Management of Bipolar Disorder During Pregnancy and the Postpartum Period
Abstract
OBJECTIVE: Bipolar disorder affects 0.5%–1.5% of individuals in the United States. The typical age at onset is late adolescence or early adulthood, placing women at risk for episodes throughout their reproductive years. General guidelines for the treatment of bipolar disorder are available from the American Psychiatric Association, but additional issues arise when these guidelines are applied in the treatment of peripartum women. The authors summarize knowledge regarding the management of bipolar disorder during pregnancy and the postpartum period, with a focus on managing mania, hypomania, and the psychotic components of the illness. METHOD: An expert panel reviewed articles that address the management of bipolar disorder and the consequences of the use of mood stabilizers during pregnancy, and a consensus document was generated. RESULTS: The treatment of bipolar disorder in pregnant women involves significant challenges. Some mood stabilizers, e.g., sodium valproate and carbamazepine, are human teratogens. On the other hand, the teratogenicity associated with lithium may have been overestimated in the past. CONCLUSIONS: Since treatment can be managed most effectively if pregnancy is planned, clinicians should discuss the issue of pregnancy and its management with every bipolar disorder patient who has childbearing potential, regardless of future reproductive plans. Additional research should address the risks of disturbed sleep to pregnant and postpartum women with bipolar disorder, as well as structural and behavioral consequences to offspring when mood stabilizers are used during pregnancy. Longitudinal and cohort studies can promote these efforts. Given the rate of bipolar disorder in the general population, research efforts will need to be broad based and include multiple collaborating centers.
Bipolar disorder is a serious psychiatric condition that affects 0.5%–1.5% of individuals in the United States. The prevalence is similar in men and women, although female patients are more likely to have the rapid-cycling form of the illness and more likely to display depressive characteristics (1). Women with bipolar disorder are typically in their teens and early 20s at onset of the illness, placing them at risk for episodes throughout their reproductive years. The issue of whether bipolar illness improves during pregnancy is controversial (2–6), but, in any case, pregnancy is not protective for all women with bipolar disorder (6), and management of the illness in pregnancy is most difficult when the pregnancy is unanticipated. Since many pregnancies are unplanned and women with bipolar disorder do not all experience symptom improvement during pregnancy, it important for clinicians to be knowledgeable about approaches to and risks associated with medication treatment during pregnancy.
Women with bipolar disorder are at high risk for symptom exacerbation during the immediate postpartum period (3, 5, 7), as indicated by a nearly sevenfold higher risk of admission for a first episode and a nearly twofold higher risk for a recurrent episode in puerperal women, compared with nonpostpartum and nonpregnant women (5). Among women with bipolar disorder who elect to discontinue lithium therapy in the puerperium, the estimated risk of relapse is threefold higher than for nonpregnant, nonpuerperal women (7). Symptom emergence is often rapid and may commence a few weeks before (2) or within the first few days to weeks after parturition (3, 5).
General guidelines for the treatment of bipolar disorder are available from the American Psychiatric Association (APA) (8), but additional issues arise when these guidelines are applied in the treatment of pregnant and postpartum women. This report summarizes knowledge regarding the management of bipolar disorder during pregnancy and the immediate postpartum period, with a focus on managing mania, hypomania, and psychotic components of the illness. We review 1) the risks to offspring of mothers treated with mood stabilizers or adjunctive medication during pregnancy and 2) clinical issues in the use of mood-stabilizing agents to treat pregnant women with bipolar disorder. We then present information on the risks of breast-feeding associated with treatments for bipolar disorder. This report was assembled by a group of psychiatrists who specialize in the treatment of pregnant women with bipolar disorder. The group was convened under the aegis of APA. Because the use of antidepressants during pregnancy has been addressed recently by APA guidelines (9), this topic is not addressed here.
Risks Associated With Mood-Stabilizing Agents During Pregnancy
The risk of fetal malformations associated with maternal drug use depends on the properties of the drug and the time period of the fetus’s exposure. Exposure up to 32 days after conception can affect neural tube development and closure; exposure 21–56 days after conception may affect heart formation; and exposure during days 42–63 may influence development of the lip and palate. Craniofacial anomalies can also occur after the first trimester. In addition, neurobehavioral teratogenicity can result from exposure after the first trimester. The following review is organized by domain of reproductive toxicity, including structural malformations, growth retardation, perinatal toxicity, and adverse neurobehavioral sequelae. The current U.S. Food and Drug Administration (FDA) classification of teratogenicity is being revised to better address and inform clinicians about the risks of fetal exposure (10). Therefore, the FDA classification is not used in this review.
Lithium
Although lithium is effective for only a subgroup of patients with bipolar disorder (11), it remains one of the mainstays for acute and maintenance treatment.
Organ dysgenesis
Shortly after lithium came into common use, concerns arose about an association between prenatal lithium exposure and congenital malformations. The Register of Lithium Babies (12), a voluntary physician-reporting database, noted a 400-fold higher rate of cardiovascular malformations, most notably Ebstein’s anomaly, in offspring exposed in utero, compared with the general population. This congenital anomaly, characterized by downward displacement of the tricuspid valve into the right ventricle and variable levels of right ventricular hypoplasia, occurs at a rate of 1:20,000 in the general population (13). Subsequent investigations identified a risk of Ebstein’s anomaly among offspring of lithium users of 1:1,000 (0.1%) to 2:1,000 (0.05%), or 20 to 40 times higher than the rate in the general population (14–16). Thus, the relative risk for Ebstein’s anomaly with prenatal lithium exposure is somewhat higher than in the general population, although the absolute risk remains small.
Intrauterine growth effects
Lithium-exposed infants were found to weigh significantly more than comparison subjects by a mean of 92 g (3475 g, compared with 3383 g) (15), even though lithium-treated women were more likely to smoke cigarettes, which typically decreases birth weight. The maternal lithium dose did not correlate with birth weight.
Neurobehavioral teratogenicity
In a follow-up study of children included in the Register of Lithium Babies, 60 children exposed to lithium either during the first trimester or throughout pregnancy did not differ behaviorally from their nonexposed siblings (17). In another study (15), the attainment of major developmental milestones for 22 lithium-exposed subjects was similar to that for nonexposed comparison subjects.
Neonatal toxicity
The most common toxicity effect in offspring exposed to lithium during labor is the “floppy baby” syndrome, characterized by cyanosis and hypotonicity (18, 19). Cases of neonatal hypothyroidism and nephrogenic diabetes insipidus have also been described (20, 21). Close monitoring of lithium levels in the mother during labor is now routine.
Use during pregnancy
Lithium has a relatively short half-life (8–10 hours) and produces substantial peak and trough serum levels. More frequent dosing (three to four times a day) allows patients to maintain therapeutic serum levels and avoid peaks, although it is unclear whether this technique benefits the fetus (22). Lithium serum levels, which may be affected by vomiting, sodium intake, and febrile illnesses, should be monitored closely (22). As pregnancy progresses, renal lithium excretion increases, generally necessitating an increase in dose (23). Lithium levels in umbilical cord blood have been found to be equivalent to maternal blood levels (23), and the concentration of the cation may be higher in amniotic fluid than in blood (24). The significance of this finding is unknown. Some experts advise decreasing the dose of lithium at the onset of labor to avoid toxicity associated with the rapid reduction of vascular volume at delivery (25). Vigilant monitoring of symptom and lithium serum levels is required to avoid relapse or toxicity during delivery and the immediate postpartum period (7, 16). Adequate hydration should be maintained, and use of intravenous fluids should be considered for patients in prolonged labor.
In the case of first-trimester exposure, anomalies can be identified with prenatal screening with a high-resolution ultrasound examination and fetal echocardiography at 16–18 weeks gestation (16, 26). This procedure aids parents in decisions regarding pregnancy termination and perinatal interventions after delivery.
Anticonvulsants
A number of anticonvulsants, most notably sodium valproate and carbamazepine, have been used in the acute treatment of bipolar disorder. Some of these anticonvulsant agents represent more potent teratogenic risks than lithium. Exposure is associated with a twofold increase in the rate of malformations (27), which include neural tube defects (spina bifida, anencephaly), craniofacial anomalies, growth retardation, microcephaly, and heart defects (28). The “anticonvulsant face,” characterized by mid-face hypoplasia, short nose with anteverted nostrils, and long upper lip, has been associated with both carbamazepine and valproate exposure (28). Teratogenic risk is higher with polytherapy than with monotherapy (29). Many experts recommend avoiding the combination of valproate and carbamazepine, particularly if there is a family history of neural tube defects (30). In contrast, lamotrigine, which was recently approved for maintenance treatment of bipolar disorder, appears to be associated with a lower rate of malformations overall and has emerged as a first-line treatment for women with epilepsy during their reproductive years (31). Nonetheless, the overall incidence of miscarriage or stillbirth is significantly higher in anticonvulsant-treated women with epilepsy, compared to unmedicated women with epilepsy (14% and 4%, respectively) (32).
Valproate
Organ dysgenesis
Sodium valproate is considered a human teratogen. Use of this compound during the first trimester is associated with neural tube defect rates of about 5%–9% (33, 34). The effect of the drug on neural tube development is related to its use 17–30 days postconception, and risk is dose related (35, 36). The neural tube defect found in exposed infants is more likely to be lumbosacral rather than anencephalic, which suggests a drug effect on neural crest closure (34).
Intrauterine growth effects
Some researchers (10) have suggested that intrauterine growth retardation is part of the “fetal valproate syndrome” (36), but intrauterine growth retardation has not been found to be an invariable consequence of valproate use during pregnancy (10).
Neurobehavioral teratogenicity
Mental retardation has been included in the “fetal valproate syndrome” (37), but the data to support its inclusion are not definitive.
Neonatal toxicity
Neonatal complications associated with valproate use near the time of delivery include heart rate decelerations (36) and withdrawal symptoms of irritability, jitteriness, feeding difficulties, and abnormal tone (34). Other complications among neonates include liver toxicity (38) and hypoglycemia (39). Reductions in neonatal fibrinogen levels have also been reported (40). It is noteworthy that the drug carries an FDA warning for use in children under the age of 2 years.
Use during pregnancy
In treatment of women with bipolar disorder or epilepsy, some experts recommend that valproate be switched to another mood stabilizer before conception (41). Women with unplanned pregnancies may not find that they are pregnant until after the deleterious effects of exposure have occurred, thus obviating most of the benefit that could occur from switching medications. Valproate is concentrated in the fetal compartment (35), and its concentration is twofold higher in cord serum than in maternal serum (42). Single daily dosing may cause unpredictably high peak levels (30).
Valproate levels are affected by the presence of other antiepileptic drugs that increase the activity of metabolic enzymes. In pregnancy, glucuronidation is induced, potentially leading to lower serum valproate levels. Patients should be monitored closely to ensure therapeutic efficacy.
Folate supplementation during pregnancy reduces the risk of neural tube defects generally, but it has not been tested as prophylaxis in pregnant women undergoing anticonvulsant treatment. Some researchers have recommended a daily dose of 5 mg of folic acid before and during pregnancy or at least through the first trimester for all women taking antiepileptic drugs (43). The American Academy of Neurology has recommended a more conservative dose of 3–4 mg/day of folic acid but has stated that the optimal dose has yet to be established (41). The patient’s level of vitamin B12 should be measured before folate supplementation to assess for concurrent pernicious anemia, which can be masked with folate treatment.
Carbamazepine
Organ dysgenesis
Carbamazepine is also considered a human teratogen. In one prospective study of 35 women treated with carbamazepine during the first trimester (28), craniofacial defects (11%), fingernail hypoplasia (26%), and developmental delay (20%) were found in live-born offspring. The rate for neural tube defects in that report and others ranged between 0.5% and 1% (28, 44).
The teratogenic potential of carbamazepine is enhanced when it is given with other agents and in particular with valproate, perhaps because the concentration of toxic epoxide metabolites is increased (45). In theory, oxcarbazepine, which does not produce the epoxide metabolite, may be less teratogenic. However, studies have not been performed to confirm this speculation.
Intrauterine growth effects
Carbamazepine is associated with reductions in birth weight (of about 250 g) (46) and mean head circumference (standardized for gestational age and sex) (47).
Neurobehavioral teratogenicity
No associations between carbamazepine and significant cognitive dysfunction have been detected in controlled studies (48).
Neonatal toxicity
In two case reports, carbamazepine was associated with transient hepatic toxicity (cholestatic hepatitis [49] and direct hyperbilirubinemia [50]) in neonates exposed to the drug during pregnancy. The hepatic dysfunction resolved after cessation of breast-feeding in both cases.
Use during pregnancy
Most experts feel carbamazepine should be used during pregnancy only if other options are lacking. As with valproate, an unplanned pregnancy may not be confirmed until the woman is in the highest risk period for a teratogenic effect. For women who continue treatment, fetal serum levels of carbamazepine are 50%–80% of maternal levels (51). Some studies show that serum levels during pregnancy remain constant for the main compound and the epoxide metabolite, although not all reports concur (10). If possible, it is best to monitor unbound levels of the compound.
Women who are started on the drug after conception incur more risk of serious side effects (agranulocytosis, hepatic failure, and Stevens-Johnson syndrome) than individuals who are undergoing carbamazepine treatment at conception, since the risks are higher within the first 8 weeks after treatment is initiated (52).
Carbamazepine can cause fetal vitamin K deficiency. Since adequate levels of vitamin K are necessary for normal mid-facial growth and for the functioning of clotting factors, carbamazepine exposure in utero could increase the risk of neonatal bleeding and mid-facial abnormalities. Most experts recommend administering 20 mg/day of oral vitamin K in the last month of pregnancy in women taking carbamazepine (30, 41). Pediatricians should also administer 1 mg i.m. of vitamin K to neonates after in utero carbamazepine exposure.
Lamotrigine
Organ dysgenesis
Lamotrigine was recently approved as a maintenance therapy for bipolar disorder. Two studies have demonstrated an increased time to subsequent mood episodes in patients treated with lamotrigine (53, 54). The obstetrical outcome data contained in the Lamotrigine Pregnancy Registry maintained by GlaxoSmithKline (54) includes data from international registries and postmarketing surveillance. As of September 30, 2003, a total of 1,081 cases have been registered and 693 birth outcomes have been obtained. Nine major defects were identified in offspring of women treated with monotherapy (N=415), yielding an estimate of 2%, while the rate was 3.4% in the 278 women undergoing treatment with several anticonvulsants. These rates are similar to the general population rate for major malformations. These defects included one instance of anencephaly and two instances of ventral septal defects. Polytherapy that included valproic acid was associated with a higher rate of malformations—10.4% (7/67).
Intrauterine growth effects
The impact of lamotrigine on intrauterine growth has not been detailed.
Neurobehavioral teratogenicity
A single follow-up of 23 infants demonstrated no alterations in development at 12 months of age (55).
Neonatal toxicity
Clinicians should be aware of reports of hepatotoxicity in adults taking lamotrigine and concerns about the development of lamotrigine-related skin rash in a fetus or neonate whose antigen characteristics are different from those of the mother. An additional concern is that lamotrigine is metabolized exclusively by means of glucuronidation, a metabolic process that is very immature in the fetus and neonate. Measurement of the clearance of lamotrigine in neonates over the first 72 hours of life demonstrated only a 25% decrease relative to umbilical cord blood concentrations (56).
Use during pregnancy
The clearance of lamotrigine during pregnancy has generated attention secondary to its increased use and the characteristics of its metabolic pathway. Two studies have found a significant increase in the clearance rate during pregnancy (i.e., a decrease in serum concentration) (57, 58). It is noteworthy that the rate of clearance returned to preconception levels rapidly after delivery, indicating the need for careful dose management in the early postpartum period.
Other Anticonvulsants
The utility of gabapentin for treatment of mania has not been established (59, 60) and is not discussed in this report. Selected newer anticonvulsants, including zonisamide and levetiracetam, are under investigation for use in the treatment of mania and depression.
First-Generation Antipsychotic Agents
One of the largest databases available is for the older, first-generation antipsychotic agents, although even this information is limited. Phenothiazines and butyrophenones have historically been used to treat hyperemesis gravidarum, nausea, and, less commonly, psychotic disorders in pregnant women. First-generation antipsychotic agents are often prescribed with anticholinergic agents such as benztropine mesylate. Risks associated with this adjunctive agent are covered in the section on ECT.
Organ dysgenesis
The best-studied phenothiazine is chlorpromazine, which was prescribed for hyperemesis gravidarum during the 1950s, usually in low doses (61, 62). In a case-control study of 315 exposed and 11,099 nonexposed women, investigators found a slightly higher rate of malformations (3.5% versus 1.6%) among exposed offspring (61). However, in a survey of more than 50,000 mother-child pairs that identified 142 first-trimester exposures and 284 total exposures to chlorpromazine, there was no elevation in the rate of physical malformations with chlorpromazine (62). The latter study also suggests that related compounds, including trifluoperazine, perphenazine, and prochlorperazine, are similarly not associated with higher-than-expected rates of malformations (62).
In one of the few studies to assess antipsychotic use in pregnant psychotic women, 52 women who took chlorpromazine throughout pregnancy were compared with 110 pregnant women with psychosis who were not exposed to chlorpromazine (63). The rates of malformations among offspring in the two groups were similar but approximately twice the rate in the general population. This finding suggests that the higher rate of anomalies may be influenced by the underlying illness. Genetic factors or behaviors such as smoking, substance abuse, and poor prenatal care may account for the higher rate of malformations.
A few case reports have suggested an association between haloperidol and limb reductions, but large case series have not supported this finding (64). A meta-analytic study of first-trimester exposure to low-potency neuroleptics found an increase of one case of malformation for every 250 pregnancies in which exposure occurred (26).
Neonatal toxicity
A withdrawal-emergent syndrome (irritability, tongue thrusting with feeding difficulty, abnormal hand posturing, and tremor of all extremities) lasting 6 months was described in an infant exposed to haloperidol during pregnancy (65). Extrapyramidal symptoms, including hypertonicity, tremors, motor restlessness, spasticity, and difficulty with feeding, have been found in infants exposed to chlorpromazine (66). Such symptoms have been reported to last up to 10 months, but most resolve within days. On the other hand, hypotonicity can occur in the neonate if the mother’s dose of chlorpromazine is high (67). Some of these complications may have been related to use of concomitant anticholinergic and antihistaminergic agents.
Behavioral teratogenicity
Investigations did not find diminished intelligence among 4-year-old progeny exposed to phenothiazines or butyrophenones (62). Children with and without histories of neuroleptic exposure showed no differences in behavioral functioning or IQ when followed up to 5 years of age (68).
Use during pregnancy
First-generation antipsychotic agents continue to have a role in the acute treatment of mania during pregnancy (69). Some experts consider the risk associated with first-generation antipsychotic agents, which have been available for decades, to be less than the risk associated with selected mood stabilizers (26). Clinicians may elect to switch a patient’s medication from lithium or an anticonvulsant to a first-generation antipsychotic either for the entire pregnancy or for the first trimester. This strategy is particularly useful for patients who have benefited from mood stabilization with antipsychotic medications in the past. First-generation antipsychotic medications may also be a choice for women with bipolar disorder who elect to discontinue medication during pregnancy but begin to experience a recurrence of symptoms while pregnant.
Second-Generation Antipsychotic Agents
Several second-generation antipsychotics, including quetiapine and risperidone, are currently under review but have not been approved by the FDA for acute and maintenance treatment of bipolar disorder. Olanzapine recently received approval from the FDA for treatment of acute mania, but experience with this drug in pregnancy is limited.
Organ dysgenesis
Olanzapine was not associated with malformations in several case reports and series (70, 71).
Neonatal toxicity
No data exist on neonatal toxicity.
Use in pregnancy
Data are limited, but olanzapine has been associated with weight gain, insulin resistance (72), gestational diabetes (73), and preeclampsia (73). Weight gain, blood sugar levels, and blood pressure should be monitored carefully in patients who are taking olanzapine.
Calcium Channel Blockers
The effects of calcium channel blockers, such as verapamil, in the treatment of bipolar disorder have been studied (74–76), although their efficacy remains unproven (77). Because verapamil is used to treat maternal hypertension and fetal arrhythmias, the effects on the fetus of exposure during the first trimester have been evaluated. In a controlled study, two of 66 subjects exposed to nifedipine or verapamil developed malformations, compared to none of a group of nonexposed comparison subjects, but the difference was not significant (p=0.27), and the study had sufficient power to rule out only a very large (fivefold) difference in risk (78). A higher rate of preterm delivery (28% versus 9% in the control group) (p=0.0003) was ascribed to maternal disease for which the drugs were prescribed. In two therapeutic trials of verapamil among hypertensive pregnant women, no adverse drug-related effects were observed among infants (79).
Benzodiazepines and Other Sedative Hypnotic Agents
Benzodiazepines are used commonly as adjunctive medications for mood stabilization or for anxiety, agitation, and sleep problems. The most commonly prescribed benzodiazepines for individuals with bipolar disorder are lorazepam and clonazepam.
Organ dysgenesis
There are no reports of malformations associated with lorazepam or clonazepam. The reproductive safety of the prototype drug in this class, diazepam, is controversial. Early reports described an increased risk of oral clefts after first-trimester exposure to drugs such as diazepam (80), but later studies have not supported this association (81, 82). A recent meta-analysis found an association between oral cleft and benzodiazepine use only among case-control studies (odds ratio=1.79, 95% confidence interval=1.13–2.82) but not in cohort studies (83). The difference in findings among studies is probably due to the greater sensitivity of case-control studies in analyzing events that are rare. Results from case-control studies of the relationship between benzodiazepine exposure and cleft lip or palate have suggested a risk rate of about 11:10,000 births, an increase of about 80% over the base risk rate of 6:10,000 births in the general population, but still a rate that yields rare events (83).
Use during pregnancy
Although the risk of physical anomalies with benzodiazepines is not greatly elevated, it may be even less for the high-potency agents in this class. The high-potency compounds may also be preferable, since they have shorter half-lives, have less accumulation, and are less likely to cause sedation.
Sleep dysregulation may be a potent trigger for illness recurrence in bipolar disorder. This idea is supported by one study that found a high rate of manic relapse among fathers with bipolar disorder during the postpartum period (84). Benzodiazepines are useful for regulating sleep and may help in prophylaxis against postpartum recurrence of manic episodes.
Intrauterine growth retardation
Intrauterine growth retardation associated with diazepam use has been reported (85), but complications with lorazepam or clonazepam have not been described.
Neonatal toxicity
Acute side effects from benzodiazepines occur in association with therapy near term. Case reports have described impaired temperature regulation, apnea, lower Apgar scores at both 1 and 5 minutes, muscular hypotonia, and failure to feed (10).
Infants born to mothers who chronically used benzodiazepines may evidence withdrawal symptoms, including tremor, irritability, diarrhea, vomiting, vigorous sucking, and hypertonicity (10). In one small study of a series of infants whose mothers (N=39) had taken clonazepam (0.5–3.5 mg/day) for treatment of panic disorder during pregnancy, neonatal toxicity was not found (86).
Behavioral teratogenicity
Systematically derived data on the long-term neurobehavioral effects of benzodiazepine exposure are sparse. Motor and developmental delays have been reported, although these reports have been criticized for having significant ascertainment biases (87). Other investigators have found no association between benzodiazepine exposure and developmental delay (88).
ECT
When used in pregnant patients, ECT has relatively few side effects and may pose fewer risks than untreated mood episodes or pharmacotherapy with a teratogenic agent.
Organ dysgenesis
While there have been occasional reports of congenital malformations in offspring exposed to ECT in utero (89), neither the number nor the pattern of these findings implicates ECT as a causal factor.
Use in pregnancy
Overall, reported complications of ECT during pregnancy are uncommon and transient (90). Barbiturates and atropine can reduce beat-to-beat variability in the fetal heart rate, and atropine can cause fetal tachycardia. The risk of fetal cardiac arrhythmias can be minimized by avoiding atropine, ensuring adequate oxygenation, avoiding excessive hyperventilation, and elevating the right hip. Fetal cardiac monitoring during ECT will allow for detection of arrhythmias and correction of any contributory problems.
Uterine smooth muscle does not routinely contract during a seizure. However, a few cases of uterine contractions have been observed after ECT (91). These contractions did not usually lead to premature labor (90). Patients who are malnourished or dehydrated may be more vulnerable to such contractions. For high-risk patients and/or for patients who cannot reliably recognize and articulate their bodily sensations, uterine tocodynamometry can be used to register uterine contractions.
Seizure threshold is decreased by estrogen and increased by progesterone. Changes in the ratio of estrogen to progesterone during pregnancy can theoretically alter seizure threshold, thereby altering the optimal stimulus parameters for administering ECT. The first treatment should be initiated with standard stimulus parameters, and subsequent treatments should be adjusted accordingly.
During pregnancy, prolonged gastric emptying time increases the risk of gastric regurgitation and aspiration pneumonitis during anesthesia. The patient can be intubated to prevent these complications. However, weight gain, hypervascularity, and edema increase the likelihood that bleeding will occur during intubation (92). The increased likelihood of bleeding can be addressed by using a small (e.g., pediatric-sized) cuffed endotracheal tube, laryngoscope, and laryngoscope blade. Alternately, many anesthesiologists choose not to intubate a pregnant patient during repeated ECT treatments, but instead decrease the risk of pneumonitis by raising gastric pH by administering a nonparticulate antacid, such as sodium citrate, before the procedure (92).
Anticholinergic agents, such as atropine and glycopyrrolate, are often given to nonpregnant patients before ECT to decrease secretions and prevent bradycardia. Both of these agents decrease lower esophageal sphincter tone, increasing the risk of gastric reflux. Therefore, neither agent is recommended for routine use during pregnancy.
Intrauterine growth effects
No intrauterine growth effects have been reported.
Neonatal toxicity
No instances of neonatal toxicity have been described.
Neurobehavioral teratogenicity
A few case reports have described developmental delays or mental retardation in offspring exposed to ECT in utero (89). However, neither the number nor the pattern of these reports suggests a causal relationship between ECT and the developmental delays. No systematic, long-term follow-up studies of neurobehavioral parameters have been conducted in offspring whose mothers received ECT during pregnancy.
Psychosocial Interventions
Psychotherapies for the manic pole of bipolar disorder focus primarily on increasing adherence to treatment with medication, improving functioning in social and occupational domains, minimizing sleep deprivation (since sleep deprivation can precipitate mania [93]), and preventing relapse. Theoretically, improved adherence to the medication regimen and strategies to recognize and avoid triggers of manic episodes (relapse prevention) may lead to a decrease in the number of manic episodes and an increase in the length of interepisode intervals. However, little is known about the direct or indirect effect of nonpharmacological interventions on mania, and no controlled clinical trials have evaluated these strategies during pregnancy. Structured daily activities, which help minimize sleep deprivation and reduce mood lability, are particularly important during pregnancy.
Treatment Planning for the Pregnant Patient With Bipolar Disorder
Preconception
Optimal treatment planning for women with bipolar disorder emphasizes overall care to promote preconception and prenatal health. Clinicians should not focus on psychotropic medication while ignoring other risk factors for poor perinatal outcome, such as obesity, smoking, and the use of alcohol or other substances of abuse. Healthy behavior, including adherence to a prenatal vitamin regimen and to a schedule of prenatal care visits, maintenance of a healthy diet, and attendance at childbirth preparation classes, must be supported.
Treatment planning is critical for minimizing the risk to the mother and fetus while limiting the morbidity from active psychiatric illness. Ideally, discussions about treatment planning should occur before the patient becomes pregnant and while the patient is euthymic. These early discussions reduce the risk for abrupt changes in therapeutic approach in the midst of an unplanned pregnancy. Informed choices coupled with close psychiatric follow-up and coordinated care with the obstetrician are the elements of an optimal model for the management of psychiatric disorders during pregnancy (9).
The most important clinical factors that influence treatment planning during pregnancy are illness history and the reproductive risks of medications. Historical factors that should be identified include the patient’s prior response to various medications, illness severity, duration of euthymia while taking medication and while not taking medication, time to relapse after medication discontinuation, and time to recover with reintroduction of pharmacotherapy.
The clinician and patient need to decide if the patient requires medication during the period before conception and during the first trimester. Stable patients may be able to discontinue taking a mood stabilizer before attempting to conceive (2, 6). Discontinuation of a maintenance pharmacologic treatment is associated with high rates of relapse, especially if discontinuation occurs abruptly, and thus the medication should be tapered slowly (94). Given the difficulty of predicting the amount of time a woman will require to conceive, a woman who discontinues medication while trying to become pregnant may be free of prophylactic treatment for many months and may be in a precarious position with regard to her risk of manic relapse. A pregravid trial of a medication taper allows the clinician and patient to assess the patient’s response and plan accordingly. Recurrence of symptoms may prompt the woman to consider the difficult choice to continue taking medication during pregnancy.
Treatment of women with severe illness presents the greatest challenge. These women are likely to continue taking medication through conception. Some women may await early documentation of pregnancy before they discontinue taking medication, and this course of action affords prophylaxis for the longest period of time up to and around the time of conception. Caution is required with some compounds, such as valproate (see earlier discussion), since teratogenic risk is greatest when exposure occurs early in pregnancy. When pregnancy is recognized, the mood stabilizer will have to be discontinued abruptly, and the abrupt discontinuation enhances the risk for illness relapse. Adjunctive (antipsychotic) medication may be used to assist in mood stabilization while medications such as valproate are tapered, but the efficacy of this approach has not been established.
Women who elect to switch their medication to an older antipsychotic or to risperidone while they are trying to conceive face other issues. These medications increase prolactin levels, thereby decreasing menstrual cyclicity and adversely affecting fertility. Since it may take several months before a woman taking these medications conceives, an agent that has less effect on prolactin may be preferable.
Early Conception
Patients who discontinue medication before pregnancy or during the first trimester and who remain well may or may not decide to restart medication later in the pregnancy. While the best option for some patients is to reintroduce medication only with early signs of relapse, other patients may opt to reinstitute medication regardless of whether relapse seems imminent. If the patient’s history includes self-harm, protracted recovery time, impaired insight, or evidence that her support system cannot tolerate another episode, pharmacological treatment may reduce overall risk to both mother and fetus.
For women who are required to or prefer to continue taking medication during the first trimester, it is important to emphasize that exposure to one psychotropic medication may be safer than exposure to multiple agents (95). The lowest effective dose of a medication must be used, and agents with the least teratogenic potential should be selected in preference to those that pose a higher risk. However, an agent known to be effective for a particular patient has already been proven useful in treating the disease, which may justify exposure to the drug for that patient. Older agents with case and cohort data are preferable, since drugs are not tested in pregnant women and assessment of the reproductive toxicity of a newer agent is often delayed for years after its introduction.
Second and Third Trimester
The periods of highest risk for teratogenicity are during the first trimester. However, other perinatal risks are related to later exposure. They include risks for minor malformations, behavioral effects, low birth weight, and preterm delivery. Given how little is known about these risks and about whether mood-stabilizing agents affect these risks, it is difficult to make recommendations. However, for a woman who is doing well, changing medication for the purpose of avoiding theoretical risks to potential offspring may place the woman’s current stability at risk and may not be the most prudent course.
Unplanned Pregnancy
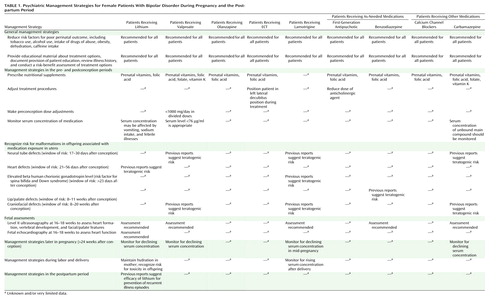
A planned conception represents the ideal for women with bipolar disorder, but only about 50% of pregnancies are planned (88). In many instances, recognition of pregnancy will occur during or after the peak risk period for some agents. Discontinuation of the medication at that point may place the woman’s clinical well-being at risk and confer minimal benefit. The patient’s stability, week of pregnancy, psychotropic agent, and treatment preferences should be considered in adjusting the treatment plan. In addition, a higher dose of folic acid (3 mg/day) should be prescribed for the woman. Principles of streamlining medication and using the minimal effective dose can be useful treatment approaches in this scenario. Management strategies are summarized in Table 1.
Treatment During the Puerperium and While Breast-Feeding
Pregnant women with bipolar disorder should be informed about the risk for relapse as they enter the postpartum period. The well-documented high rate of recurrence in this period underscores the need to consider prophylactic intervention (7). Medication prophylaxis during the immediate postpartum period should be considered, although data to support this practice are available only for treatment with lithium. Lithium postpartum prophylaxis has been found to reduce the rate of relapse from near 50% to less than 10% (96, 97). Since many women prefer to breast-feed, information about the use of mood stabilizers during breast-feeding is also needed.
Lithium
Lithium is secreted into breast milk and levels achieved are nearly half of maternal serum levels (19). The diminished renal clearance in neonates can elevate serum levels of lithium. In a study of breast-fed offspring of women who were taking lithium, Schou and Amdisen (19) reported that lithium concentrations were detectable in the sera of neonates in all cases and were equivalent to concentrations in breast milk (ranging from 0.1 mmol/liter to 0.6 mmol/liter). One concern regarding appreciable lithium levels is the propensity for rapid dehydration in neonates with febrile illnesses. Another consideration is that the longer-term effects on the infant of sustained lithium levels are not known. The American Academy of Pediatrics recommends that breast-feeding be undertaken with caution by women undergoing lithium treatment (98). In a breast-fed infant exposed to lithium, lithium serum concentrations and the CBC should be monitored.
Valproate
Serum concentrations of valproate found in breast-fed neonates whose mothers took valproate during pregnancy ranged from 4% to 40% of maternal levels but declined from the time of birth (99). Breast-fed infants whose mothers did not take valproate during pregnancy had substantially lower serum concentrations (6% or less of maternal serum levels) (9, 100). No adverse effects have been reported among infants whose mothers were treated with valproate solely during breast-feeding (100). The American Academy of Neurology advocates breast-feeding for mothers maintained on antiepileptic agents (101).
Carbamazepine
Concentrations of carbamazepine in breast milk are detectable but low. Most data on serum concentrations of carbamazepine in breast-feeding infants have been based on measurements in offspring of mothers who took the drug during pregnancy. Serum concentrations in such infants ranged from 6% to 65% of maternal levels (102). The American Academy of Neurology supports breast-feeding while undergoing carbamazepine treatment in selected cases (101). The American Academy of Pediatrics committee on medications in breast-feeding lists both valproic acid and carbamazepine as compatible with breast-feeding (98).
Lamotrigine
Lamotrigine is excreted into human breast milk with a milk/plasma ratio of 0.61. Serum concentrations in nursing infants (N=10) were approximately 30% of maternal serum concentrations. No adverse effects were noted in this study, but the authors recommended that the baby be monitored for rash (103).
First-Generation Antipsychotic Agents
Women with bipolar disorder who relapse during the immediate postpartum period (or the third trimester) may require antipsychotic medications. Ten reports have addressed a total of 28 infants’ exposure to antipsychotic agents through nursing (99). In the majority of cases, no adverse events were observed.
Benzodiazepines
Like other psychotropic medications, benzodiazepines are secreted into breast milk. Although complications associated with lorazepam and clonazepam have not been described, one case report has associated diazepam with sedation in the neonate (104).
Conclusions
Bipolar disorder is common among women of childbearing age. Experts agree that acute and maintenance management of bipolar disorder requires somatic prophylaxis. Unfortunately, a number of the medications used to treat acute mania and prevent episodes of depression and mania are associated with structural teratogenicity. On the other hand, there is scant evidence of enduring neurobehavioral teratogenicity associated with these agents, although the data are extremely limited. This document outlines available data on the risks and benefits of treatment and summarizes management strategies. Since treatment can be managed most effectively if pregnancy is planned, clinicians should discuss the issue of pregnancy and its management with every patient with bipolar disorder who has childbearing potential, regardless of future reproductive plans.
Managing bipolar disorder during pregnancy can also be enhanced by additional research. Such an agenda might include studies that examine 1) the sleep of women with bipolar disorder during the childbearing years; 2) the maternal and fetal effects of agents used during pregnancy, including detailed gestational timing and exposure analyses; 3) novel therapies, including calcium channel blockers, omega-3 fatty acids, rapid transcranial magnetic stimulation, and light therapy, for women who cannot tolerate or refuse agents with known efficacy; and 4) ways in which professional education can be enhanced to improve the likelihood that pregnant women with bipolar disorder will receive appropriate care. If such explorations are supported, it is likely that they will shed more light on the disorder and benefit all patients with bipolar illness.
 |
Received March 31, 2003; revision received Aug. 5, 2003; accepted Aug. 9, 2003. From the Department of Psychiatry, Yale University School of Medicine; the Departments of Psychiatry, Obstetrics and Gynecology, and Pediatrics, University of Pittsburgh, Pittsburgh; Western Psychiatric Institute and Clinic, University of Pittsburgh, Pittsburgh; the Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta; the Pediatrics and Developmental Neuropsychiatry Branch, Mood and Anxiety Disorders Program, National Institute of Mental Health, Bethesda, Md.; the Department of Psychiatry, Harvard Medical School and Massachusetts General Hospital, Boston; the Department of Psychiatry, University of Illinois at Chicago, Chicago; the Department of Psychiatry and Behavioral Science, Stanford University, Stanford, Calif.; the Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas; and the Department of Psychiatry, David Geffen School of Medicine at the University of California, Los Angeles. Address reprint requests to Dr. Yonkers, Department of Psychiatry, Yale University School of Medicine, 142 Temple St., Suite 301, New Haven, CT 06510; [email protected] (e-mail). The authors thank the Bipolar Disorder Subcommittee of the American Psychiatric Association Corresponding Committee on Research on Psychiatric Treatments for its support.
1. Leibenluft E: Women with bipolar illness: clinical and research issues. Am J Psychiatry 1996; 153:163–173Link, Google Scholar
2. Grof P: Protective effect of pregnancy in women with lithium-responsive bipolar disorder. J Affect Disord 2000; 61:31–39Crossref, Medline, Google Scholar
3. Kendell RE, Chalmers JC, Platz C: Epidemiology of puerperal psychoses. Br J Psychiatry 1987; 150:662–673Crossref, Medline, Google Scholar
4. Pugh TF, Jerath BK, Schmidt WM, Reed RB: Rates of mental disease related to childbearing. N Engl J Med 1963; 268:1224–1228Crossref, Medline, Google Scholar
5. Terp IM, Mortensen PB: Postpartum psychoses: clinical diagnoses and relative risk of admission after parturition. Br J Psychiatry 1998; 172:521–526Crossref, Medline, Google Scholar
6. Freeman M, Smith K, Freeman S, McElroy S, Kmetz G, Wright R, Keck P: The impact of reproductive events on the course of bipolar disorder in women. J Clin Psychiatry 2002; 63:284–287Crossref, Medline, Google Scholar
7. Viguera AC, Nonacs R, Cohen LS, Tondo L, Murray A, Baldessarini RJ: Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry 2000; 157:179–184Link, Google Scholar
8. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Bipolar Disorder (Revision). Am J Psychiatry 2002; 159(April suppl)Google Scholar
9. Wisner KL, Zarin D, Holmboe E, Appelbaum P, Gelenberg AJ, Leonard HL, Frank E: Risk-benefit decision making for treatment of depression during pregnancy. Am J Psychiatry 2000; 157:1933–1940Link, Google Scholar
10. Briggs G, Freeman R, Yaffe S: Drugs in Pregnancy and Lactation. Philadelphia, Lippincott Williams & Wilkins, 2002Google Scholar
11. Moncrieff J: Lithium revisited: a re-examination of the placebo-controlled trials of lithium prophylaxis in manic-depressive disorder. Br J Psychiatry 1995; 167:569–574Crossref, Medline, Google Scholar
12. Schou M, Goldfield MD, Weinstein MR, Villeneuve A: Lithium and pregnancy, I: report from the Register of Lithium Babies. Br Med J 1973; 2:135–136Crossref, Medline, Google Scholar
13. Nora JJ, Nora AH, Toews WH: Lithium, Ebstein’s anomaly, and other congenital heart defects (letter). Lancet 1974; 2:594–595Crossref, Medline, Google Scholar
14. Edmonds LD, Oakley GP: Ebstein’s anomaly and maternal lithium exposure during pregnancy. Teratology 1990; 41:551–552Google Scholar
15. Jacobson SJ, Jones K, Johnson K, Ceolin L, Kauer P, Sahn K, Konnenfeld AE, Rieder M, Santelli R, Smythe J, Pastuszak A, Einarson T, Koren G: Prospective multicentre study of pregnancy outcome after lithium exposure during first trimester. Lancet 1992; 339:530–533Crossref, Medline, Google Scholar
16. Cohen LS, Friedman JM, Jefferson JW, Johnson EM, Weiner ML: A reevaluation of risk of in utero exposure to lithium. JAMA 1994; 271:146–150Crossref, Medline, Google Scholar
17. Schou M: What happened later to the lithium babies? a follow-up study of children born without malformations. Acta Psychiatr Scand 1976; 54:193–197Crossref, Medline, Google Scholar
18. Ananth J: Side effects in the neonate from psychotropic agents excreted through breast-feeding. Am J Psychiatry 1978; 135:801–805Link, Google Scholar
19. Schou M, Amdisen A: Lithium and pregnancy, 3: lithium ingestion by children breast-fed by women on lithium treatment. Br Med J 1973; 2:138Crossref, Medline, Google Scholar
20. Mizrahi EM, Hobbs JF, Goldsmith DI: Nephrogenic diabetes insipidus in transplacental lithium intoxication. J Pediatr 1979; 94:493–495Crossref, Medline, Google Scholar
21. Nars PW, Girard J: Lithium carbonate intake during pregnancy leading to large goiter in a premature infant. Am J Dis Child 1977; 131:924–925Medline, Google Scholar
22. Goldfield MD, Weinstein MR: Lithium carbonate in obstetrics: guidelines for clinical use. Am J Obstet Gynecol 1973; 116:15–22Crossref, Medline, Google Scholar
23. Schou M, Amdisen A, Steenstrup OR: Lithium and pregnancy, II: hazards to women given lithium during pregnancy and delivery. Br Med J 1973; 2:137–138Crossref, Medline, Google Scholar
24. MacKay AVP, Loose R, Glen AIM: Labour on lithium. Br Med J 1976; 1:878Crossref, Medline, Google Scholar
25. Weinstein MR: Lithium treatment of women during pregnancy and in the post-delivery period, in Handbook of Lithium Therapy. Edited by Johnson FN. Baltimore, University Park Press, 1980, pp 421–429Google Scholar
26. Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J: Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153:592–606Link, Google Scholar
27. Crawford P, Appleton R, Betts T, Duncan J, Guthrie E, Morrow J: Best practice guidelines for the management of women with epilepsy. Seizure 1999; 8:201–217Crossref, Medline, Google Scholar
28. Jones KL, Lacro RV, Johnson KA, Adams J: Pattern of malformations in the children of women treated with carbamazepine during pregnancy. N Engl J Med 1989; 320:1661–1666Crossref, Medline, Google Scholar
29. Lindhout D, Omtzigt J: Teratogenic effects of antiepileptic drugs: implications for the management of epilepsy in women of childbearing age. Epilepsia 1994; 35:S19-S28Google Scholar
30. Delgado-Escueta A, Janz D: Consensus guidelines: preconception counseling, management, and care of pregnant women with epilepsy. Neurology 1992; 42:149–160Crossref, Medline, Google Scholar
31. Karceski S, Morrell M, Carpenter D: The expert consensus guideline series: treatment of epilepsy. Epilepsy Behav 2001; 2:A1-A50Google Scholar
32. Nakane Y, Okuma T, Takahashi R: Multi-institutional study on teratogenicity and fetal toxicity of antiepileptic drugs: a report of a collaborative study group in Japan. Epilepsia 1980; 21:663–680Crossref, Medline, Google Scholar
33. Omtzigt J: Prenatal diagnosis of spina bifida aperta after first-trimester valproate exposure. Prenat Diagn 1992; 12:893–897Crossref, Medline, Google Scholar
34. Kennedy D, Koren G: Valproic acid use in psychiatry: issues in treating women of reproductive age. J Psychiatry Neurosci 1998; 23:223–228Medline, Google Scholar
35. Omtzigt J: The disposition of valproate and its metabolites in the late first trimester and early second trimester of pregnancy in maternal serum, urine, and amniotic fluid: effect of dose, co-medication, and the presence of spina bifida. Eur J Clin Pharmacol 1992; 43:381–388Crossref, Medline, Google Scholar
36. Jager-Roman E: Fetal growth, major malformations, and minor anomalies in infants born to women receiving valproic acid. J Pediatr 1986; 108:997–1004Crossref, Medline, Google Scholar
37. DiLiberti J, Farndon P, Dennis N, Curry C: The fetal valproate syndrome. Am J Med Genet 1984; 19:473–481Crossref, Medline, Google Scholar
38. Felding I, Rane A: Congenital liver damage after treatment of mother with valproic acid and phenytoin. Acta Paediatr Scand 1984; 73:565–568Crossref, Medline, Google Scholar
39. Thisted E, Ebbesen F: Malformations, withdrawal manifestations, and hypoglycaemia after exposure to valproate in utero. Arch Dis Child 1993; 69:288–291Crossref, Medline, Google Scholar
40. Majer R, Green P: Neonatal afibrinogenaemia due to sodium valproate. Lancet 1987; 2:740–741Crossref, Medline, Google Scholar
41. American Academy of Neurology: Practice parameter: management issues for women with epilepsy (summary statement): report of the Quality Standard Subcommittee of the American Academy of Neurology. Neurology 1998; 51:944–948Crossref, Medline, Google Scholar
42. Nau H, Rating D, Koch S, Hauser I, Helge H: Valproic acid and its metabolites: placental transfer, neonatal pharmacokinetics, transfer via mother’s milk and clinical status in neonates of epileptic mothers. J Pharmacol Exp Ther 1981; 219:768–777Medline, Google Scholar
43. Crawford P, Appleton R, Betts T, Duncan J, Guthrie E, Morrow J (Women With Epilepsy Guidelines Development Group): Best practice guidelines for the management of women with epilepsy. Seizure 1999; 8:201–217Crossref, Medline, Google Scholar
44. Rosa FW: Spina bifida in infants of women treated with carbamazepine during pregnancy. N Engl J Med 1991; 324:674–677Crossref, Medline, Google Scholar
45. Lindhout D: Antiepileptic drugs and teratogenesis in two consecutive cohorts: changes in prescription policy paralleled by changes in pattern of malformations. Neurology 1992; 42:94–110Medline, Google Scholar
46. Diav-Citrin O, Shechtman S, Arnon J, Ornoy A: Is carbamazepine teratogenic? a prospective controlled study of 210 pregnancies. Neurology 2001; 57:321–324Crossref, Medline, Google Scholar
47. Hiilesmaa VK, Teramo K, Granstrom ML, Bardy AH: Fetal head growth retardation associated with maternal antiepileptic drugs. Lancet 1981; 2:165–167Crossref, Medline, Google Scholar
48. Scolnick D: Neurodevelopment of children exposed in utero to phenytoin and carbamazepine monotherapy. JAMA 1994; 271:767–770Crossref, Medline, Google Scholar
49. Frey B, Schubiger G, Musy J: Transient cholestatic hepatitis in a neonate associated with carbamazepine exposure during pregnancy and breastfeeding. Eur J Pediatr 1990; 150:136–138Crossref, Medline, Google Scholar
50. Merlob P, Mor N, Litwin A: Transient hepatic dysfunction in an infant of an epileptic mother treated with carbamazepine during pregnancy and breastfeeding. Ann Pharmacother 1992; 26:1563–1565Crossref, Medline, Google Scholar
51. Nau H, Kuhnz W, Egger H, Rating D, Hedge H: Anticonvulsants during pregnancy and lactation: transplacental, maternal and neonatal pharmacokinetics. Clin Pharmacokinet 1982; 6:508–543Crossref, Google Scholar
52. Rzany B, Correia O, Kelly JP, Naldi L, Auquier A, Stern R: Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis during first weeks of antiepileptic therapy: a case-control study. Lancet 1999; 353:2190–2194Crossref, Medline, Google Scholar
53. Calabrese J, Suppes T, Bowden C, Sachs G, Swann A, McElroy S, Kusumakar V, Ascher J, Earl N, Greene P, Monaghan E: A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. J Clin Psychiatry 2000; 61:841–850Crossref, Medline, Google Scholar
54. Calabrese JR, Shelton MD, Rapport DJ, Kimmel SE, Elhaj O: Long-term treatment of bipolar disorder with lamotrigine. J Clin Psychiatry 2002; 63(suppl 10):18–22Google Scholar
55. Mackay F, O’Brien T, Hitchcock A: Safety of long-term lamotrigine in epilepsy. Epilepsia 1997; 38:881–886Crossref, Medline, Google Scholar
56. Ohman I, Vitols S, Tomson T: Lamotrigine in pregnancy, pharmacokinetics during delivery, in the neonate, and during lactation. Epilepsia 2000; 41:709–713Crossref, Medline, Google Scholar
57. Tran TA, Leppik IE, Blesi K, Sathanandan ST, Remmel R: Lamotrigine clearance during pregnancy. Neurology 2002; 59:251–255Crossref, Medline, Google Scholar
58. Pennel PB, Montgomery JQ, Clements SD, Newport DJ: Lamotrigine clearance markedly increases during pregnancy (abstract). Epilepsia 2002; 43(suppl 7):234Google Scholar
59. Pande A: Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000; 20:467–471Crossref, Medline, Google Scholar
60. Frye M: A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000; 20:607–617Crossref, Medline, Google Scholar
61. Rumeau-Rouquette C, Goujard J, Huel G: Possible teratogenic effect of phenothiazines in human beings. Teratology 1976; 15:57–64Crossref, Google Scholar
62. Slone D, Siskind V, Heinonen OP, Monson RR, Kaufman DW, Shapiro S: Antenatal exposure to the phenothiazines in relation to congenital malformations, perinatal mortality rate, birth weight, and intelligence quotient score. Am J Obstet Gynecol 1977; 128:486–488Crossref, Medline, Google Scholar
63. Sobel D: Fetal damage due to ECT, insulin coma, chlorpromazine or reserpine. Arch Gen Psychiatry 1960; 2:606–611Crossref, Google Scholar
64. Van Waes A, Van de Velde E: Safety evaluation of haloperidol in the treatment of hyperemesis gravidarum. J Clin Pharmacol 1969; 9:224–227Google Scholar
65. Sexson WR, Barak Y: Withdrawal emergent syndrome in an infant associated with maternal haloperidol therapy. J Perinatol 1989; 9:170–172Medline, Google Scholar
66. Ayd F: Is psychiatry in a crisis because of the complications of the psychopharmaceuticals? Dis Nerv Syst 1968; 29:23–25Medline, Google Scholar
67. Hammond J, Toseland P: Placental transfer of chlorpromazine. Arch Child Dis 1970; 45:139–140Crossref, Medline, Google Scholar
68. Edlund MJ, Craig TJ: Antipsychotic drug use and birth defects: an epidemiologic reassessment. Compr Psychiatry 1984; 25:32–37Crossref, Medline, Google Scholar
69. McElroy S, Keck P, Stanton S, Tugrul K, Bennett J, Strakowski S: A randomized comparison of divalproex oral loading versus haloperidol in the initial treatment of acute psychotic mania. J Clin Psychiatry 1996; 57:142–146Medline, Google Scholar
70. Goldstein D: Olanzapine-exposed pregnancies and lactation: early experience. J Clin Psychopharmacol 2000; 20:399–403Crossref, Medline, Google Scholar
71. Littrell KH, Johnson CG, Peabody CD, Hilligoss N: Antipsychotics during pregnancy (letter). Am J Psychiatry 2000; 157:1342Link, Google Scholar
72. Dickson R: Olanzapine and pregnancy. Can J Psychiatry 1998; 43:196–197Medline, Google Scholar
73. Kirchheiner J, Berghofer A, Bolk-Weischedel D: Healthy outcome under olanzapine treatment in a pregnant woman. Pharmacopsychiatry 2000; 33:78–80Crossref, Medline, Google Scholar
74. Dose M, Emrich HM, Cording-Tommel C, von Zerssen D: Use of calcium antagonists in mania. Psychoneuroendocrinology 1986; 11:241–243Crossref, Medline, Google Scholar
75. Dubovsky SL, Franks RD, Allen S, Murphy J: Calcium antagonists in mania: a double-blind study of verapamil. Psychiatry Res 1986; 18:309–320Crossref, Medline, Google Scholar
76. Janicak PG, Sharma RP, Pandey G, Davis JM: Verapamil for the treatment of acute mania: a double-blind, placebo-controlled trial. Am J Psychiatry 1998; 155:972–973Link, Google Scholar
77. Wisner K, Peindl K, Perel J, Hanusa B, Piontek C, Baab S: Verapamil treatment for women with bipolar disorder. Biol Psychiatry 2002; 51:745–752Crossref, Medline, Google Scholar
78. Magee L, Schick B, Donnenfeld A, Sage R, Conover B, Cook L, McElhatton P, Schmidt M, Koren G: The safety of calcium channel blockers in human pregnancy: a prospective multicenter study. Am J Obstet Gynecol 1996; 174:823–828Crossref, Medline, Google Scholar
79. Marlettini M, Cassani A, Morselli-Labate A, Crippa S, Contarini A, Miniero R, Plate L, Orlandi C: Maternal and fetal prolactin in pregnancy-induced hypertension. Arch Gynecol Obstet 1990; 247:73–81Crossref, Medline, Google Scholar
80. Aarskog D: Association between maternal intake of diazepam and oral clefts (letter). Lancet 1975; 2:921Crossref, Medline, Google Scholar
81. Heinonen OP, Slone D, Shapiro S: Birth Defects and Drugs in Pregnancy. Littleton, Mass, Publishing Sciences Group, 1999Google Scholar
82. McElhatton PR: The effects of benzodiazepine use during pregnancy and lactation. Reprod Toxicol 1994; 8:461–475Crossref, Medline, Google Scholar
83. Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR: Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. Br Med J Clin Res Ed 1998; 317:839–843Crossref, Medline, Google Scholar
84. Davenport YB, Adland ML: Postpartum psychoses in female and male bipolar manic-depressive patients. Am J Orthopsychiatry 1982; 52:288–297Crossref, Medline, Google Scholar
85. Laegreid L, Olegard R, Wahlstrom J, Conradi N: Abnormalities in children exposed to benzodiazepines in utero (letter). Lancet 1987; 1:108–109Crossref, Medline, Google Scholar
86. Weinstock L: Obstetrical and neonatal outcome following clonazepam use during pregnancy: a case series. Psychother Psychosom 1996; 70:158–162Crossref, Google Scholar
87. Laegreid L, Hagberg G, Lundberg A: Neurodevelopment in late infancy after prenatal exposure to benzodiazepines—a prospective study. Neuropediatrics 1992; 23:60–67Crossref, Medline, Google Scholar
88. Bergman U, Rosa FW, Baum C, Wiholm BE, Faich GA: Effects of exposure to benzodiazepine during fetal life. Lancet 1992; 340:694–696Crossref, Medline, Google Scholar
89. Impastato D, Gabriel A, Lardaro H: Electric and insulin shock therapy during pregnancy. Dis Nerv Syst 1964; 25:542–546Medline, Google Scholar
90. Miller LJ: Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry 1994; 45:444–450Abstract, Google Scholar
91. Polster D, Wisner K: ECT-induced premature labor: a case report. J Clin Psychiatry 1999; 60:53–54Crossref, Medline, Google Scholar
92. Diaz JH: The physiologic changes of pregnancy have anesthetic implications for both mother and fetus, in Prenatal Anesthesia and Critical Care. Edited by Diaz JH. Philadelphia, WB Saunders, 1991, pp 210–215Google Scholar
93. Ehlers CL, Frank E, Kupfer DJ: Social zeitgebers and biological rhythms: a unified approach to understanding the etiology of depression. Arch Gen Psychiatry 1988; 45:948–952Crossref, Medline, Google Scholar
94. Suppes T, Baldessarini RJ, Faedda GL, Tohen M: Risk of recurrence following discontinuation of lithium treatment in bipolar disorder. Arch Gen Psychiatry 1991; 48:1082–1088Crossref, Medline, Google Scholar
95. Laegreid L, Kyllerman M, Hedner T, Hagberg B, Viggedahl G: Benzodiazepine amplification of valproate teratogenic effects in children of mothers with absence epilepsy. Neuropediatrics 1993; 24:88–92Crossref, Medline, Google Scholar
96. Stewart DE, Klompenhouwer JL, Kendell RE, van Hulst AM: Prophylactic lithium in puerperal psychosis: the experience of three centers. Br J Psychiatry 1991; 158:393–397Crossref, Medline, Google Scholar
97. Cohen LS, Sichel DA, Robertson LM, Heckscher E, Rosenbaum JF: Postpartum prophylaxis for women with bipolar disorder. Am J Psychiatry 1995; 152:1641–1645Link, Google Scholar
98. Gartner L: Breastfeeding and the use of human milk. Pediatrics 1997; 100:1035–1039Crossref, Medline, Google Scholar
99. von Unruh G, Froescher W, Hoffmann F, Niesen M: Valproic acid in breast milk: how much is really there? Ther Drug Monit 1984; 6:272–276Crossref, Medline, Google Scholar
100. Wisner K, Perel J: Serum levels of valproate and carbamazepine in breastfeeding mother-infant pairs. Psychopharmacology (Berl) 1998; 18:167–169Google Scholar
101. Holmes L, Harvey E, Coull B, Huntington K, Khoshbin S, Ailish M, Ryan L: The teratogenicity of anticonvulsant drugs. N Engl J Med 2001; 344:1132–1138Crossref, Medline, Google Scholar
102. Chaudron L: Mood stabilizers during breastfeeding: a review. J Clin Psychiatry 2000; 61:79–90Crossref, Medline, Google Scholar
103. Ohman I, Tomson T, Vitols S: Lamotrigine levels in plasma and breast milk in nursing women and their infants (abstract). Epilepsia 1998; 21(suppl 2):39Google Scholar
104. Patrick M, Tilstone W, Reavey P: Diazepam and breastfeeding. Lancet 1972; 1:542–543Crossref, Medline, Google Scholar



