Randomized, Placebo-Controlled Trial of Exposure and Ritual Prevention, Clomipramine, and Their Combination in the Treatment of Obsessive-Compulsive Disorder
Abstract
OBJECTIVE: The purpose of the study was to test the relative and combined efficacy of clomipramine and exposure and ritual prevention in the treatment of obsessive-compulsive disorder (OCD) in adults. Serotonin reuptake inhibitors (SRIs) and cognitive behavior therapy by exposure and ritual prevention are both established treatments for OCD, yet their relative and combined efficacy have not been demonstrated conclusively. METHOD: A double-blind, randomized, placebo-controlled trial comparing exposure and ritual prevention, clomipramine, their combination (exposure and ritual prevention plus clomipramine), and pill placebo was conducted at one center expert in pharmacotherapy, another with expertise in exposure and ritual prevention, and a third with expertise in both modalities. Participants were adult outpatients (N=122 entrants) with OCD. Interventions included intensive exposure and ritual prevention for 4 weeks, followed by eight weekly maintenance sessions, and/or clomipramine administered for 12 weeks, with a maximum dose of 250 mg/day. The main outcome measures were the Yale-Brown Obsessive Compulsive Scale total score and response rates determined by the Clinical Global Impression improvement scale. RESULTS: At week 12, the effects of all active treatments were superior to placebo. The effect of exposure and ritual prevention did not differ from that of exposure and ritual prevention plus clomipramine, and both were superior to clomipramine only. Treated and completer response rates were, respectively, 62% and 86% for exposure and ritual prevention, 42% and 48% for clomipramine, 70% and 79% for exposure and ritual prevention plus clomipramine, and 8% and 10% for placebo. CONCLUSIONS: Clomipramine, exposure and ritual prevention, and their combination are all efficacious treatments for OCD. Intensive exposure and ritual prevention may be superior to clomipramine and, by implication, to monotherapy with the other SRIs.
Obsessive-compulsive disorder (OCD) is characterized by recurrent obsessive thoughts, images, or impulses that evoke anxiety and by compulsive behaviors (e.g., handwashing) or mental acts (e.g., ritualistic praying) aimed at decreasing discomfort. Six-month prevalence is estimated at 1%–2% (1) and lifetime prevalence at 2%–3% (2, 3). OCD’s relatively high prevalence, the typically long gap between onset and treatment, and pervasive associated dysfunction (4–7) highlight the importance of developing and disseminating effective treatments.
Cognitive behavior therapy by exposure and response (ritual) prevention (8) is now considered the best available psychotherapy for OCD (9). Pharmacotherapy with the serotonin reuptake inhibitor (SRI) clomipramine (10) and the selective serotonin reuptake inhibitors (SSRIs) sertraline (11), fluvoxamine (12), paroxetine (13), fluoxetine (14), and citalopram (15) has also proven efficacious. Although side effects limit its use as a first-line treatment, clomipramine remains both the best studied and possibly the most efficacious medication for OCD (16, 17). Although exposure and ritual prevention and SRIs are each efficacious treatments, some patients do not benefit from these interventions and most remain at least somewhat symptomatic.
An important question concerns the relative and combined efficacy of these treatments. To our knowledge, five previous studies with adults have included medication and exposure and ritual prevention, but none have directly compared exposure and ritual prevention, medication, and their combination relative to placebo (18–22). Marks et al. (18) compared the effects of clomipramine and placebo over 4 weeks, followed by an additional 3 weeks of exposure and ritual prevention or relaxation. In this study, as well as a subsequent study (19), the combination of clomipramine with exposure and ritual prevention had a small transitory additive effect, compared to the combination of placebo with exposure and ritual prevention. However, the designs of both studies did not allow for a direct comparison of exposure and ritual prevention and clomipramine alone. Cottraux et al. (20) found that the combination of exposure and ritual prevention with fluvoxamine produced similar acute and 6-month reductions in OCD symptoms; exposure and ritual prevention plus fluvoxamine led to slightly greater short-term, but not long-term, improvement in depression than did exposure and ritual prevention alone. The use of antiexposure instructions in the fluvoxamine alone condition limits the conclusions that can be drawn from this study. More recently, Hohagen et al. (21) found that exposure and ritual prevention plus fluvoxamine was superior to exposure and ritual prevention plus placebo, but van Balkom et al. (22) failed to detect any additive effect for fluvoxamine over exposure and ritual prevention alone. Differences in sampling, experimental design, and exposure and ritual prevention procedures (e.g., number and spacing of sessions) compromise direct comparison of these results. Generally speaking, design and procedural issues in existing studies do not permit strong conclusions regarding the relative efficacy of exposure and ritual prevention, SRIs, and their combination, which are vital for guiding the clinical practice of OCD treatment.
To avoid previous pitfalls, in the present study we used a manual-based, empirically validated version of exposure and ritual prevention; an adequate dose of clomipramine and an adequate duration of clomipramine treatment; and a straightforward one-by-four study design. The study was conducted at three centers: one known for its expertise in exposure and ritual prevention (Center for the Treatment and Study of Anxiety, University of Pennsylvania, Philadelphia), one for its expertise in psychopharmacological treatment (Anxiety Disorders Clinic, New York State Psychiatric Institute, New York), and one experienced with both modalities (St. Boniface General Hospital, Winnipeg, Manitoba, Canada). By having each site conduct all treatments after extensive training and with ongoing supervision of research staff, we sought to ensure that treatments were consistently administered in an expert fashion across sites. We hypothesized that 1) exposure and ritual prevention, clomipramine, and exposure and ritual prevention plus clomipramine would each be superior to placebo; 2) exposure and ritual prevention plus clomipramine would be superior to exposure and ritual prevention alone or clomipramine alone; and 3) exposure and ritual prevention would be superior to clomipramine.
Method
Study Participants
Inclusion and exclusion criteria are presented in Table 1. Participants were recruited through self-referrals, professional referrals, and media advertisements and needed to live within a commutable distance from their study site. Active recruitment into the trial occurred in 1990–2000.
Randomization and Blinding
Treatment assignment was done randomly within blocks of four. If a patient withdrew from the trial after learning his/her assignment but before baseline assessment or treatment, another patient was assigned to that condition within the block of four. In mid-trial, preliminary analyses revealed that a smaller placebo group would afford sufficient power. Thereafter, patients were randomly assigned in blocks of seven with one placebo slot. Independent evaluators, who remained blind to treatment assignment, conducted the assessments. Psychiatrists were blind to patients’ medication assignment and therapy status. The therapists who provided exposure and ritual prevention were blind to patients’ medication status.
Study Design
Patients were recruited in Philadelphia, New York, and at the satellite site in Winnipeg. The institutional review boards at each site approved the study, and written informed consent was obtained from study participants after a full explanation of procedures.
Procedures
Initial screening
Potential patients underwent a psychiatric evaluation by senior clinicians, were assessed with the Structured Clinical Interview for DSM-IV (23) to confirm psychiatric diagnosis, and received a comprehensive medical evaluation. Patients who were taking psychoactive medication at intake underwent a drug-free period before the pretreatment assessment (6 weeks for fluoxetine, 4 weeks for other SSRIs, and 2 weeks for other psychotropic medications). Eligible participants were randomly assigned to receive 1) exposure and ritual prevention, 2) clomipramine, 3) exposure and ritual prevention plus clomipramine, or 4) placebo. After random assignment to treatment conditions, patients were scheduled for the pretreatment assessment.
Measures
Independent evaluators rated OCD symptom severity at weeks 0, 4, 8, and 12. The primary outcome measure was the mean total score on the Yale-Brown Obsessive Compulsive Scale (24–26), a 10-item interviewer measure of symptom severity with a range from 0 to 40. A score of 16 or greater indicates clinically relevant OCD; a score below 11 indicates mild symptoms. Other OCD measures included the Clinical Global Impression (CGI) scales for severity (1=no symptoms to 7=very severe symptoms) and improvement (1=very much improved to 7=very much worse) (27), and the NIMH Global Obsessive-Compulsive Scale (28–32), a single-item clinician rated index of OCD illness severity ranging from 1 (normal) to 15 (very severe). Response status was defined by a score of 1 (very much improved) or 2 (much improved) on the CGI improvement scale. Depression was measured with the 17-item version of the Hamilton Depression Rating Scale (33, 34).
Treatments
The manual-based treatment procedures are summarized in the following sections.
Exposure and ritual prevention
After two information-gathering sessions, exposure sessions, each lasting 2 hours, were conducted each weekday over a 3-week period (15 sessions), and daily exposure and ritual prevention homework (up to 2 hours a day) were assigned. Imaginal and in vivo exposure exercises were used in each treatment session. Objects or situations evoking moderate obsessional distress were introduced at first, with progression to the most feared situation by the sixth exposure session. During sessions, therapists discussed patients’ OCD-related beliefs and the disconfirmatory evidence provided by exposure exercises. Daily homework was designed by the therapist in collaboration with the patient and consisted of self-monitoring and further exposure to stimuli similar to those confronted in that day’s session. Ritual prevention entailed instructions to abstain from ritualistic behavior throughout the 3-week period (35). Therapists visited the patients’ homes twice (4 hours total) in the fourth week to promote generalizability of treatment gains by conducting exposures in contexts relevant to the patient’s functioning. For the remaining 8 weeks, 45-minute sessions were conducted weekly to promote maintenance. The patient and therapist discussed the patient’s remaining OCD symptoms and how to combat them; no new in-session exposure exercises were assigned.
Clomipramine or placebo
Patients were seen weekly for 30 minutes by their psychiatrist for medication adjustment. The dosage schedule was fixed for the first 5 weeks, starting at 25 mg/day and increasing to 200 mg/day with an optional increase thereafter to 250 mg/day if the patient tolerated the dose and if a higher dose was indicated. Increases could be delayed or doses lowered for adverse events. Patients were encouraged to expose themselves to situations that evoked their obsessions while refraining from ritualizing, without systematic exposure instructions or homework.
Exposure and ritual prevention plus clomipramine
The patients began and continued both treatments simultaneously, according to the procedures described for exposure and ritual prevention alone and for clomipramine alone.
Quality Control Procedures
Exposure and ritual prevention therapists received training and ongoing weekly supervision from faculty from the Philadelphia site. Training included observing experts who conducted exposure and ritual prevention and completing at least one training case of exposure and ritual prevention. Psychiatrists received training and ongoing supervision from faculty from the New York site. Independent evaluators received training and ongoing supervision from Philadelphia faculty and performed practice ratings of taped interviews intermittently during the study. Throughout, independent evaluators from New York and Philadelphia met to discuss assessment issues and rated specific assessments together to ensure interrater reliability. Before each assessment by the independent evaluator, patients were reminded not to discuss their treatment in order to maintain the blind.
Statistical Methods
First, to detect possible pretreatment differences among conditions, we conducted one-way analyses of variance (ANOVAs) on each of the OCD outcome measures. Second, we examined the differential efficacy of the conditions at weeks 4 and 12 using linear mixed-effects models from the SAS procedure MIXED (SAS Institute, Cary, N.C.). Piecewise linear growth curve models with a change point at week 4 and an unstructured variance model provided the best fit for the data. These models were applied to each of the three continuous OCD outcome measures. We estimated week 4 and week 12 scores and compared outcome using the same linear mixed-effects models. Endpoint analyses were selected over change from baseline to allow comparison of the study results with those of prior outcome trials and to get an estimation of posttreatment severity. Because no pretreatment differences among conditions were detected among of the measures, endpoint analyses and change scores were equivalent. We chose linear mixed-effects models for our primary outcome analyses because this method is more powerful and may also be less biased than traditional ANOVA methods (36, 37).
Third, we examined site and demographic effects by including an indicator and its interactions with treatment and time in the piecewise linear mixed-effects models. When the interaction of site by treatment or site by time was significant, we conducted follow-up analyses to explain these interactions. Because of the small number of subjects at the Winnipeg site (N=14), data for the Winnipeg subjects were not included in follow-up site analyses. Fourth, we used both chi-square analyses and generalized linear models (SAS procedure GENMOD) for the ordinal data from the CGI improvement scale to examine response status (very much improved and/or much improved versus other response statuses). These analyses used the default model option for GENMOD in SAS. They were based on the complementary log-log link for ordinal responses and were conducted separately for patients who completed at least one treatment session (treated group) and patients who completed the acute 12-week phase of the study (completer group). Alpha was set to 0.05, two-tailed, for all analyses.
Results
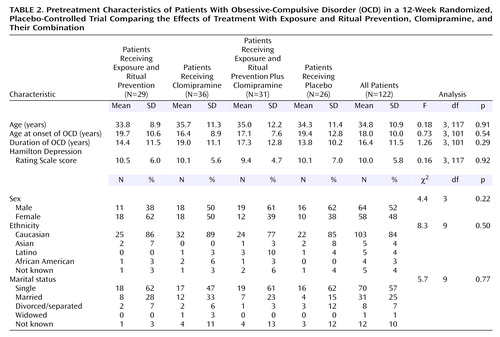
Pretreatment Clinical and Demographic Characteristics
One hundred forty-nine patients were randomly assigned to treatment groups (see flowchart in Figure 1). Of these, 27 dropped out after learning their treatment assignment and before receiving pretreatment assessments or any treatment and thus are not included in analyses. Of the 122 patients who were randomly assigned to treatment groups and who entered treatment, 56 were treated in Philadelphia, 52 in New York, and 14 in Winnipeg. The only pretreatment difference between patients at the three sites was in the NIMH Global Obsessive-Compulsive Scale score (the mean score for the Philadelphia site was higher than that for the New York site, F=4.93, df=1, 105, p<0.05). There were no other differences among conditions (Table 2) or sites on demographic variables nor in pretreatment scores on the Yale-Brown Obsessive Compulsive Scale (F=1.0, df=3, 118, n.s.), CGI severity scale (F=0.7, df=3, 118, n.s.), NIMH Global Obsessive-Compulsive Scale (F=0.4, df=3, 118, n.s.), or Hamilton depression scale (F=0.2, df=3, 118, n.s.).
Treatment Efficacy
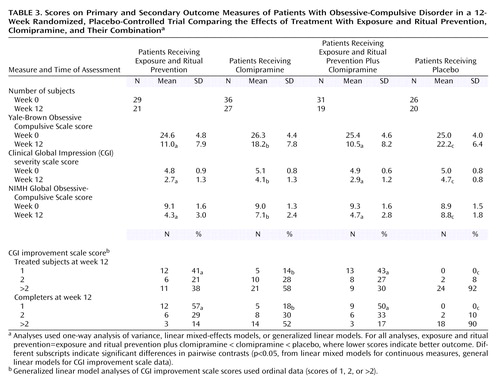
Results of the primary analyses comparing the four conditions are presented in Table 3 and in Figure 2.
Endpoint Analyses (week 12)
Linear mixed-effects model analyses
Linear mixed-effects model analyses indicated treatment differences in response over the 12-week period for scores on the Yale-Brown Obsessive Compulsive Scale (weeks 0–4-by-condition: F=30.4, df=3, 118, p<0.0001; weeks 4–12-by-condition: F=2.8, df=3, 118, p=0.04), CGI severity scale (weeks 0–4-by-condition: F=17.1, df=3, 118, p<0.0001; weeks 4–12-by-condition: F=1.6, df=3, 118, p=0.21), and NIMH Global Obsessive-Compulsive Scale (weeks 0–4-by-condition: F=23.4, df=3, 118, p<0.0001; weeks 4–12-by-condition: F=2.0, df=3, 118, p=0.12).
At week 12, the scores on all measures of patients receiving active treatments were significantly lower than those of patients receiving placebo (all F>4.0, df=1, 118, all p<0.05). Exposure and ritual prevention was superior to clomipramine on all measures (all F>6.6, df=1, 118, all p≤0.01). Exposure and ritual prevention plus clomipramine was superior to clomipramine on all measures (all F>10, df=1, 118, all p<0.01). No differences between exposure and ritual prevention and exposure and ritual prevention plus clomipramine were found on any measure (all F<1.0, df=1, 118, p>0.20).
Response status analyses
Response status was analyzed in two ways: 1) by responder status (CGI improvement=1 or 2 versus ≥3) and 2) by stratifying according to response status, as follows: excellent response (CGI improvement=1), response (CGI improvement=2), and nonresponse (CGI improvement ≥3). Conditions differed with respect to responder status (χ2=25.5, df=4, p<0.001 for the treated group; χ2=29.3, df=4, p<0.001 for the completer group) and for excellent response (χ2=33.6, df=3, p<0.001 for the treated group; χ2=38.8, df=3, p<0.001 for the completer group).
There were more responders and more excellent responders in each of the active treatment conditions, compared to the placebo condition, in both the treated group and the completer group. The exposure and ritual prevention condition and the clomipramine condition did not differ in the number of responders in the treated group (χ2=2.7, df=1, p=0.10) but did differ in the number of responders in the completer group (χ2=7.3, df=1, p<0.01). The exposure and ritual prevention condition had a greater number of excellent responders than the clomipramine condition in both the treated group and the completer group (χ2=5.3, df=1, p=0.02; χ2=10.8, df=1, p=0.001, respectively). The exposure and ritual prevention plus clomipramine condition had more responders than the clomipramine condition in the treated group (χ2=4.5, df=1, p<0.05) and the completer group (χ2=4.4, df=1, p<0.05). There were more excellent responders in the exposure and ritual prevention plus clomipramine condition than in the clomipramine condition in both the treated group and the completer group (χ2=7.1, df=1, p=0.008; χ2=7.6, df=1, p=0.006, respectively). No differences in the number of responders or excellent responders were found in comparison of the exposure and ritual prevention condition and the exposure and ritual prevention plus clomipramine condition in either the treatment group or the completer group (all p>0.50).
Week 4 Analyses
Linear mixed-effects model analyses at week 4 revealed that scores on the Yale-Brown Obsessive Compulsive Scale for patients who received all active treatments were lower than the scores for patients who received placebo (F>4.0, df=1, 118, all p<0.05), but no differences in CGI improvement scores or NIMH Global Obsessive-Compulsive Scale scores were found between the patients who received clomipramine and those who received placebo (F<1.4, df=1, 117, p>0.20). Exposure and ritual prevention and exposure and ritual prevention plus clomipramine both yielded superior outcomes, compared to placebo (F>4.0, df=1, 118, all p<0.05). Exposure and ritual prevention and exposure and ritual prevention plus clomipramine were superior to clomipramine on all measures (all F>15.0, df=1, 118, all p<0.0001; all F>9.0, df=1, 118, all p<0.01). No differences were found on any measure between exposure and ritual prevention and exposure and ritual prevention plus clomipramine (all F<1.0, df=1, 118, p>0.20).
Site Effects
The only significant interaction of time, treatment condition, and site that emerged in the linear mixed-effects model analyses was for the NIMH Global Obsessive-Compulsive Scale score from weeks 0–4 (site-by-treatment-by-week 0–4: F=2.3, df=6, 118, p=0.04; all other analyses: F<1.0, df=1, 118, p>0.10). The interaction reflected more change on the NIMH Global Obsessive-Compulsive Scale from weeks 0 to 4 in the exposure and ritual prevention plus clomipramine condition at the Philadelphia site than at the New York site (F=10.5, df=1, 110, p=0.002); the difference was not significant at week 12 (F=1.8, df=1, 110, p=0.18). Also, outcome at week 12, as measured by the NIMH Global Obsessive-Compulsive Scale score, for the New York patients who received clomipramine was superior to that for the Philadelphia patients who received clomipramine (New York: mean=6.1, SD=2.8; Philadelphia: mean=8.2, SD=1.8; F=4.8, df=1, 110, p=0.03).
Pharmacotherapy Doses
Mean daily doses during the last week in the acute phase for the treated group and the completer group were, respectively, 196 mg (SD=82) and 235 mg (SD=34) for the patients who received clomipramine, 209 mg (SD=76) and 245 mg (SD=23) for the patients who received placebo, and 163 mg (SD=65) and 194 mg (SD=48) for the patients who received exposure and ritual prevention plus clomipramine. The patients who received exposure and ritual prevention plus clomipramine received lower doses of medication than the clomipramine or placebo patients (p<0.05) in both the treated group and the completer group. Most completers who received clomipramine alone (77%) or placebo (95%) received the maximum 250 mg/day dose at week 12. In contrast, only eight completers who received exposure and ritual prevention plus clomipramine (37.5%) received the maximum 250 mg/day dose; 12.5% received 200 mg/day, and 50% received 150 mg/day.
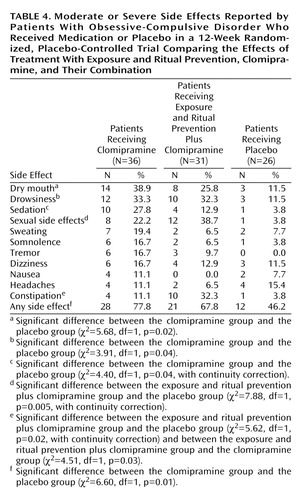
Adverse Events
Adverse events related to medication were recorded on a symptom checklist by the psychiatrist at each visit. No data were obtained for adverse events associated with exposure and ritual prevention. Of the 36 patients who received clomipramine alone, 28 (78%) reported at least one moderate or severe side effect during the acute phase of treatment, compared to 21 (68%) of the 31 patients in the exposure and ritual prevention plus clomipramine group and 12 (46%) of the 26 patients in the placebo group. Side effects reported by more than 10% of any group are presented in Table 4. On the average, clomipramine patients experienced three side effects, and exposure and ritual prevention plus clomipramine patients experienced two side effects; the frequency of side effects was significantly higher for both groups than for the placebo group (p<0.05).
Patient Recruitment and Treatment Retention
Figure 1 presents subject flow from point of initial contact. The overall dropout rate of those entering treatment was 29%. There were no differences in rates across conditions (χ2=1.9, df=3, p=0.58), and patients dropped for various reasons. Among the eight patients who dropped out of the exposure and ritual prevention condition, reasons for dropout were dislike of exposure (N=5), worsening OCD (N=1), a new skin disorder (N=1), and loss to follow-up (N=1). Among the nine patients who dropped out of the clomipramine condition, reasons were side effects or medication noncompliance (N=6), dislike of medication (N=1), improvement and a desire to stop medication (N=1), and unknown reasons (N=1). Among the 12 patients who dropped out of the exposure and ritual prevention plus clomipramine condition, reasons were desire to stop exposure and ritual prevention (N=4), clomipramine side effects or noncompliance (N=6), worsening OCD (N=1), and unknown reasons (N=1). Among the six patients who dropped out of the placebo condition, the reasons for dropout were lack of benefit (N=3), worsening symptoms (N=2), and unknown reasons (N=1).
Patients who dropped out of the study did not differ from completers on demographic or clinical characteristics, including OCD severity. The majority (82%) of dropouts occurred within the first 4 weeks, with no differences in the number of weeks completed before dropout across conditions or sites. However, New York had significantly more dropouts than Philadelphia (26 [43%] versus 10 [16%]; χ2=10.4, df=1, p=0.001). New York had a significantly higher percentage of dropouts, compared with Philadelphia, in the exposure and ritual prevention condition (six [54%] versus two [13%]; p<0.04, Fisher’s exact test) and placebo condition (five [46%] versus none [0%]; p<0.02, Fisher’s exact test) but not in the exposure and ritual prevention plus clomipramine condition (four [27%] versus eight [61%]; p=0.13, Fisher’s exact test) or clomipramine condition (two [14%]versus five [29%]; p=0.41, Fisher’s exact test). Eight (22%) of the 36 dropouts were responders; six of those patients were in the combined condition and two were in the clomipramine condition. Seven of these eight were at the New York site.
Discussion
Summary of Results
As hypothesized, after 12 weeks of treatment, all three active treatment groups differed significantly from the placebo group on all measures. Also as hypothesized, intensive exposure and ritual prevention was more effective than clomipramine. It is noteworthy that, on average, the posttreatment Yale-Brown Obsessive Compulsive Scale scores of the clomipramine group would have qualified for entry to the study, whereas the scores of the patients who received exposure and ritual prevention would have been considered, on average, too mild to meet the entry criteria.
Our hypothesis that combined treatment would be superior to both monotherapies was only partly supported: exposure and ritual prevention plus clomipramine was superior to clomipramine alone on all outcome measures, but the combined treatment failed to show superiority over exposure and ritual prevention alone on any analyses. However, several factors may have limited the sensitivity of the present design to potential combined treatment effects: failure to maximize clomipramine doses for exposure and ritual prevention plus clomipramine patients (most patients in the combined group did not achieve maximum doses of clomipramine), the potency of intensive exposure and ritual prevention alone (leaving little room for further improvement), the practical nonlinearity of the Yale-Brown Obsessive Compulsive Scale (asymptomatic patients rarely receive scores lower than 6), and the simultaneous instigation of intensive exposure and ritual prevention and slow upward titration of clomipramine (the intensive phase of exposure and ritual prevention was completed before the 4–6 weeks needed for clomipramine action). The added benefit of clomipramine might be most evident in individuals for whom exposure and ritual prevention alone is too distressing, and pretreatment with SRIs might make exposure tasks less difficult.
Other investigations have found exposure and ritual prevention to be superior to various psychosocial comparison conditions (38–40) in groups of patients who were matched for treatment length and amount of therapist contact. Thus, nonspecific factors associated with psychotherapy probably do not account for the superiority of exposure and ritual prevention over clomipramine and placebo in the present study. Because clomipramine was also superior to placebo and its effect size was comparable to those reported in previous controlled studies of SRIs (16, 17), it cannot be argued that the present patient group was, as a whole, nonresponsive to medication (41). Thus, we conclude that the present study was a fair test of the relative and combined efficacy of exposure and ritual prevention and clomipramine.
Generalizability of Findings
As in all randomized, controlled trials, the question of generalizability arises because patients have to agree to be randomly assigned to treatments rather than choose them and because exclusion criteria render the subjects somewhat different from the population of treatment seekers. Indeed, the ratio between the number of individuals who were initially assessed for eligibility and the number of completers is large, albeit consistent with that in other randomized, controlled trials comparing medication, psychosocial treatments, and their combination (42, 43). In addition to the other reasons for exclusion, refusal, or dropout (see Figure 1), the diversity of treatments that patients were required to accept increased refusal rates because of patients’ preferences. It is important to note that many patients in standard clinical care also refuse or terminate treatment prematurely; moreover, the dropout rate in our study was similar to that found in other large randomized, controlled trials with similar designs (44).
The generalizability of our findings is suggested by two results: 1) there were virtually no significant site differences, and 2) the degree of improvement for the two monotherapies and for placebo was consistent with the findings of previous studies (10, 20–22, 38, 39). The finding that exposure and ritual prevention seems to be superior to SRI pharmacotherapy for OCD is consistent with the findings of Marks et al. (18) but not with those reported by Cottraux et al. (20). The inconsistency may be related to the attenuated outcome of exposure and ritual prevention in the latter study, possibly because Cottraux et al. used a once-weekly exposure and ritual prevention program, whereas the Marks et al. study (18) and the present study used intensive (daily) exposure and ritual prevention programs. We compared our data with those from the study by Cottraux et al. (20) and found similar effect sizes for the combined and medication-alone treatments but a substantially larger effect size for exposure and ritual prevention alone in our study. Given that weekly treatment regimens such as that delivered by Cottraux et al. better reflect routine clinical practice and given that the combined treatment outcome in that study was similar to the combined treatment outcome with intensive exposure and ritual prevention, the findings of Cottraux et al. may point to the potential benefit for combined treatment in settings where exposure and ritual prevention is not conducted intensively.
In order to enhance generalizability to clinical practice, we intentionally allowed session length and visit frequency to vary between the exposure and ritual prevention and the medication-only conditions, as they would in clinical practice. As a result, we cannot exclude the possibility that the differential session length and visit frequency for the exposure and ritual prevention condition and the clomipramine condition account for the superiority of exposure and ritual prevention in the current study. Other studies of intensive exposure and ritual prevention that have carefully controlled for time have found clear evidence of a specific exposure and ritual prevention effect (39), however, and thus we felt that repeating this experiment here at the expense of external validity was unwarranted scientifically.
With respect to generalizability of the present findings to nonresearch contexts, the data are encouraging although by no means definitive. Philadelphia outpatients who refused or were ineligible for randomized, controlled trials but who received exposure and ritual prevention on an outpatient fee-for-service basis achieved substantial symptom reduction that was comparable to that reported in randomized, controlled trials of exposure and ritual prevention (45). Thus, the benefits of exposure and ritual prevention are not restricted to those who receive treatment in carefully controlled randomized trials. However, whether exposure and ritual prevention would retain its superiority over medication in nonexpert clinics is unknown.
Patients with comorbid depression were excluded from this study. Such exclusion is the rule rather than the exception in OCD studies, especially those involving medication, in order to eliminate the possibility that the medication affects OCD symptoms by its effects on depression (18). In earlier trials, the effects of clomipramine were studied in depressed and nondepressed OCD patients with no difference in outcome (10, 46), and only the most severely depressed patients (the upper 10th percentile) appeared less responsive to exposure and ritual prevention (47). Nevertheless, it is possible that for patients who have both OCD and depression, combined treatment or medication monotherapy would fare better than they did here.
Implications for Care
Many OCD patients benefit from SRI medications that are widely available and require less time commitment and efforts than exposure and ritual prevention. The average effect of medication still leaves many patients clinically symptomatic, however. Because, on average, patients who received both treatments (despite receiving less medication) benefited more than those who received medication only, exposure and ritual prevention can be used to augment the benefit from medication, to reduce the use of medication for those who suffer side effects, or as a first-line treatment for those who refuse medication.
One important question addressed in this study was the transportability of treatment modalities across clinics with different expertise. Our results generally support transportability, in that there were virtually no site effects. It should be noted, however, that the expert sites provided regular supervision to the other sites throughout the study, which may have minimized any “home court” advantages.
Expertise in exposure and ritual prevention is uncommon among clinicians in the community, perhaps because typical psychotherapists, even with cognitive behavior therapy training, do not treat sufficient numbers of OCD patients to acquire the experience necessary for effective intervention for patients with difficult-to-treat OCD. One solution is to develop regional specialty clinics similar to centers for heart disease and cancer. Intensive exposure and ritual prevention is the ideal treatment model for such centers because it is suitable for patients who live too far away to commute once or twice weekly during treatment; instead, they can make short-term living arrangements in close proximity to the center and receive daily treatment for 3–4 weeks.
Furthermore, while 1-hour weekly treatments have generally produced inferior results, compared to intensive exposure and ritual prevention (48), a twice-weekly exposure and ritual prevention program that was otherwise identical to the intensive treatment studied here produced results comparable to those of intensive treatment at 3-month follow-up (49). Thus, intensive exposure and ritual prevention should be considered for patients who fail to respond to twice-weekly treatment or for those who seek treatment at expert centers.
This study began in 1990 when the only medication indicated for OCD by the U.S. Food and Drug Administration (FDA) was clomipramine. Since then, several SSRIs were approved for OCD by the FDA and are usually used before clomipramine, despite possibly being less effective, because of clomipramine’s potentially more serious side effect profile (e.g., toxicity in overdose, heart block, and increased seizure risk) (16, 17). However, as argued earlier, it is reasonable to assume that the findings for clomipramine versus and combined with exposure and ritual prevention can be generalized to the SSRIs.
Study Limitations
Several study limitations merit consideration, all of which resulted from changes that have occurred in standard methods for randomized, controlled trials since this trial began. First, data were not collected on patients who dropped out of treatment after randomization but before treatment, and they could not be included in last-observation-carried-forward analyses. Second, although care was taken to ensure cross-site comparability in implementing assessments and assessors met regularly to maintain reliability, formal data on interrater reliability were not collected. Third, systematic data on prior treatment history were not collected, preventing us from exploring the relationship between treatment history and outcome. Fourth, the assessments did not include instruments to measure functional impairment and quality of life that are currently in standard use in randomized, controlled trials.
 |
 |
 |
 |
Presented in part in a poster session at the 155th annual meeting of the American Psychiatric Association, Philadelphia, May 18–23, 2002. Received Feb. 17, 2004; accepted March 10, 2004. From the Department of Psychiatry, University of Pennsylvania School of Medicine; New York State Psychiatric Institute, New York; Columbia University College of Physicians and Surgeons, New York; the Department of Psychiatry, Medical College of Pennsylvania, Philadelphia; and St. Boniface General Hospital, Winnipeg, Man., Canada. Address correspondence and reprint requests to Dr. Foa, Center for the Treatment and Study of Anxiety, University of Pennsylvania, 3535 Market St., Suite 600N, Philadelphia, PA 19104; [email protected] (e-mail). Supported by NIMH grants MH-45404 (Dr. Foa) and MH-45436 (Dr. Liebowitz). The authors thank Donald F. Klein, M.D., for comments on earlier drafts of the manuscript; Ning Zhao, Ph.D., for assistance with data management; and the patients, therapists, psychopharmacologists, evaluators, and research assistants who participated in the study.
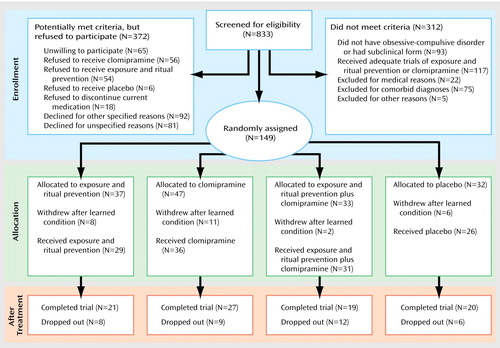
Figure 1. Flowchart of a 12-Week Randomized, Placebo-Controlled Trial Comparing the Effects of Exposure and Ritual Prevention, Clomipramine, and Their Combination in the Treatment of Patients With Obsessive-Compulsive Disorder
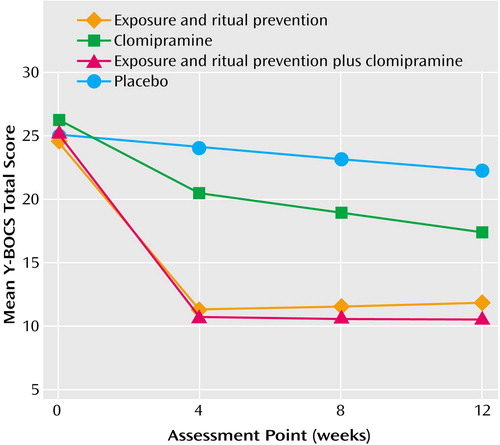
Figure 2. Yale-Brown Obsessive Compulsive Scale (Y-BOCS) Scores of Patients With Obsessive-Compulsive Disorder in a 12-Week Randomized, Placebo-Controlled Trial Comparing the Effects of Treatment With Exposure and Ritual Prevention, Clomipramine, and Their Combinationa
aLinear mixed-effects model analyses.
1. Myers J, Weissman M, Tischler G, Holzer CE, Leaf PJ, Orvaschel H, Anthony JC, Boyd JH, Burke JD, Kramer M, Stoltzman R: Six-month prevalence of psychiatric disorders in three communities: 1980–1982. Arch Gen Psychiatry 1984; 41:959–967Crossref, Medline, Google Scholar
2. Karno M, Golding JM, Sorenson SB, Burnam MA: The epidemiology of obsessive-compulsive disorder in five US communities. Arch Gen Psychiatry 1988; 45:1094–1099Crossref, Medline, Google Scholar
3. Robins LN, Helzer JE, Weissman MM, Orvaschel H: Lifetime prevalence of specific psychiatric disorders in three sites. Arch Gen Psychiatry 1984; 41:949–958Crossref, Medline, Google Scholar
4. Rasmussen SA, Tsuang MT: Clinical characteristics and family history in DSM-III obsessive-compulsive disorder. Am J Psychiatry 1986; 143:317–322Link, Google Scholar
5. Koran LM, Thienemann ML, Davenport R: Quality of life for patients with obsessive-compulsive disorder. Am J Psychiatry 1996; 153:783–788Link, Google Scholar
6. Leon AC, Portera L, Weisman MM: The social costs of anxiety disorders. Br J Psychiatry 1995; 166(suppl 27):19–22Google Scholar
7. Riggs DS, Hiss H, Foa EB: Marital distress and the treatment of obsessive-compulsive disorder. Behav Ther 1992; 23:585–597Crossref, Google Scholar
8. Meyer V: Modification of expectations in cases with obsessional rituals. Behav Res Ther 1966; 4:273–280Crossref, Medline, Google Scholar
9. March JS, Frances A, Carpenter D, Kahn D: The Expert Consensus Guidelines Series: Treatment of Obsessive-Compulsive Disorder. J Clin Psychiatry 1997; 58(suppl 4)Google Scholar
10. Clomipramine Collaborative Group: Clomipramine in the treatment of patients with obsessive-compulsive disorder. Arch Gen Psychiatry 1991; 48:730–738Crossref, Medline, Google Scholar
11. Greist JH, Chouinard G, DuBoff E, Halaris A, Kim SW, Koran L, Liebowitz M, Lydiard RB, Rasmussen S, White K, Sikes C: Double-blind parallel comparison of three dosages of sertraline and placebo in outpatients with obsessive-compulsive disorder. Arch Gen Psychiatry 1995; 52:289–295Crossref, Medline, Google Scholar
12. Goodman WK, Price LH, Rassmussen SA, Delgado PL, Heninger GR, Charney DS: Efficacy of fluvoxamine in obsessive-compulsive disorder: a double-blind comparison with placebo. Arch Gen Psychiatry 1989; 46:36–44Crossref, Medline, Google Scholar
13. Hollander E, Allen A, Steiner M, Wheadon DE, Oakes R, Burnham DB, Paroxetine OCD Study Group: Acute and long-term treatment and prevention of relapse of obsessive-compulsive disorder with paroxetine. J Clin Psychiatry 2003; 64:1113–1121Crossref, Medline, Google Scholar
14. Tollefson GD, Rampey AH, Potvin JH, Jenike MA, Rush AJ, Dominguez RA, Koran LM, Shear MK, Goodman W, Genduso LA: A multicenter investigation of fixed dose fluoxetine in the treatment of obsessive-compulsive disorder. Arch Gen Psychiatry 1994; 51:559–567Crossref, Medline, Google Scholar
15. Montgomery SA, Kasper S, Stein DJ, Hedegaard K, Bang LOM: Citalopram 20 mg, 40 mg and 60 mg are all effective and well tolerated compared with placebo in obsessive-compulsive disorder. Int Clin Psychopharmacol 2001; 16:75–86Crossref, Medline, Google Scholar
16. Kobak KA, Greist JH, Jefferson JW, Katzelnick DJ: Behavioral versus pharmacological treatments of obsessive compulsive disorder: a meta-analysis. Outcomes Manage 1998; 136:205–216Google Scholar
17. Ackerman D, Greenland S: Multivariate meta-analysis of controlled drug studies for obsessive-compulsive disorder. J Clin Psychopharmacol 2002; 22:309–317Crossref, Medline, Google Scholar
18. Marks IM, Stern RS, Mawson D, Cobb J, McDonald R: Clomipramine and exposure for obsessive-compulsive rituals. Br J Psychiatry 1980; 136:1–25Crossref, Medline, Google Scholar
19. Marks IM, Lelliott P, Basoglu M, Noshirvani H, Monteiro W, Cohen D, Kasvikis Y: Clomipramine, self-exposure, and therapist-aided exposure for obsessive-compulsive rituals. Br J Psychiatry 1988; 152:522–534Crossref, Medline, Google Scholar
20. Cottraux J, Mollard E, Bouvard M, Marks I, Sluys M, Nury AM, Douge R, Cialdella P: A controlled study of fluvoxamine and exposure in obsessive-compulsive disorder. Int Clin Psychopharmacol 1990; 5:17–30Crossref, Medline, Google Scholar
21. Hohagen F, Winkelmann G, Rasche-Rauchle H, Hand I, Koenig A, Muenchau N, Hiss H, Geiger-Kabisch C, Kaeppler C, Schramm P, Rey E, Aldenhoff J, Berger M: Combination of behaviour therapy with fluvoxamine in comparison with behaviour therapy and placebo: results of a multicentre study. Br J Psychiatry 1998; 173:71–78Crossref, Google Scholar
22. van Balkom AJLM, de Haan E, van Oppen P, Spinhoven P, Hoogduin KAL, van Dyck R: Cognitive and behavioral therapies alone and in combination with fluvoxamine in the treatment of obsessive compulsive disorder. J Nerv Ment Dis 1998; 186:492–499Crossref, Medline, Google Scholar
23. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
24. Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS: The Yale-Brown Obsessive Compulsive Scale, II: validity. Arch Gen Psychiatry 1989; 46:1012–1016Crossref, Medline, Google Scholar
25. Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS: The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Medline, Google Scholar
26. Goodman WK, Price LH: Assessment of severity and change in obsessive compulsive disorder. Psychiatr Clin North Am 1992; 15:861–869Crossref, Medline, Google Scholar
27. Hiss H, Foa EB, Kozak MJ: Relapse prevention program for treatment of obsessive-compulsive disorder. J Consult Clin Psychol 1994; 62:801–808Crossref, Medline, Google Scholar
28. Insel TR, Donnelly LF, Lalakea ML, Alterman IS, Murphy DL: Neurological and neuropsychological studies of patients with obsessive-compulsive disorder. Biol Psychiatry 1983; 18:741–751Medline, Google Scholar
29. Murphy DL, Pickar D, Alterman IS: Methods for the quantitative assessment of depressive and manic behavior, in The Behavior of Psychiatric Patients. Edited by Burdock EI, Sudilovsky A, Gershon S. New York, Marcel Dekker, 1982, pp 355–392Google Scholar
30. Kim SW, Dysken MW, Kuskowski M: The Symptom Checklist-90 obsessive compulsive subscale: a reliability and validity study. Psychiatr Res 1992; 41:37–44Crossref, Medline, Google Scholar
31. Black DW, Kelly M, Myers C, Noyes R Jr: Tritiated imipramine binding in obsessive-compulsive volunteers and psychiatrically normal controls. Biol Psychiatry 1990; 27:319–327Crossref, Medline, Google Scholar
32. Taylor S: Assessment of obsessions and compulsions: reliability, validity, and sensitivity to treatment effects. Clin Psychol Rev 1995; 15:261–296Crossref, Google Scholar
33. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
34. Hedlund J, Vieweg B: The Hamilton Rating Scale for Depression: a comprehensive review. J Operational Psychiatry 1979; 10:149–165Google Scholar
35. Kozak MJ, Foa EB: Mastery of Obsessive-Compulsive Disorder: A Cognitive Behavioral Approach. San Antonio, Tex, Graywind Publications, 1997Google Scholar
36. Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, Shea MT, Imber SD, Sotsky SM, Watkins JT: Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Arch Gen Psychiatry 1993; 50:739–750Crossref, Medline, Google Scholar
37. Nich C, Carroll K: Now you see it, now you don’t: a comparison of traditional versus random-effects regression models in the analysis of longitudinal follow-up data from a clinical trial. J Consult Clin Psychol 1997; 65:252–261Crossref, Medline, Google Scholar
38. Fals-Stewart W, Marks AP, Schafer J: A comparison of behavioral group therapy and individual behavior therapy in treating obsessive-compulsive disorder. J Nerv Ment Dis 1993; 181:189–193Crossref, Medline, Google Scholar
39. Lindsay M, Crino R, Andrews G: Controlled trial of exposure and response prevention in obsessive-compulsive disorder. Br J Psychiatry 1997; 171:135–139Crossref, Medline, Google Scholar
40. Marks IM, Hodgson R, Rachman S: Treatment of chronic obsessive-compulsive neurosis by in-vivo exposure: a two-year follow-up and issues in treatment. Br J Psychiatry 1975; 127:349–364Crossref, Medline, Google Scholar
41. Klein DF: Preventing hung juries about therapy studies. J Consult Clin Psychol 1996; 64:81–87Crossref, Medline, Google Scholar
42. Hofmann SG, Barlow DH, Papp LA, Detweiler MF, Ray SE, Shear MK, Woods SW, Gorman JM: Pretreatment attrition in a comparative treatment outcome study on panic disorder. Am J Psychiatry 1998; 155:43–47Link, Google Scholar
43. Huppert JD, Franklin ME, Foa EB, Davidson JR: Study refusal and exclusion from a randomized treatment study of generalized social phobia. J Anxiety Disord 2003; 17:683–693Crossref, Medline, Google Scholar
44. Barlow DH, Gorman JM, Shear MK, Woods SW: Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA 2000; 283:2529–2536Crossref, Medline, Google Scholar
45. Franklin ME, Abramowitz JS, Kozak MJ, Levitt JT, Foa EB: Effectiveness of exposure and ritual prevention for obsessive-compulsive disorder: randomized compared with nonrandomized samples. J Consult Clin Psychol 2000; 68:594–602Crossref, Medline, Google Scholar
46. DeVeaugh-Geiss J, Landau P, Katz R: Treatment of OCD with clomipramine. Psychiatr Annals 1989; 19:97–101Crossref, Google Scholar
47. Abramowitz JS, Franklin ME, Street GP, Kozak MJ, Foa EB: Effects of comorbid depression on response to treatment for obsessive-compulsive disorder. Behav Ther 2000; 31:517–528Crossref, Google Scholar
48. Abramowitz JS: Variants of exposure and response prevention in the treatment of obsessive-compulsive disorder: a meta-analysis. Behav Ther 1996; 27:583–600Crossref, Google Scholar
49. Abramowitz JS, Foa EB, Franklin ME: Exposure and ritual prevention for obsessive-compulsive disorder: effects of intensive versus twice-weekly sessions. J Consult Clin Psychol 2003; 71:394–398Crossref, Medline, Google Scholar



