline, Google Scholar
67. Hawley RJ, Major LF, Schulman EA, Linnoila M: Cerebrospinal fluid 3-methoxy-4-hydroxyphenylglycol and norepinephrine levels in alcohol withdrawal: correlations with clinical signs. Arch Gen Psychiatry 1985; 42:1056–1062Crossref, Medline, Google Scholar
68. Charney DS, Redmond DE Jr, Galloway MP, Kleber HD, Heninger GR, Murberg M, Roth RH: Naltrexone precipitated opiate withdrawal in methadone addicted human subjects: evidence for noradrenergic hyperactivity. Life Sci 1984; 35:1263–1272Crossref, Medline, Google Scholar
69. Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB: Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci 1986; 6:2908–2914Crossref, Medline, Google Scholar
70. Zhang JJ, Swiergiel AH, Palamarchouk VS, Dunn AJ: Intracerebroventricular infusion of CRF increases extracellular concentrations of norepinephrine in the hippocampus and cortex as determined by in vivo voltammetry. Brain Res Bull 1998; 47:277–284Crossref, Medline, Google Scholar
71. Lavicky J, Dunn AJ: Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis. J Neurochem 1993; 60:602–612Crossref, Medline, Google Scholar
72. Raber J, Koob GF, Bloom FE: Interleukin-2 (IL-2) induces corticotropin-releasing factor (CRF) release from the amygdala and involves a nitric oxide-mediated signaling: comparison with the hypothalamic response. J Pharmacol Exp Ther 1995; 272:815–824Medline, Google Scholar
73. Koob GF: Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry 1999; 46:1167–1180Crossref, Medline, Google Scholar
74. Anand A, Shekhar A: Brain imaging studies in mood and anxiety disorders: special emphasis on the amygdala. Ann NY Acad Sci 2003; 985:370–388Crossref, Medline, Google Scholar
75. Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC: Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry 2001; 158:86–95Link, Google Scholar
76. Biederman J, Munir K, Knee D: Conduct and oppositional disorder in clinically referred children with attention deficit disorder: a controlled family study. J Am Acad Child Adolesc Psychiatry 1987; 26:724–727Crossref, Medline, Google Scholar
77. Simonoff E, Pickles A, Meyer JM, Silberg JL, Maes HH, Loeber R, Rutter M, Hewitt JK, Eaves LJ: The Virginia Twin Study of Adolescent Behavioral Development: influences of age, sex, and impairment on rates of disorder. Arch Gen Psychiatry 1997; 54:801–808Crossref, Medline, Google Scholar
78. Iacono WG, Carlson SR, Malone SM, McGue M: P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry 2002; 59:750–757Crossref, Medline, Google Scholar
79. Crowley TJ, Riggs PD: Adolescent substance use disorder with conduct disorder and comorbid conditions. NIDA Res Monogr 1995; 156:49–111Medline, Google Scholar
80. Thompson LL, Riggs PD, Mikulich SK, Crowley TJ: Contribution of ADHD symptoms to substance problems and delinquency in conduct-disordered adolescents. J Abnorm Child Psychol 1996; 24:325–347Crossref, Medline, Google Scholar
81. Lambert NM, Hartsough CS: Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil 1998; 31:533–544Crossref, Medline, Google Scholar
82. Milberger S, Biederman J, Faraone SV, Chen L, Jones J: ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997; 36:37–44Crossref, Medline, Google Scholar
83. Barkley RA: Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 1997; 121:65–94Crossref, Medline, Google Scholar
84. Burt SA, Krueger RF, McGue M, Iacono W: Parent-child conflict and the comorbidity among childhood externalizing disorders. Arch Gen Psychiatry 2003; 60:505–513Crossref, Medline, Google Scholar
85. Wills TA, McNamara G, Vaccaro D, Hirky AE: Escalated substance use: a longitudinal grouping analysis from early to middle adolescence. J Abnorm Psychol 1996; 105:166–180Crossref, Medline, Google Scholar
86. Arnsten AF, Steere JC, Jentsch DJ, Li BM: Noradrenergic influences on prefrontal cortical cognitive function: opposing actions at postjunctional alpha 1 versus alpha 2-adrenergic receptors. Adv Pharmacol 1998; 42:764–767Crossref, Medline, Google Scholar
87. Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF: A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry 1999; 46:1266–1274Crossref, Medline, Google Scholar
88. Carter CS, Botvinick MM, Cohen JD: The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci 1999; 10:49–57Crossref, Medline, Google Scholar
89. Kerns JG, Cohen JD, MacDonald AW III, Cho RY, Stenger VA, Carter CS: Anterior cingulate conflict monitoring and adjustments in control. Science 2004; 303:1023–1026Crossref, Medline, Google Scholar
90. Paus T: Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2001; 2:417–424Crossref, Medline, Google Scholar
91. Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P: The anterior cingulate cortex: the evolution of an interface between emotion and cognition. Ann NY Acad Sci 2001; 935:107–117Crossref, Medline, Google Scholar
92. Waschbusch DA, Pelham WE Jr, Jennings JR, Greiner AR, Tarter RE, Moss HB: Reactive aggression in boys with disruptive behavior disorders: behavior, physiology, and affect. J Abnorm Child Psychol 2002; 30:641–656Crossref, Medline, Google Scholar
93. Moss HB, Vanyukov M, Yao JK, Kirillova GP: Salivary cortisol responses in prepubertal boys: the effects of parental substance abuse and association with drug use behavior during adolescence. Biol Psychiatry 1999; 45:1293–1299Crossref, Medline, Google Scholar
94. Shoal GD, Giancola PR, Kirillova GP: Salivary cortisol, personality, and aggressive behavior in adolescent boys: a 5-year longitudinal study. J Am Acad Child Adolesc Psychiatry 2003; 42:1101–1107Crossref, Medline, Google Scholar
95. Shidara M, Richmond BJ: Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science 2002; 296:1709–1711Crossref, Medline, Google Scholar
96. Matsumoto K, Suzuki W, Tanaka K: Neuronal correlates of goal-based motor selection in the prefrontal cortex. Science 2003; 301:229–232Crossref, Medline, Google Scholar
97. Capriles N, Rodaros D, Sorge RE, Stewart J: A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003; 168:66–74Crossref, Medline, Google Scholar
98. Kalivas PW, Pierce RC, Cornish J, Sorg BA: A role for sensitization in craving and relapse in cocaine addiction. J Psychopharmacol 1998; 12:49–53Crossref, Medline, Google Scholar
99. Iaboni F, Douglas VI, Ditto B: Psychophysiological response of ADHD children to reward and extinction. Psychophysiology 1997; 34:116–123Crossref, Medline, Google Scholar
100. Cherek DR, Lane SD: Fenfluramine effects on impulsivity in a sample of adults with and without history of conduct disorder. Psychopharmacology (Berl) 2000; 152:149–156Crossref, Medline, Google Scholar
101. Lou HC, Henriksen L, Bruhn P, Borner H, Nielsen JB: Striatal dysfunction in attention deficit and hyperkinetic disorder. Arch Neurol 1989; 46:48–52Crossref, Medline, Google Scholar
102. Zametkin AJ, Liebenauer LL, Fitzgerald GA, King AC, Minkunas DV, Herscovitch P, Yamada EM, Cohen RM: Brain metabolism in teenagers with attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1993; 50:333–340Crossref, Medline, Google Scholar
103. Matochik JA, Liebenauer LL, King AC, Szymanski HV, Cohen RM, Zametkin AJ: Cerebral glucose metabolism in adults with attention deficit hyperactivity disorder after chronic stimulant treatment. Am J Psychiatry 1994; 151:658–664Link, Google Scholar
104. Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, Arnsten AFT, Cohen DJ, Leckman JF: A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry 2001; 158:1067–1074Link, Google Scholar
105. Shaham Y, Highfield D, Delfs J, Leung S, Stewart J: Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci 2000; 12:292–302Crossref, Medline, Google Scholar
106. Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J: Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology 2000; 23:138–150Crossref, Medline, Google Scholar
107. Shaner A, Khalsa ME, Roberts L, Wilkins J, Anglin D, Hsieh SC: Unrecognized cocaine use among schizophrenic patients. Am J Psychiatry 1993; 150:758–762Link, Google Scholar
108. Ziedonis D, Kosten T, Glazer W, Frances R: Nicotine dependence and schizophrenia. Hosp Community Psychiatry 1994; 45:204–206Abstract, Google Scholar
109. Brady K, Anton R, Ballenger JC, Lydiard RB, Adinoff B, Selander J: Cocaine abuse among schizophrenic patients. Am J Psychiatry 1990; 147:1164–1167Link, Google Scholar
110. Dixon L, Haas G, Weiden P, Sweeney J, Frances A: Acute effects of drug abuse in schizophrenic patients: clinical observations and patients’ self-reports. Schizophr Bull 1990; 16:69–79Crossref, Medline, Google Scholar
111. Chambers RA, Krystal JH, Self DW: A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry 2001; 50:71–83Crossref, Medline, Google Scholar
112. Mathalon DH, Pfefferbaum A, Lim KO, Rosenbloom MJ, Sullivan EV: Compounded brain volume deficits in schizophrenia-alcoholism comorbidity. Arch Gen Psychiatry 2003; 60:245–252Crossref, Medline, Google Scholar
113. Ziedonis DM, George TP: Schizophrenia and nicotine use: report of a pilot smoking cessation program and review of neurobiological and clinical issues. Schizophr Bull 1997; 23:247–254Crossref, Medline, Google Scholar
114. Adler LE, Hoffer LD, Wiser A, Freedman R: Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 1993; 150:1856–1861Link, Google Scholar
115. Olincy A, Ross RG, Young DA, Roath M, Freedman R: Improvement in smooth pursuit eye movements after cigarette smoking in schizophrenic patients. Neuropsychopharmacology 1998; 18:175–185Crossref, Medline, Google Scholar
116. George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, Kosten TR, Wexler BE: Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology 2002; 26:75–85Crossref, Medline, Google Scholar
117. Weiser M, Reichenberg A, Grotto I, Yasvitzky R, Rabinowitz J, Lubin G, Nahon D, Knobler HY, Davidson M: Higher rates of cigarette smoking in male adolescents before the onset of schizophrenia: a historical-prospective cohort study. Am J Psychiatry 2004; 161:1219–1223Link, Google Scholar
118. Noordsy DL, Green AI: Pharmacotherapy for schizophrenia and co-occurring substance use disorders. Curr Psychiatry Rep 2003; 5:340–346Crossref, Medline, Google Scholar
119. Kosten TA, Nestler EJ: Clozapine attenuates cocaine conditioned place preference. Life Sci 1994; 55:PL9-PL14Google Scholar



