Depressive Symptoms and 24-Hour Urinary Norepinephrine Excretion Levels in Patients With Coronary Disease: Findings From the Heart and Soul Study
Abstract
OBJECTIVE: Depressive symptoms are associated with an increased risk of cardiac events in patients with heart disease. Elevated catecholamine levels may contribute to this association, but whether depressive symptoms are associated with catecholamine levels in patients with heart disease is unknown. METHOD: The authors examined the association between depressive symptoms (defined by a Patient Health Questionnaire score ≥10) and 24-hour urinary norepinephrine, epinephrine, and dopamine excretion levels in 598 subjects with coronary disease. RESULTS: A total of 106 participants (18%) had depressive symptoms. Participants with depressive symptoms had greater mean norepinephrine excretion levels than those without depressive symptoms (65 μg/day versus 59 μg/day, with adjustment for age, sex, body mass index, smoking, urinary creatinine levels, comorbid illnesses, medication use, and cardiac function). In logistic regression analyses, participants with depressive symptoms were more likely than those without depressive symptoms to have norepinephrine excretion levels in the highest quartile and above the normal range. Depressive symptoms were not associated with dopamine or epinephrine excretion levels. CONCLUSIONS: In patients with coronary disease, depressive symptoms are associated with elevated norepinephrine excretion levels. Future longitudinal studies are needed to determine whether elevations in norepinephrine contribute to adverse cardiac outcomes in patients with depressive symptoms.
Coronary disease and major depression are the two leading causes of disability worldwide (1). Depressive symptoms occur in about 20% of patients with coronary disease (2, 3) and are associated with an increased risk of future cardiac events and mortality (4–7). However, the mechanisms linking depressive symptoms with subsequent cardiac events are unknown (8).
Enhanced activity of the sympathetic nervous system with increased concentrations of catecholamines has been proposed as one possible mechanism by which depressive symptoms may increase morbidity and mortality (7–9). This hypothesis is based on evidence suggesting that depressed patients without heart disease have elevated catecholamine levels (10–12). Previous studies have also found alterations of the sympathetic nervous system in depressed patients with coronary heart disease, including increased heart rate (13) and decreased heart rate variability (14). High catecholamine levels can damage cardiac myocytes (15, 16) and have been associated with cardiac events and mortality in a variety of clinical and population-based samples (17–22). Thus, altered autonomic tone may contribute to adverse cardiac outcomes in patients with depression.
The Heart and Soul Study is an ongoing prospective cohort study of psychosocial factors and health outcomes in patients with coronary disease (3). We examined the association of depressive symptoms with 24-hour levels of urinary norepinephrine, epinephrine, and dopamine excretion at baseline. We hypothesized that depressive symptoms would be associated with increased levels of excretion of urinary catecholamines.
Method
Participants
Details regarding our recruitment procedures have been published previously (3). In brief, we used administrative databases to identify outpatients with documented coronary disease at two Veterans Affairs (VA) Medical Centers (the San Francisco VA Medical Center and the VA Palo Alto Health Care System, California), one university medical center (the University of California, San Francisco), and nine public health clinics in the Community Health Network of San Francisco. The patients were eligible to participate if they had at least one of the following: a history of myocardial infarction, angiographic evidence of ≥50% stenosis in one or more coronary vessels, prior evidence of exercise-induced ischemia by treadmill or nuclear testing, a history of coronary revascularization, or a diagnosis of coronary disease by an internist or cardiologist (based on a positive angiogram or an exercise treadmill test in >98% of the cases). The subjects received a nominal reimbursement for their participation.
Between September 2000 and December 2002, a total of 1,024 participants enrolled and completed a day-long study appointment at the San Francisco VA Medical Center. Of these, we evaluated the association between depressive symptoms and 24-hour urinary catecholamine excretion levels in the participants whose urine collection we verified as having been refrigerated during the 24-hour collection. Refrigeration is important for the preservation of catecholamines. Although all participants were instructed to refrigerate their urine, we verified urine refrigeration for only the last 630 study participants. Of these 630 subjects, we further excluded 24 participants whose 24-hour urine collections were deemed inadequate (because of incomplete collection), seven participants with low urine volumes (<500 ml), and one participant with pheochromocytoma, leaving 598 participants for the analysis. Our protocol was approved by the appropriate institutional review boards, and all participants provided written informed consent.
Depressive Symptoms
We measured depressive symptoms by using the 9-item Patient Health Questionnaire (20), a self-report checklist of depressive symptoms derived from the well-validated Primary Care Evaluation of Mental Disorders interview (23). When compared with a structured psychiatric interview by mental health professionals as a criterion standard, a score on the Patient Health Questionnaire ≥10 has been reported to be 88% sensitive and 88% specific for major depression (24). We used this standard cutoff point of ≥10 to define depressive symptoms. We also categorized scores on this scale as representing none to minimal depressive symptoms (Patient Health Questionnaire score=0–3), mild to moderate depressive symptoms (score=4–9), and symptoms consistent with major depression (score ≥10). The participants found to have high levels of depressive symptoms were informed that they may be suffering from depression, were instructed to discuss these symptoms with their primary care provider, and were provided a list of local resources available for further evaluation and treatment.
24-Hour Urinary Catecholamine Measures
To ensure urine collection during a typical day under typical circumstances, the participants were asked to collect urine in their home environments. They were instructed to collect all urine for 24 hours between the end of their study appointment and the time when a researcher visited their house the next day and to keep the urine collection jugs refrigerated at all times. In our pilot testing, we found that this procedure was more likely to yield complete 24-hour collections than asking participants to start their 24-hour collection at 8 a.m. the next day. No preservatives were added to the urine jugs.
The subjects were instructed to void immediately before leaving the study appointment and beginning the urine collection. Research personnel arrived at the patients’ homes exactly 24 hours after their study appointments ended to ensure accurately timed specimens and to enhance compliance with the protocol, including verifying that the urine jugs were stored in the refrigerator. If more than 1 hour had passed since the participants’ last void, the subjects were asked to void again to complete the collection.
All patients were asked whether they were able to collect all urine or if some fraction had been inadvertently discarded. If the sample was reported to be incomplete, if it was not refrigerated throughout the procedure, or if the volume was less than 1 liter, the subjects were asked to repeat the collection, and research personnel returned 24 hours later to recollect the urine. Similarly, if the 3-liter collection jugs were completely full, the subjects were given two new jugs and asked to repeat the collection to ensure that no urine had been inadvertently discarded. If the subjects were unable to collect all urine for any reason or had urinary incontinence, their samples were deemed inadequate, and no urinary catecholamine data were recorded for these subjects.
Urinary catecholamine excretion levels (norepinephrine, epinephrine, and dopamine) were measured with gas chromatography-mass spectrometry at the Associated Regional and University Pathologists laboratories, with headquarters in Salt Lake City (25). The normal reference range for these assays is 60–440 μg/day for dopamine, 0–25 μg/day for epinephrine, and 0–100 μg/day for norepinephrine. Because the detection limit was 1.0 μg/dl, catecholamine levels for the participants whose excretion level was below this detection limit were coded as 1.0 μg/dl. To ensure adequate sampling, we measured urinary creatinine levels in parallel and included only the participants who had a urinary creatinine value within the normal range (0.8–2.1 g/day).
Potential Confounding Variables
Self-reported age, gender, medical history (with a checklist of 30 common medical disorders), smoking, and alcohol use were determined by questionnaire. Body mass index was calculated as weight in kilograms divided by the square of height in meters, and obesity was defined as a body mass index ≥30 kg/m2(26). To assess physical activity, we asked the participants, “Which of the following statements best describes how physically active you have been during the last month?” Those who answered fairly, quite, very, or extremely active (versus not at all or a little active) were considered physically active. The participants received written instructions and were reminded by telephone the night before their appointments to bring their medication bottles to the study appointments, and study personnel carefully recorded all current medications.
We assessed cardiac function by using a resting ECG for the measurement of left ventricular ejection fraction, an exercise treadmill test for the measurement of exercise capacity, and a stress ECG for the assessment of ischemia. We measured resting blood pressure with a standard sphygmomanometer and performed a symptom-limited, graded exercise treadmill test according to standard Bruce protocol. We defined exercise capacity as the total number of metabolic equivalents and calculated the wall motion score index at the peak of exercise as our measure of ischemia (27).
Statistical Analysis
The goal of this study was to examine the association between depressive symptoms and 24-hour urinary catecholamine excretion levels in patients with coronary heart disease. Differences in characteristics between participants with and without depressive symptoms were compared by using t tests (or a nonparametric equivalent) for continuous variables and chi-square tests for dichotomous variables. Analysis of covariance was used to compare mean levels of catecholamine excretion in participants with and without depressive symptoms.
To determine the unadjusted and adjusted associations between depressive symptoms and catecholamine levels, we used logistic regression analyses with depressive symptoms (Patient Health Questionnaire score ≥10) as the predictor variable. Our dichotomous outcome variables were 1) highest quartile of catecholamine levels and 2) catecholamine value above the normal range. To obtain adjusted risk estimates, we entered all variables from Table 1 into a backward-elimination logistic regression model, with depressive symptoms forced into the model. The variables that were associated with elevated levels of catecholamine excretion at p<0.15 were retained in the models so as not to miss any associations between potentially confounding variables and the variables of interest. The likelihood ratio test was used to assess the fit of the model and to determine if additional variables should be dropped from the models. We also tested for interactions between depressive symptoms and all other variables that were associated with elevated levels of catecholamine excretion. The analyses were performed with Statistical Analysis Software, version 8 (SAS Institute, Cary, N.C.).
Results
Of the 598 participants, 106 (18%) had depressive symptoms (Patient Health Questionnaire score ≥10). Compared with the participants who did not have depressive symptoms, those with depressive symptoms were younger, more likely to be female, and more likely to have hypertension, diabetes, or congestive heart failure (Table 1). The participants with depressive symptoms were more likely to be using antidepressant medications, to have a lower exercise capacity, and to smoke. There was no difference in 24-hour levels of urine creatinine excretion between the participants with and without depressive symptoms. The proportion with depressive symptoms was similar to the Heart and Soul Study participants who were included or excluded from the analysis (18% versus 19%) (χ2=0.28, df=1, p=0.74).
Mean Levels of Catecholamine Excretion
The participants with depressive symptoms had greater 24-hour levels of urinary free norepinephrine excretion than the patients without depressive symptoms (Table 2). This difference in levels of norepinephrine excretion persisted after we adjusted for age, sex, body mass index, smoking, urinary creatinine level, comorbid illnesses, medication use, physical activity, and cardiac function (Table 2). We observed no interactions between depressive symptoms and the other variables that entered the model (all p values for interaction >0.05). The participants with depressive symptoms also had greater levels of dopamine excretion than those who did not have depressive symptoms, but this difference did not persist after adjustment for age, sex, body mass index, smoking, urinary creatinine level, comorbid illnesses, medication use, physical activity, and cardiac function. We did not observe an association between depressive symptoms and epinephrine levels (Table 2).
Levels in Highest Quartile of Catecholamine Excretion
Depressive symptoms were not associated with elevated levels of epinephrine or dopamine excretion. However, depressive symptoms were strongly associated with elevated levels of norepinephrine excretion. Among the participants with depressive symptoms, 32% (34 of 106) had a norepinephrine excretion level in the highest quartile (>65 μg/day), compared with 23% (112 of 492) of the participants who did not have depressive symptoms (odds ratio=1.6, 95% confidence interval [CI]=1.0–2.5, p=0.04). This association remained after we controlled for comorbid illnesses, medication use, cardiac function, physical activity, and urinary creatinine level (adjusted odds ratio=1.6, 95% CI=1.0–2.7, p=0.06), although the confidence interval overlapped one. There were no interactions between depressive symptoms and other predictors of elevated levels of norepinephrine excretion (all p values for interaction >0.05).
Abnormal Levels of Catecholamine Excretion
Depressive symptoms were not associated with abnormal levels of epinephrine or dopamine excretion. However, 9.4% (10 of 106) of the participants with depressive symptoms had a norepinephrine value above the normal range (>100 μg/day), compared with 3.3% (16 of 492) of the participants who did not have depressive symptoms (odds ratio=3.1, 95% CI=1.4–7.0, p=0.007) (Figure 1). Again, this association persisted after adjustment for comorbid illness, medication use, physical activity, and cardiac function (adjusted odds ratio=2.9, 95% CI=1.2–6.9, p=0.02).
Norepinephrine and Cardiac Function
Norepinephrine excretion level (entered either as the highest quartile or as a continuous variable) was not associated with systolic blood pressure, diastolic blood pressure, diabetes, congestive heart failure, exercise capacity, or ischemia. Likewise, the highest quartile level of norepinephrine was not associated with left ventricular ejection fraction. However, when entered as a continuous variable, each standard deviation increase in norepinephrine level (25 μg/day) was associated with a 1% decrease in left ventricular ejection fraction (parameter estimate=–0.04, SE=0.02, t=–2.37, p=0.02). This association remained significant after adjustment for male sex, history of myocardial infarction, history of congestive heart failure, use of antiarrhythmics, and urinary creatinine level.
Discussion
We found that depressive symptoms were associated with elevated levels of norepinephrine excretion in 598 subjects with coronary heart disease. The participants with depressive symptoms were more likely than those without to have increased levels of 24-hour mean norepinephrine excretion, to have norepinephrine excretion levels in the highest quartile, and to have norepinephrine excretion levels above the normal range. The presence of depressive symptoms was not associated with 24-hour urinary epinephrine or dopamine levels.
Because a high norepinephrine level is an established risk factor for cardiac events and mortality (17–22), our findings suggest that elevated norepinephrine may contribute to the increased risk of cardiac events associated with depressive symptoms (7). It is well known that norepinephrine is increased in patients with heart failure, correlates with the severity of heart failure, and is associated with increased mortality in cardiac patients (28). In our sample, a high level of norepinephrine excretion was associated with the lower left ventricular ejection fraction. These adverse effects of norepinephrine levels have been shown not only in patients with heart failure (21, 28, 29) but also in population-based samples of apparently healthy elderly subjects (20, 22), in asymptomatic patients without clinically relevant heart failure (18), in patients with end-stage renal disease without heart failure (19), and in patients with tachyarrhythmias without heart failure (17, 30). These findings argue for a more causative role of norepinephrine in increasing the risk for cardiac events and mortality. Possible mechanisms include direct toxic effects of norepinephrine on cardiocytes (16, 31), its ability to trigger tachyarrhythmia (17, 30), and its role in the promotion of platelet aggregation and platelet thrombi formation (18).
To our knowledge, only one previous study has examined the association between depressive symptoms and norepinephrine levels in patients with coronary heart disease (13). Carney et al. (13) measured plasma norepinephrine levels in 50 depressed and 39 nondepressed subjects with coronary heart disease but did not find an association between depressive symptoms and plasma norepinephrine levels. Unlike our study, their measurement of norepinephrine was based on a series of plasma norepinephrine levels at baseline and at three time points within 10 minutes after orthostatic challenge. It is possible that integrated 24-hour urinary measurement of norepinephrine is a more sensitive measure for the detection of differences in norepinephrine levels.
The causal direction between depressive symptoms and norepinephrine levels cannot be determined by our cross-sectional study. However, a plausible mechanism linking depressive symptoms with increased sympathetic activity is the enhanced activity of hypothalamic and extrahypothalamic corticotropin-releasing factor (CRF) in depressed patients. It is well established that CRF is increased in medically healthy patients with depressive symptoms (10, 32), leading to increased cortisol levels (33–35). Moreover, a large body of evidence suggests that both hypothalamic and extrahypothalamic CRF activate the locus ceruleus in the brain, leading to an increase in norepinephrine (36–39).
Accordingly, earlier studies have found that depressed patients who were cortisol nonsuppressors after receiving dexamethasone had higher norepinephrine levels. Moreover, administration of a CRF antagonist led to a diminished norepinephrine response to stress in primates (40). Thus, high CRF activity may simultaneously lead to elevated cortisol and norepinephrine levels (41). Moreover, levels of CSF norepinephrine and plasma cortisol were increased and highly correlated in depressed patients, which is also consistent with the increased CRF activity causing these alterations (11). In turn, norepinephrine enhances forebrain CRF activity, possibly closing a feed-forward loop leading to higher activity of both norepinephrine and CRF in depressed patients (37). Indeed, in this study group, we also found increased cortisol levels in depressed patients (42), consistent with this model of norepinephrine-CRF interaction (10).
We did not find an association between depressive symptoms and epinephrine or dopamine levels in this group, which is in accordance with an earlier study that also found increased norepinephrine but similar epinephrine levels in depressed patients (12). This might suggest that depressive symptoms are more closely related to increased sympathetic nervous system activity, as reflected in increased norepinephrine levels compared with the adrenomedullary system and reflected levels of epinephrine excretion. Indeed, it has been shown that there is a differential response of the sympathetic nervous and adrenomedullary hormonal systems, depending on the type and severity of a stressor (43).
Several limitations should be considered in interpreting the results of our study. Only 58% (598 of 1,024) of the Heart and Soul Study participants were included in this analysis. However, the prevalence of depressive symptoms was similar in the participants who were included or excluded from the analysis. Although we made every effort to systematically assess medication use and to ensure complete urine collection, our results were limited by self-reported medication use and compliance with the urine collection procedure. Only 17% of our participants were women, thereby reducing our power to detect interactions by gender and limiting the generalizability of our results. Finally, most of the catecholamine values in our group were within the normal range, and their clinical significance is unclear. However, the Heart and Soul Study is an ongoing prospective study that will follow patients to determine whether greater norepinephrine levels at baseline contribute to the association of depressive symptoms with worse outcomes in patients with coronary heart disease.
In summary, we found that depressive symptoms are associated with elevated levels of norepinephrine excretion but not with epinephrine or dopamine excretion levels in medical outpatients with heart disease. Increased levels of norepinephrine excretion may contribute to an increased risk of future cardiac events in patients with depressive symptoms.
 |
 |
Received June 10, 2004; revision received Sept. 10, 2004; accepted Oct. 26, 2004. From the Department of Psychiatry, the Department of Medicine, and the Department of Epidemiology and Biostatistics, University of California, San Francisco; the Veterans Affairs Medical Center, San Francisco; the Department of Psychiatry, University Hospital Hamburg-Eppendorf, Germany; and the Research Institute, California Pacific Medical Center, San Francisco. Address correspondence and reprint requests to Dr. Whooley, Veterans Affairs Medical Center, 4150 Clement St. (111A1), San Francisco, CA 94121; [email protected] (e-mail). Funded by grants from the Department of Veterans Affairs (Epidemiology Merit Review Program), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), and the Ischemia Research and Education Foundation. Dr. Otte was supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft (grant Ot 209/2–1). Dr. Whooley was supported by a Research Career Development Award from the Department of Veterans Affairs Health Services Research and Development Service. None of the funding sources had any role in the collection of data, the interpretation of results, or the preparation of this manuscript.
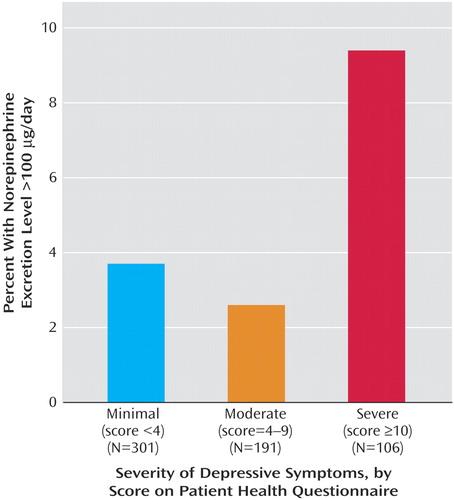
Figure 1. Proportion of Patients With Coronary Heart Disease With a 24-Hour Norepinephrine Excretion Level Above the Normal Range, According to the Severity of Depressive Symptomsa
aTest for trend (p=0.05).
1. Murray CJ, Lopez AD: Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997; 349:1498–1504Crossref, Medline, Google Scholar
2. Rudisch B, Nemeroff CB: Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry 2003; 54:227–240Crossref, Medline, Google Scholar
3. Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA: Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA 2003; 290:215–221Crossref, Medline, Google Scholar
4. Blumenthal JA, Lett HS, Babyak MA, White W, Smith PK, Mark DB, Jones R, Mathew JP, Newman MF: Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet 2003; 362:604–609Crossref, Medline, Google Scholar
5. Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG: Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation 2002; 105:1049–1053Crossref, Medline, Google Scholar
6. Frasure-Smith N, Lesperance F, Talajic M: Depression following myocardial infarction: impact on 6-month survival. JAMA 1993; 270:1819–1825Crossref, Medline, Google Scholar
7. Carney RM, Freedland KE: Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry 2003; 54:241–247Crossref, Medline, Google Scholar
8. Musselman DL, Evans DL, Nemeroff CB: The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry 1998; 55:580–592Crossref, Medline, Google Scholar
9. Chrousos GP, Gold PW: A healthy body in a healthy mind—and vice versa—the damaging power of “uncontrollable” stress. J Clin Endocrinol Metab 1998; 83:1842–1845Medline, Google Scholar
10. Gold PW, Chrousos GP: Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry 2002; 7:254–275Crossref, Medline, Google Scholar
11. Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD Jr, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW: Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci USA 2000; 97:325–330Crossref, Medline, Google Scholar
12. Veith RC, Lewis N, Linares OA, Barnes RF, Raskind MA, Villacres EC, Murburg MM, Ashleigh EA, Castillo S, Peskind ER, et al: Sympathetic nervous system activity in major depression: basal and desipramine-induced alterations in plasma norepinephrine kinetics. Arch Gen Psychiatry 1994; 51:411–422Crossref, Medline, Google Scholar
13. Carney RM, Freedland KE, Veith RC, Cryer PE, Skala JA, Lynch T, Jaffe AS: Major depression, heart rate, and plasma norepinephrine in patients with coronary heart disease. Biol Psychiatry 1999; 45:458–463Crossref, Medline, Google Scholar
14. Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O’Connor C, Stone PH, Freedland KE: Depression, heart rate variability, and acute myocardial infarction. Circulation 2001; 104:2024–2028Crossref, Medline, Google Scholar
15. Mann DL, Kent RL, Parsons B, Cooper GT: Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation 1992; 85:790–804Crossref, Medline, Google Scholar
16. Communal C, Singh K, Pimentel DR, Colucci WS: Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the β-adrenergic pathway. Circulation 1998; 98:1329–1334Crossref, Medline, Google Scholar
17. Meredith IT, Broughton A, Jennings GL, Esler MD: Evidence of a selective increase in cardiac sympathetic activity in patients with sustained ventricular arrhythmias. N Engl J Med 1991; 325:618–624Crossref, Medline, Google Scholar
18. Benedict CR, Shelton B, Johnstone DE, Francis G, Greenberg B, Konstam M, Probstfield JL, Yusuf S: Prognostic significance of plasma norepinephrine in patients with asymptomatic left ventricular dysfunction. Circulation 1996; 94:690–697Crossref, Medline, Google Scholar
19. Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cataliotti A, Stancanelli B, Malatino LS: Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation 2002; 105:1354–1359Crossref, Medline, Google Scholar
20. Christensen NJ, Schultz-Larsen K: Resting venous plasma adrenalin in 70-year-old men correlated positively to survival in a population study: the significance of the physical working capacity. J Intern Med 1994; 235:229–232Crossref, Medline, Google Scholar
21. Anand IS, Fisher LD, Chiang Y-T, Latini R, Masson S, Maggioni AP, Glazer RD, Tognoni G, Cohn JN (Val-HeFT Investigators): Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT). Circulation 2003; 107:1278–1283Crossref, Medline, Google Scholar
22. Reuben DB, Talvi SL, Rowe JW, Seeman TE: High urinary catecholamine excretion predicts mortality and functional decline in high-functioning, community-dwelling older persons: MacArthur Studies of Successful Aging. J Gerontol A Biol Sci Med Sci 2000; 55:M618-M624Google Scholar
23. Spitzer RL, Kroenke K, Williams JBW: Validation and utility of a self-report version of PRIME-MD: the PHQ Primary Care Study. JAMA 1999; 282:1737–1744Crossref, Medline, Google Scholar
24. Kroenke K, Spitzer RL, Williams JB: The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613Crossref, Medline, Google Scholar
25. ARUP Laboratories: Catecholamines: fractionated, plasma and urine free. http://www.aruplab.com/guides/clt/tests/clt_a151.jsp#1059229Google Scholar
26. Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB: Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 2002; 162:1867–1872Crossref, Medline, Google Scholar
27. Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al (American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms): Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr 1989; 2:358–367Crossref, Medline, Google Scholar
28. Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T, Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L: Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984; 311:819–823Crossref, Medline, Google Scholar
29. Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L (CONSENSUS Trial Study Group): Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. Circulation 1990; 82:1730–1736Crossref, Medline, Google Scholar
30. Podrid PJ, Fuchs T, Candinas R: Role of the sympathetic nervous system in the genesis of ventricular arrhythmia. Circulation 1990; 82(2 suppl):I103-I113Google Scholar
31. Mann DL, Kent RL, Parsons B, Cooper G IV: Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation 1992; 85:790–804Crossref, Medline, Google Scholar
32. Holsboer F: The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res 1999; 33:181–214Crossref, Medline, Google Scholar
33. Deuschle M, Schweiger U, Weber B, Gotthardt U, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I: Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab 1997; 82:234–238Crossref, Medline, Google Scholar
34. Rothschild A: The hypothalamic-pituitary-adrenal axis and psychiatric illness, in Psychoneuroendocrinology. Edited by Wolkowitz O, Rothschild A. Washington, DC, American Psychiatric Publishing, 2003, pp 139–163Google Scholar
35. Wolkowitz OM, Epel ES, Reus VI: Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry 2001; 2:115–143Crossref, Medline, Google Scholar
36. Arlt J, Jahn H, Kellner M, Strohle A, Yassouridis A, Wiedemann K: Modulation of sympathetic activity by corticotropin-releasing hormone and atrial natriuretic peptide. Neuropeptides 2003; 37:362–368Crossref, Medline, Google Scholar
37. Koob GF: Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry 1999; 46:1167–1180Crossref, Medline, Google Scholar
38. Valentino RJ, Page M, Van Bockstaele E, Aston-Jones G: Corticotropin-releasing factor innervation of the locus coeruleus region: distribution of fibers and sources of input. Neuroscience 1992; 48:689–705Crossref, Medline, Google Scholar
39. Melia KR, Duman RS: Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc Natl Acad Sci USA 1991; 88:8382–8386Crossref, Medline, Google Scholar
40. Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, Chrousos GP, McCann SM, Suomi SJ, Higley JD, Gold PW: Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci USA 2000; 97:6079–6084Crossref, Medline, Google Scholar
41. Roy A, Pickar D, De Jong J, Karoum F, Linnoila M: Norepinephrine and its metabolites in cerebrospinal fluid, plasma, and urine: relationship to hypothalamic-pituitary-adrenal axis function in depression. Arch Gen Psychiatry 1988; 45:849–857Crossref, Medline, Google Scholar
42. Otte C, Marmar CR, Pipkin SS, Moos R, Browner WS, Whooley MA: Depression and 24-hour urinary cortisol in medical outpatients with coronary heart disease: the Heart and Soul Study. Biol Psychiatry 2004; 56:241–247Crossref, Medline, Google Scholar
43. Goldstein DS: Catecholamines and stress. Endocr Regul 2003; 37:69–80Medline, Google Scholar



