Perception of Complex Sounds in Autism: Abnormal Auditory Cortical Processing in Children
Abstract
OBJECTIVE: The authors have previously described less activation of left speech-related temporal areas in adults with autism when listening to speech-like sounds than in normal adults. Here, they investigated whether this abnormal cortical processing was also present in children with primary autism. METHOD: Regional cerebral blood flow was measured with positron emission tomography after premedication in 11 autistic children and six nonautistic mentally retarded children during rest and while they were listening to speech-like sounds. RESULTS: As with autistic adults, direct comparison between the two groups revealed significantly less activation in the autistic group localized in left speech-related areas. CONCLUSIONS: For the first time to their knowledge, an activation study was performed in children with autism and has confirmed previous results obtained in adults. The abnormal cortical auditory processing observed in both children and adults with autism could be involved in inadequate behavioral responses to sounds and in language impairments characteristic of autism.
We have recently studied auditory cortical processing in adults with autism using complex speech-like sounds (1) and found less activation of the left temporal word processing network in autistic adults than in healthy comparison subjects. The results obtained in autistic adults suggest a dysfunction of specific temporal regions specializing in the perception and integration of complex sounds (2). Such stimuli are never recognized as speech; therefore, an abnormal pattern of activation found in autistic adults could reflect basic anomalies of prelinguistic auditory processing rather than a consequence of abnormal language development (3). Since autism is a developmental disorder, we believed it important to investigate the cortical response to sounds in children with autism. All reported activation studies in autism have been performed in adults, but no data are available for children, to our knowledge. Therefore, we performed a positron emission tomography (PET) auditory activation study in autistic children using the same speech-like stimuli previously described in the autistic adult study. Autism is associated with abnormal auditory behavior, including aversive reactions to everyday life sounds. In addition, autistic children are often initially misdiagnosed as deaf (4). An abnormal perception of speech-like sounds in childhood may account for inadequate behavioral response to sounds and thus for language impairments typical of autism.
Method
Eleven autistic children (10 boys) ages 4 to 10 years (mean=6.6, SD=1.6) with primary autism were selected. Autism was diagnosed according to DSM-IV criteria. The Autism Diagnostic Interview—Revised (5) confirmed the diagnosis: mean social interaction score=27.3 (SD=6.6); mean nonverbal communication score=11.9 (SD=3.5); mean stereotypy score=7.9 (SD=4.7); mean age-onset criteria=4.3 (SD=0.8). Four children had verbal communication; their mean score was 17 (SD=7). Their IQ or development quotient was determined with the WISC-R or Brunet-Lézine test (6). Their mean IQ or development quotient was 43 (SD=21).
Six children (four boys) ages 3 to 9 years (mean=7.2 years, SD=2.4) with idiopathic mental retardation (mean IQ or development quotient=64, SD=15) were selected as control children since regional cerebral blood flow (rCBF) studies are not performed on normal children in our institute for ethical reasons. The diagnosis of mental retardation conformed to DSM-IV criteria. No etiology has been found after extensive investigations.
We excluded from this study children with known infectious, metabolic, neurological, or genetic diseases; chromosomal abnormality; seizures; and abnormal magnetic resonance imaging (MRI).
All subjects were free of medication. An ethics committee approved this study, and examinations were performed with the written informed consent of the parents.
Relative rCBF was determined from the radioactivity distribution measured with PET (ECAT-EXACT-HR+) after bolus intravenous injections of [15O]H2O (7). The protocol included three rCBF measurements carried out in a single session, performed at 10-minute intervals during three conditions: the first at rest and the second and third during passive listening to complex speech-like sounds (1).
We used a subset of the synthetic nonverbal speech-like auditory stimuli previously published elsewhere (1, 3). These stimuli contain spectral maxima (speech-like formants) that change over time. They consist of sounds with a central 200-msec steady-state period surrounded by initial and final changes in spectral maxima frequency. Their acoustic structure was similar to consonant-vowel-consonant, but as stated, normal volunteers never recognized them as speech.
PET studies were performed during sleep induced by premedication (7 mg/kg of sodium pentobarbital) for both autistic and control children to obtain immobility.
rCBF images were analyzed by using Statistical Parametric Mapping 99 for image realignment, transformation into standard stereotactic anatomical space, smoothing, and statistical analysis (8). State-dependent differences in global flow were covaried by using proportional scaling. Comparisons across conditions were made by using the t statistic subsequently transformed into a normally distributed z statistic in a multistudy design. Two statistical comparisons were performed: 1) a within-group comparison of activation for listening to complex sounds versus resting and 2) a between-group comparison of activations.
Results
Passive listening to speech-like sounds versus resting was assessed in each group independently (z=3.67, df=28, p<0.0005). We found significant activation of the auditory cortex in the bilateral superior temporal gyrus (Brodmann’s area 22) in both groups while subjects were listening to speech-like stimuli. However, the activation pattern was different between two groups. The control children activated the superior temporal cortex bilaterally with left-biased asymmetry, as we have previously observed in normal adult comparison subjects (1). This left dominance was not observed in the autistic group. In addition, autistic children had additional significant activation outside the auditory cortex: the left temporal pole (Brodmann’s area 38), the bilateral cingulum (Brodmann’s areas 32 and 24), the bilateral posterior parietal (Brodmann’s area 19), the cerebellar hemispheres, and the brainstem.
The direct comparison showed a significant difference between the autistic and control groups in three areas in the left cortical network. The left middle temporal gyrus (Brodmann’s areas 21 and 39) and the left precentral gyrus (Brodmann’s area 43/6) (z>3.41, df=28, p<0.001) were significantly less activated in the autistic children than in the nonautistic control children (Figure 1, top). In addition, we obtained exactly the same results by performing an additional analysis and excluding the girls from the autistic and control groups. We found no region that was significantly more activated in the autistic group.
Discussion
The present findings of an abnormal cortical pattern of auditory activation in autistic children are similar to those previously described in autistic adults when using the same auditory paradigm (1). The direct comparison between the two groups of children while they were listening to speech-like stimuli revealed less activation in left speech-related areas, including Wernicke’s area, in autistic children. Similar results were found in autistic adults in relation to normal comparison adults (1). The middle and inferior temporal gyrus (Brodmann’s area 21) were significantly less activated in autistic adults than in comparison subjects. Figure 1 shows the similarity of the results obtained in children and adults with autism compared to nonautistic age-matched control subjects.
By comparing speech-like sounds to the resting state in each group independently, we also observed remarkable differences in the activation pattern between the two groups. Autistic and nonautistic children activated the temporal auditory cortex bilaterally, but the nonautistic children activated the left side more than the autistic group. This left dominance was also observed in normal adults (1). In addition, we observed in autistic children diffuse activation outside the temporal lobe, located in the bilateral cingulum, the posterior parietal, the brainstem, and the cerebellum. Thus, in autistic children, listening to complex sounds induced an abnormal cortical activation including an aberrant functional network. This may explain why autistic children have exaggerated behavioral responses to sounds, one of the most pronounced signs of autism. However, we did not observe in autistic children the abnormal right frontotemporal activation previously described in autistic adults (1). This difference may be related to relatively small group sizes in both studies or, more likely, may indicate that a marked right-side asymmetry emerges in autistic patients across their development.
To perform activation studies in typical mentally retarded autistic children, we needed to use premedication. Even under sedation, we observed in both autistic and nonautistic children a bilateral activation of the temporal auditory cortex that was induced by complex sounds. These results suggest that the sound’s perception with the subjects premedicated is effective and opens new perspectives about the passive activation paradigm in children with developmental disorder. Likewise, auditory functional MRI in pentobarbital-sedated children showed auditory cortical activation (9). Since both groups were studied under same conditions, the findings should reflect true differences. However, the present results need to be replicated in a larger well-matched population of autistic and control children.
The present results, together with the adult study, indicate that autism in both children and adults is associated with a dysfunction of specific temporal regions specialized in perception and the integration of complex sounds. The areas found to be less activated by complex sounds in autistic children (Brodmann’s areas 21 and 39) are thought to be auditory associative areas that are involved in word processing (10) and are also presumed to act as an interface between word perception and long-term representations of familiar words in memory (10, 11). In the dominant hemisphere for language, these areas play a critical role in the ability to understand and produce meaningful speech. Thus, a dysfunction of left speech-related cortical areas could be at the origin of the language developmental impairments observed in autism.
Finally, the resting bilateral temporal lobe dysfunction previously described in autistic children (12, 13) could be implicated in the developmental disorganization of temporal neural circuits leading to severe perturbation in the auditory activation pattern.
In conclusion, the present results extend to autistic children previous findings obtained in adults and are compatible with the general hypothesis of disturbances in the establishment of neural circuits in the autistic brain.
Received Dec. 4, 2003; revisions received Feb. 12 and Feb. 26, 2004; accepted March 4, 2004. From the Equipe Recherche Methodologique 0205 Institut National de la Santé et de la Recherche Médicale—Commissariat a l’Energie Atomique, Service Hospitalier Frédéric Joliot, DSV, DRM, Commissariat a l’Energie Atomique; the Service de Radiologie Pédiatrique, the Service de Neurochirurgie Pédiatrique, and the Département de Génétique Médicale, Necker Enfants Malades, Assistance Public—Hopitaux de Paris, Paris; the Service de Pédopsychiatrie, Hôpital Robert Debré, Assistance Public—Hopitaux de Paris, Paris; the Department of Psychology, Université de Montreal, Montreal; the Institut National de la Santé et de la Recherche Médicale Unité 316, CHU Bretonneau, Tours, France; and the Service des Urgences Cerebro-Vasculaires, Hôpital La Salpêtrière, Assistance Public—Hopitaux de Paris, Paris, France. Address reprint requests to Dr. Zilbovicius, Commissariat a l’Energie Atomique, Service Hospitalier Frédéric Joliot, 4 place du Général Leclerc, 91406, Orsay, France; [email protected] (e-mail). Supported by the Programme Hospitalier de Recherche Clinique, Ministère de la Santé, the Fondation France, and the Télecom and Fondation de France. The authors thank the nurses and the technical staff of the Orsay Brain Imaging Center for their assistance and the families for their cooperation.
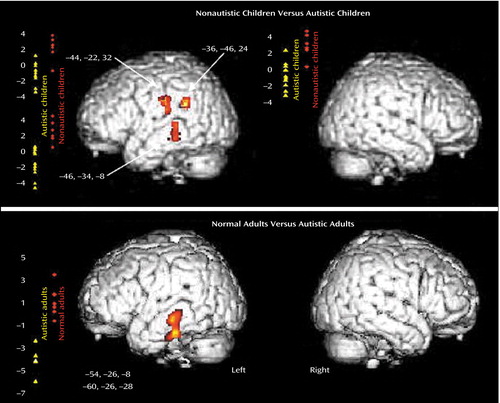
Figure 1. Abnormal Activation of Left Speech-Related Brain Areas in Children and Adults With Autisma
aResults are represented in a three-dimensional rendering of magnetic resonance imaging of the left and right surfaces of the lateral cortex. Diagrams indicate individual regional changes in cerebral blood flow (auditory activations versus rest time) for autistic subjects (yellow triangles) and nonautistic control subjects (red diamonds). Talairach coordinates of peaks are also indicated. In the present study, the left middle temporal gyrus (Brodmann’s areas 21 and 39) and the precentral frontal gyrus (Brodmann’s area 43/6) were significantly less activated in autistic children than in nonautistic control children (z=3.67, df=28, p<0.0005). In a previous study, the left middle and inferior temporal gyrus (Brodmann’s area 21) was significantly less activated in autistic adults than in normal adult comparison subjects (z=3.09, df=22, p<0.001) (1).
1. Boddaert N, Belin P, Chabane N, Poline J-B, Barthélémy C, Mouren-Simeoni M-C, Brunelle F, Samson Y, Zilbovicius M: Perception of complex sounds: abnormal pattern of cortical activation in autism. Am J Psychiatry 2003; 160:2057–2060Link, Google Scholar
2. Samson Y, Belin P, Thivard L, Boddaert N, Crozier S, Zilbovicius M: Auditory perception and language: functional imaging of speech sensitive auditory cortex. Rev Neurol 2001; 157:837–846Medline, Google Scholar
3. Thivard L, Belin P, Zilbovicius M, Poline JB, Samson Y: A cortical region sensitive to auditory spectral motion. Neuroreport 2000; 11:2969–2972Crossref, Medline, Google Scholar
4. Rapin I, Katzman R: Neurobiology of autism. Ann Neurol 1998; 43:7–14Crossref, Medline, Google Scholar
5. Lord C, Rutter M, Le Couteur A: Autism Diagnostic Interview—Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24:659–685Crossref, Medline, Google Scholar
6. Brunet O, Lezine I: Echelle de developpement psychomoteur de la premiere enfance, 2nd ed. Paris, Presses Universitaires de France, 1976Google Scholar
7. Fox PT, Mintun MA, Raichle ME, Herscovitch P: A non-invasive approach to quantitative functional brain mapping with H215O and positron emission tomography. J Cereb Blood Flow Metab 1984; 4:329–333Crossref, Medline, Google Scholar
8. Friston K, Holmes AP, Worsley KJ, Poline JB, Heather JD, Frackowiak R: Statistical parametric mapping in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210Crossref, Google Scholar
9. Altman NR, Bernal B: Brain activation in sedated children: auditory and visual functional MR imaging. Radiology 2001; 221:56–63Crossref, Medline, Google Scholar
10. Price CJ, Wise RJ, Warburton EA, Moore CJ, Howard D, Patterson K, Frackowiak RS, Friston KJ: Hearing and saying: the functional neuro-anatomy of auditory word processing. Brain 1996; 119:919–931Crossref, Medline, Google Scholar
11. Wise RJ, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA: Separate neural subsystems within “Wernicke’s area.” Brain 2001; 124:83–95Crossref, Medline, Google Scholar
12. Zilbovicius M, Boddaert N, Belin P, Poline J-B, Remy P, Mangin J-F, Thivard L, Barthélémy C, Samson Y: Temporal lobe dysfunction in childhood autism: a PET study. Am J Psychiatry 2000; 157:1988–1993Link, Google Scholar
13. Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, Sasaki M: Abnormal regional cerebral blood flow in childhood autism. Brain 2000; 123:1838–1844Crossref, Medline, Google Scholar



