Hippocampal Volume, PTSD, and Alcoholism in Combat Veterans
Abstract
Studies imposing rigorous control over lifetime alcohol intake have usually not found smaller hippocampal volumes in persons with posttraumatic stress disorder. Because the majority of negative studies have used adolescent samples, it has been suggested that chronicity is a necessary condition for such findings. To test the hypothesis that a smaller hippocampus in PTSD is unrelated to comorbid alcoholism or to chronicity, this study estimated hippocampal volume in a relatively large group (N=99) of combat veterans in which PTSD, lifetime alcohol abuse/dependence, and Vietnam versus Gulf War service were crossed. In subjects with histories of alcoholism, unadjusted hippocampal volume was 9% smaller in persons with PTSD than in those without PTSD. In nonalcoholic subjects, the PTSD-related difference in hippocampal volume was 3%. The failure to observe a strong association between PTSD and hippocampal volume in nonalcoholic subjects was not ascribable to younger age, reduced PTSD chronicity, or lower PTSD symptom severity. The possibility that smaller hippocampal volume is limited to groups in which PTSD is compounded by comorbid alcoholism is not necessarily incompatible with results suggesting a smaller hippocampus is predispositional to PTSD. Further examination of the role of alcoholism and other comorbid conditions in studies of brain structure and function in PTSD appears warranted.
Most neuroimaging studies of adult posttraumatic stress disorder (PTSD) have sought to reduce possible alcoholism-related confounders but have ultimately relied on comparisons in which the PTSD groups reported more alcohol exposure than the comparison subjects (1 – 7) . Conversely, studies that have rigorously excluded comorbid alcohol abuse/dependence have usually not found smaller hippocampal volumes (8 – 12 , but see 13 ). Smaller hippocampal volumes have also been reported in individuals with primary alcohol abuse/dependence (14 – 17) , and although this effect rarely persists after adjustment for global tissue volume (18 – 21) , there is a consensus that comorbid alcohol abuse/dependence is relevant to a complete understanding of the neurobiology of PTSD. A common approach to the problem of comorbid alcoholism has been to adjust brain volumes for lifetime consumption (3 – 6) ; however, the alcohol literature shows a scarcity of linear relationships between consumption and indices of brain structure or function (22 – 27) . This scarcity could derive from the low reliability of retrospective self-reports (28 – 30) and/or the possibility that binge/withdrawal episodes, rather than “typical” drinking, account disproportionately for alcohol-related brain damage (31) . To overcome these limitations, we recruited subjects from two large VA catchments with the aim of accruing a substantial number of participants diagnosed free of lifetime alcohol abuse/dependence (32) .
One proposed explanation for the absence of smaller hippocampal volumes in groups that did not confounding PTSD and alcoholism is that their subjects have often been children and adolescents. To provide a partial test of this hypothesis, we compared Vietnam and Gulf War veterans with mean ages of 56 and 38 years and mean years since military trauma of approximately 36 and 9 years, respectively. Numerous effects of aging on brain morphometry have been documented (33 – 37) . Because aging was confounded with other factors known to influence PTSD, including trauma severity and socioeconomic status, the term “cohort” was used.
Method
Recruitment and Screening
The subjects were recruited through advertising and contacts with current and past patients and research volunteers. To improve the recruitment of female Gulf War veterans, large mailings were sent to candidates identified through the Defense Manpower Database and the Fort Devens Study of Gulf War Veterans. Initial screening established that the subjects were combat-exposed U.S. military veterans of the Vietnam Conflict (Aug. 1964 to May 1975) or the Persian Gulf War (August 1990 to March 1991) reporting no current or past CNS disease, no psychosis, and no alcohol or substance abuse/dependence in the last 6 months. Initial exclusions were based upon current alcohol or substance use, high fevers, loss of consciousness requiring medical attention, or known contraindications to magnetic resonance imaging (MRI). The subjects provided written informed consent in accordance with procedures of the institutional review boards of either Stanford University Medical School/Veterans Administration (VA) Palo Alto Healthcare System or Boston VA Medical Center and McLean Hospital. The subjects meeting screening criteria were administered the Clinician-Administered PTSD Scale (CAPS) (38) for PTSD symptoms and selected axis I modules of the Structured Clinical Interview for DSM–IV (SCID; mood episodes, psychotic and associated symptoms, alcohol and other substance use disorders, and anxiety and other disorders) (39) . Self-report instruments included the Combat Exposure Scale (40) , the Life Events Checklist (41) , the Mississippi Scale for Combat-Related PTSD (42) , the Beck Depression Inventory (43) , and the Michigan Alcoholism Screening Test—Short Form (44) . Eighty-seven subjects also underwent a structured interview to determine which Life Events Checklist endorsements fulfilled PTSD criterion A and at what age they occurred. Formally assessed participants were excluded if they were determined to be negative for current military PTSD but positive for lifetime civilian PTSD (18) or were positive for current/recent alcohol/drug abuse (14) , probable brain damage (6) , or psychosis (2) . Four subjects later withdrew because of fatigue or nicotine withdrawal; two missed their scanning appointments and were unreachable; and five withdrew because of claustrophobia. After scanning, 11 subjects were excluded because of an imaging artifact and two to previously undiagnosed brain injury.
Subjects
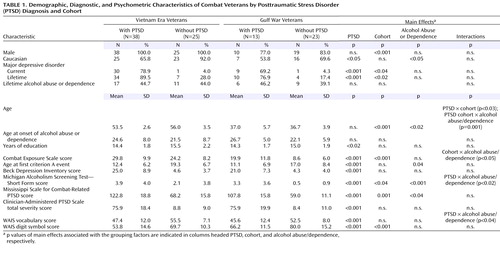
The final study group included 99 military veterans. PTSD-positive subjects met criteria for current PTSD as a result of experiencing one or more military traumas. PTSD-negative subjects were free of diagnosable PTSD, current or lifetime. The subjects who were positive for alcohol abuse/dependence were classified based upon meeting lifetime, but not current, alcohol abuse or dependence criteria of the SCID. Additional characteristics of the group are presented in Table 1 .
Current psychotropic medications were not discontinued. Seventy-nine percent of the PTSD-positive participants were taking some form of psychotropic medication versus 21% of the PTSD-negative participants. Sixty-one percent of the PTSD-positive participants were taking antidepressant medications versus 6% of the PTSD-negative participants. Twenty-eight percent of the PTSD-positive participants were taking selective serotonin reuptake inhibitors (SSRIs) versus 2% of the PTSD-negative participants. Twenty-six percent of the PTSD-positive participants were taking anticonvulsant/mood-stabilizing medications versus 2% of the PTSD-negative participants. The subjects who were positive for alcohol abuse/dependence were not significantly more likely than the participants who were negative for alcohol abuse/dependence to be taking some form of psychotropic medication (50% versus 36%, respectively; χ 2 =1.86, df=1, n.s.). Multiway contingency analyses confirmed that alcohol abuse/dependence did not interact with any other between-subjects factor (including PTSD status) to influence medication status.
Brain Imaging
MRI was performed by using two 1.5 T General Electric Signa (Milwaukee) systems at similar revisions, one at the Diagnostic Radiology Center of Veterans Affairs Palo Alto Health Care System and one at the Brain Imaging Center of McLean Hospital in Belmont, Mass. During scanning, the subjects’ heads were stabilized by using tape and a pump-evacuated cushion (Vac-Pac, Olympic Medical, Seattle). Following locator scans, a 124-slice volumetric spoiled gradient echo series was acquired (TR=35 msec, TE=6 msec, flip angle=45°, field of view=24 cm, number of excitations=1, image matrix size=256×192). Slice thickness ranged from 1.5 mm to 1.7 mm depending upon head size. All cases were screened for gross structural abnormalities by a board-certified neuroradiologist.
The raw spoiled gradient data were imported into BrainImage (A.L. Reiss, BrainImage 5.x, Stanford University, Stanford, Calif.) for image optimization, including correction for inhomogeneity artifacts, resampling to cubic voxels (0.9375 mm 3 ), positional normalization by reference to the anterior and posterior commissures and intrahemispheric fissure, skull stripping, tissue segmentation based upon a constrained fuzzy algorithm (45) , and parcellation according to a modified Talairach grid (46 , 47) . Manual delineation of the hippocampus followed a protocol described in Kates et al. (48) in which the anterior limit is defined by the hippocampal sulcus or alveus and the posterior limit by the fusion of the fornix with the splenium. Delineation was performed by a single rater (W.K.S.) who was trained to interrater criterion within the Stanford Psychiatry Neuroimaging Laboratory and blinded to subject identity and diagnosis.
Morphometric studies often include adjustment for body size. This study employed two indices, supratentorial cerebral tissue volume and supratentorial cranial volume. The former is the sum of gray and white matter volumes following skull stripping and tissue segmentation. The latter is the volume of the cranium as estimated from linear measurements applied, as much as possible, to bone. Cranial volume was estimated directly because the skull stripping performed upon T 1 -weighted images removes meningeal tissue and nonsulcal CSF, leaving as much as 6% of intracranial volume unaccounted for in young adults (49) . Furthermore, cranial volume is stable after early childhood, whereas adult cerebral tissue volume reflects continuing developmental and exogenous influences. Estimation of supratentorial cranial volume involved measuring the height and width of the cranial vault at anterior, central, and posterior slices; multiplying the areas of those rectangles by 33% of cranial length; and summing them ( Figure 1 ). This procedure was applied to a positionally normalized, non-skull-stripped version of the spoiled gradient series. Because the T 1 -weighted images did not allow reliable discrimination of the inner table of the skull from underlying CSF, boundaries between the outer table of the skull and overlying muscle were employed whenever possible. Anterior, posterior, and ventral landmarks were based upon brain tissue. Detailed landmarks are provided elsewhere (unpublished study by Woodward et al.). These measurements were also performed by a single rater (N.J.A.) who was blind to subject identity and diagnosis.
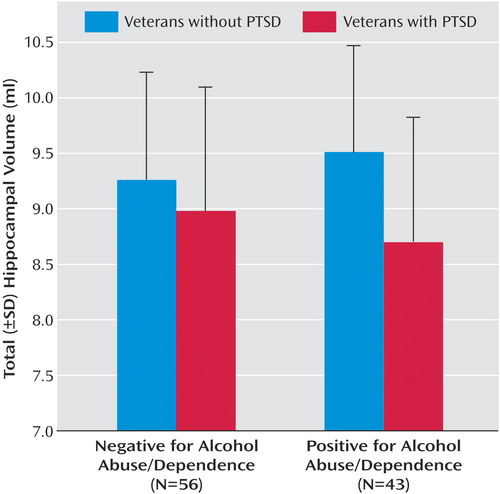
a The y axis was scaled to the range of total hippocampal volumes observed.
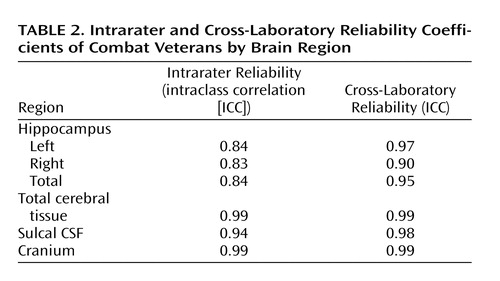
Intrarater reliability was assessed by a blinded reanalysis of 16 subjects, eight from each site, including de novo importation, segmentation, and region-of-interest delineation. Cross-site reliability was assessed by obtaining scans in both magnets from seven study staff during the period of subject data acquisition. Intrarater and cross-site reliabilities for the hippocampal, total cerebral tissue, and cranial volumes were high ( Table 2 ).
Statistical Analyses
Two controversies surround adjustment for “nuisance” variance in body size. Such adjustment should, under ideal circumstances, increase power and reduce the likelihood of type II error. Arndt et al. (50) argued that the reliabilities of volume ratios and residualized volumes are reduced when the correlations between components exceed r=0.90 and component reliabilities fall below 0.80; however, in this study, interregional volume correlations never exceeded r=0.80, whereas reliabilities exceeded 0.90. The second controversy concerns the validity of the most common method for “removing” differences between study groups: analysis of covariance (ANCOVA). Under standard assumptions that subjects are randomly assigned to groups and groups do not differ on covariates, ANCOVAs function only to increase power (51) . If, however, groups differ on the covariate, Miller and Chapman (52) argued that there is no guarantee that the residualized grouping variable will remain faithful to the original. Here we propose that a residualized grouping variable that bears the same relations to the demographic, diagnostic, and psychometric indices as the original upholds the validity of ANCOVA even when groups differ on the covariate. In this study, some of the covariates exhibited main effects that are the focus of another article (unpublished study by Woodward et al.); however, in no case did a residualized grouping variable diverge substantially from the original in its relations to the obtained demographic, diagnostic, and psychometric indices. In view of these arguments, statistical tests included ANOVAs applied to unadjusted hippocampal volumes and ANCOVAs adjusting for cerebral tissue volume, cranial volume, and WAIS vocabulary score. Omnibus analyses are accompanied by tests of PTSD in the alcohol abuse or dependence subgroup. Finally, selected analyses of covariation are reported.
Results
Combat-related PTSD was strongly associated with comorbid major depression, elevated Beck Depression Inventory scores, and reduced WAIS vocabulary scores. PTSD-positive subjects also performed much worse on the WAIS digit symbol substitution subtest (F=25.5, df=1, 91, p<0.001). Because of oversampling, PTSD was not associated with an elevated frequency of alcohol abuse/dependence; nevertheless, Michigan Alcoholism Screening Test—Short Form scores exhibited both a main effect of PTSD and a PTSD-by-alcohol abuse/dependence interaction deriving from especially high scores in PTSD-positive, alcohol abuse/dependence-positive subjects. Even within the alcohol abuse/dependence-negative subgroup, PTSD was associated with a small but significant elevation of Michigan Alcoholism Screening Test—Short Form scores (PTSD-positive subjects: 1.19, PTSD-negative subjects: 0.18). Generally speaking, the alcohol abuse/dependence-positive and alcohol abuse/dependence-negative subgroups were closely matched, and PTSD and alcohol abuse/dependence did not interact to influence psychometric indices. Being alcohol abuse/dependence-positive was not associated with an elevated incidence of major depressive disorder and did not interact with other factors to influence a diagnosis of major depressive disorder. Being alcohol abuse/dependence-positive was also not associated with an elevated Beck Depression Inventory score or an elevated Combat Exposure Scale score. Alcohol abuse/dependence-positive subjects did not differ in years of education and did not exhibit worse performance on the WAIS digit symbol substitution subtest. In contrast, Gulf War and Vietnam cohorts exhibited large differences in combat exposure, current PTSD severity, and digit symbol substitution performance. The former were consistent with the differing conditions of the two conflicts and the known impact of trauma severity on PTSD (53) and the latter with normal aging (54) . The Vietnam cohort also exhibited more lifetime major depressive disorder and higher scores on the Michigan Alcoholism Screening Test—Short Form.
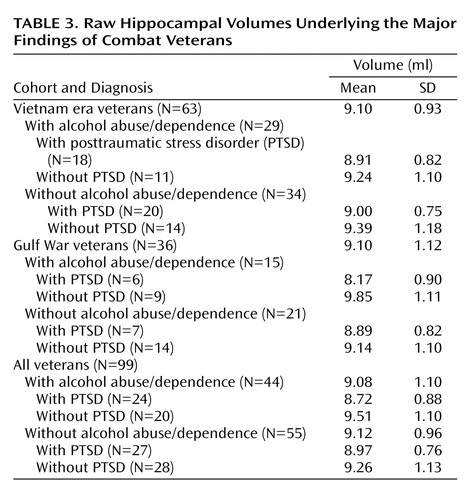
A three-factor multiple analysis of variance (MANOVA) (PTSD × cohort × alcohol abuse/dependence) performed on left and right hippocampal volumes found a significant multivariate F value for PTSD (F=4.89, df=2, 90, p=0.01). As well, the hippocampus was slightly larger in the right hemisphere (4.67 ml versus 4.44 ml; t=7.30, df=98, p<0.001), but this difference exhibited no interactions with grouping factors. Hence, group effects and interactions were reestimated on total hippocampal volume. (Although prior findings of smaller hippocampal volumes in PTSD have often been unilateral, no systematic directionality has emerged.) Mean unadjusted total hippocampal volumes are presented for all comparisons and for selected contrasts in Table 3 . The main effect of PTSD on total hippocampal volume (F=9.83, df=1, 91, p=0.002) was accompanied by a near-significant two-way interaction of PTSD and alcohol abuse/dependence (F=2.69, df=1, 91, p=0.11) and a near-significant three-way interaction of PTSD, cohort, and alcohol abuse/dependence (F=2.87, df=1, 91, p<0.09). To account for the three-way interaction, PTSD and cohort interacted significantly in the alcohol abuse/dependence-positive subjects (F=4.63, df=1, 40, p<0.04) but not the alcohol abuse/dependence-negative subgroup. In the former, PTSD was associated with smaller hippocampal volume in the Gulf War cohort (9.85 ml versus 8.17 ml) but not in the Vietnam cohort (9.24 versus 8.91 ml). To account for the two-way interaction, the effects of PTSD on hippocampal volume varied in the alcohol abuse/dependence-positive and alcohol-dependence-negative subgroups. In the former, the hippocampal volumes of PTSD patients were 9% smaller than those of veterans without PTSD (8.72 versus 9.51 ml; F=10.44, df=1, 40, p=0.002). In the alcohol abuse/dependence-negative subgroup, hippocampal volume did not differ significantly between subjects with and without PTSD (8.97 ml versus 9.26 ml; F=1.25, df=1, 51, p=0.27) ( Figure 1 ), although the tendency (3%) was in the expected direction. Effect sizes were also estimated with pooled d+ (55) to accommodate the different numbers of Gulf War and Vietnam cohort members across PTSD-positive and PTSD-negative groups. In the alcohol abuse/dependence-positive subgroup, pooled d+ for the effect of PTSD on hippocampal volume was –0.69, with a two-tailed 95% confidence interval (CI) of –0.06 to –1.33. In the alcohol abuse/dependence-negative subgroup, d+ was –0.34, with a 95% CI of –0.89 to 0.21. (These values correspond to a PTSD-positive minus PTSD-negative contrast.)
There were no main effects of alcohol abuse/dependence or cohort on hippocampal volume. Adjustment for cranial volume, total cerebral tissue volume, and WAIS vocabulary score all failed to reinstate an effect of PTSD on hippocampal volume in the alcohol abuse/dependence-negative subgroup. Rather, adjustment for cranial volume eliminated the effect of PTSD on hippocampal volume in the alcohol abuse/dependence-positive subgroup (F=1.97, df=1, 38, p=0.17) and in the group as a whole (F=1.34, df=1, 90, p=0.25). None of these results was modified by the exclusion of women or of PTSD-positive subjects with CAPS total severity scores below 65.
Hippocampal volume was not correlated with combat trauma exposure as indexed by Combat Exposure Scale scores (r=–0.12, df=99, p=0.23) in the whole group; however, because the Vietnam and Gulf War veterans had experienced different distributions of combat exposure, these relationships were reestimated for each cohort. In the Vietnam cohort only, there was a moderately significant inverse correlation between hippocampal volume and Combat Exposure Scale score (r=–0.28, df=61, p<0.03). In the whole group, after prior entry of age and vocabulary scores, both cerebral tissue volume (β=0.303, t=2.04, df=94, p<0.05) and cranial volume (β=0.356, t=2.29, df=94, p<0.03) accounted for unique variance in hippocampal volume.
Discussion
The subjects with PTSD and histories of comorbid alcoholism exhibited effects on hippocampal volume similar in direction and magnitude to those reported in studies in which PTSD-positive subjects had greater lifetime exposure to alcohol than comparison subjects. Nonalcoholic PTSD-positive subjects exhibited a nonsignificant tendency (3%) toward a smaller hippocampus. Adjustment for cranial volume, total cerebral tissue volume, or vocabulary failed to uncover an effect of PTSD on hippocampal volume in nonalcoholic veterans. A comparison of PTSD effect sizes in alcoholics and nonalcoholics confirmed the possible role of alcoholism as a facilitator of the effect of PTSD on the hippocampus but introduced important caveats. The observed confidence intervals surrounding these effects are large and overlapping. The CI in the alcoholic subgroup includes small effects, whereas the CI in the nonalcoholic subgroup includes large effects. The absence of a statistically significant effect in nonalcoholics could represent a type II error.
Acknowledging these caveats, the present results raise doubts regarding certain explanations that have been advanced to explain earlier failures to find smaller hippocampal volumes in nonalcoholic PTSD-positive groups. A 27-year differential in chronicity did not result in a PTSD × cohort interaction, and although power was limited by the small size of the Gulf War PTSD comparison, the observed tendencies were contrary to a chronicity effect. Exclusion of PTSD-positive subjects with CAPS total severity scores below 65 also did not influence the results. The alcoholic and nonalcoholic subgroups had similar Combat Exposure Scale scores, CAPS total severity scores, and Beck Depression Inventory scores. Statistical power for comparisons performed within the nonalcoholic subgroup was comparable to multiple studies reporting positive findings.
A role for comorbid alcohol abuse/dependence in prior observations of smaller hippocampal volume in PTSD is tentatively supported in these data. At the same time, lifetime alcoholism was not independently associated with smaller hippocampal volume even before adjustment for total cerebral tissue volume. Deployed U.S. military veterans who do and do not meet criteria for lifetime alcoholism may have less contrastive alcohol histories than groups sampled from civilian populations. Nevertheless, the observed reversal of the aging-alcohol interaction could arise only if the effects of alcoholism on the hippocampus were accentuated in the Gulf War cohort, attenuated in the Vietnam cohort, or both. It is possible that biased attrition-attenuated selected group effects involved Vietnam-era PTSD-positive alcoholic subjects in this study. Drescher et al. (56) demonstrated that a contemporaneous Vietnam-era sample drawn from the VA Palo Alto Healthcare System PTSD inpatient population exhibited excess age-adjusted mortality in association with alcohol and substance abuse. The survivor population would be expected to exhibit attenuated versions of neurobiological concomitants of alcohol and substance abuse preferentially associated with premature mortality. The possibility that a smaller hippocampus participates with alcohol/substance abuse to confer a predisposition to premature mortality cannot be ruled out, particularly if a smaller hippocampus is predispositional to PTSD (2) , itself a consequence of exposure to life threat. This study found modest support for an inverse relationship between hippocampal volume and exposure to potentially traumatic combat events, as reported by Gurvits et al. (3) .
The current observation of normal hippocampal volume in PTSD uncomplicated by alcoholism appears to contradict the findings of Gilbertson et al. (2) . In a study of monozygotic twins, these authors obtained evidence that a smaller hippocampus represents an inherited predisposition to develop PTSD after trauma rather than being a consequence of trauma. These findings are not incompatible if the data of Gilbertson et al. are interpreted to indicate that a smaller hippocampus is predispositional to PTSD with comorbid alcohol abuse/dependence. Eighty-two percent of the PTSD-positive subjects of Gilbertson et al. met criteria for comorbid alcoholism. As well, the unexposed twins of their PTSD-positive alcohol abuse/dependence-positive subjects tended to exhibit higher rates of alcohol abuse/dependence (47% versus 30%) and higher scores on the Michigan Alcoholism Screening Test (6.8 versus 2.5; p=0.09; reference 44 ) than the unexposed twins of PTSD-negative subjects, both observations compatible with an elevated risk for primary alcoholism. Evidence of shared genetic vulnerability to combat exposure/PTSD and alcoholism (57) has been obtained from other samples drawn from the Vietnam Era Twin Registry (58) .
Among the covariates used to increase the power of group comparisons of hippocampal volume, an estimate of cranial volume had the opposite effect, eliminating PTSD effects even in the alcoholic subgroup. This result might have been achieved if adjustment for cranial volume had simply added a random variate to hippocampal volume; however, cranial volume accounted for a significant unique variance in hippocampal volume. This observation is remarkable in light of the fact that the cranium expands little after age 5 or 6 (49 , 59) . Systematic effects on cranial volume noted in this group are considered in a separate article (unpublished study by Woodward et al.).
1. Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B: Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 1997; 27:951–959Google Scholar
2. Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK: Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002; 5:1242–1247Google Scholar
3. Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, Orr SP, Kikinis R, Jolesz FA, McCarley RW, Pitman RK: Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry 1996; 40:1091–1099Google Scholar
4. Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, Kodituwakku PW, Hart BL, Escalona R, Brooks WM: Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry 2002; 52:119–125Google Scholar
5. Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB: MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995; 152:973–981Google Scholar
6. Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS: Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol Psychiatry 1997; 41:23–32Google Scholar
7. Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Duncan JS, Charney DS: MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 2003; 160:924–932Google Scholar
8. Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL: Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry 2001; 50:943–951Google Scholar
9. De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND: A.E. Bennett Research Award: developmental traumatology part II: Brain Development Biol Psychiatry 1999; 45:1271–1284Google Scholar
10. De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G: Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry 2002; 52:1066–1078Google Scholar
11. Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL: Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry 2002; 52:1089–1101Google Scholar
12. Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, Weiner MW: Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry 2001; 50:952–959Google Scholar
13. Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, den Heeten GJ, Gersons BP: Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol Psychiatry 2004; 56:356–363Google Scholar
14. Jernigan TL, Schafer K, Butters N, Cermak LS: Magnetic resonance imaging of alcoholic Korsakoff patients. Neuropsychopharmacology 1991; 4:175–186Google Scholar
15. Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A: Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res 1995; 19:110–122Google Scholar
16. Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW: Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry 1999; 56:356–363Google Scholar
17. Laakso MP, Vaurio O, Savolainen L, Repo E, Soininen H, Aronen HJ, Tiihonen J: A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behav Brain Res 2000; 109:177–186Google Scholar
18. Pfefferbaum A, Rosenbloom M, Serventi KL, Sullivan EV: Corpus callosum, pons, and cortical white matter in alcoholic women. Alcohol Clin Exp Res 2002; 26:400–406Google Scholar
19. Sullivan EV, Lane B, Deshmukh A, Rosenbloom MJ, Desmond JE, Lim KO, Pfefferbaum A: In vivo mammillary body volume deficits in amnesic and nonamnesic alcoholics. Alcohol Clin Exp Res 1999; 23:1629–1636Google Scholar
20. Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO: Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res 1997; 21:521–529Google Scholar
21. Mann K, Agartz I, Harper C, Shoaf S, Rawlings RR, Momenan R, Hommer DW, Pfefferbaum A, Sullivan EV, Anton RF, Drobes DJ, George MS, Bares R, Machulla HJ, Mundle G, Reimold M, Heinz A: Neuroimaging in alcoholism: ethanol and brain damage. Alcohol Clin Exp Res 2001; 25(5 suppl ISBRA):104S–109SGoogle Scholar
22. Neiman J: Alcohol as a risk factor for brain damage: neurologic aspects. Alcohol Clin Exp Res 1998; 22(7 suppl):346S–351SGoogle Scholar
23. Adams KM, Grant I: Failure of nonlinear models of drinking history variables to predict neuropsychological performance in alcoholics. Am J Psychiatry 1984; 141:663–667Google Scholar
24. Adams KM, Brown G, Grant I: Analysis of covariance as a remedy for demographic mismatch of research subject groups: some sobering simulations. J Clin Exp Neuropsychol 1985; 7:445–462Google Scholar
25. Grant I, Adams K, Reed R: Normal neuropsychological abilities of alcoholic men in their late thirties. Am J Psychiatry 1979; 136:1263–1269Google Scholar
26. Acker W, Ron MA, Lishman WA, Shaw GK: A multivariate analysis of psychological, clinical, and CT scanning measures in detoxified chronic alcoholics. Br J Addictions 1984; 79:293–301Google Scholar
27. Grant I: Alcohol and the brain: neuropsychological correlates. J Consult Clin Psychol 1987; 55:310–324Google Scholar
28. Hilton ME: A comparison of a prospective diary and two summary recall techniques for recording alcohol consumption. Br J Addict 1989; 84:1085–1092Google Scholar
29. Midanik LT: Validity of self-reported alcohol use: a literature review and assessment. Br J Addict 1988; 83:1019–1030Google Scholar
30. Sobell LC, Cellucci T, Nirenberg TD, Sobell MB: Do quantity-frequency data underestimate drinking-related health risks? Am J Public Health 1982; 72:823–828Google Scholar
31. Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A: Relationship between alcohol withdrawal seizures and temporal lobe white matter volume deficits. Alcohol Clin Exp Res 1996; 20:348–354Google Scholar
32. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1988Google Scholar
33. Pfefferbaum A, Zatz LM, Jernigan TL: Computer-interactive method for quantifying cerebrospinal fluid and tissue in brain CT scans: effects of aging. J Comput Assist Tomogr 1986; 10:571–578Google Scholar
34. Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR: Cerebral structure on MRI, part I: localization of age-related changes. Biol Psychiatry 1991; 29:55–67Google Scholar
35. Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR: Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 2001; 22:581–594Google Scholar
36. Pfefferbaum A, Sullivan EV, Jernigan TL, Zipursky RB, Rosenbloom MJ, Yesavage JA, Tinklenberg JR: A quantitative analysis of CT and cognitive measures in normal aging and Alzheimer’s disease. Psychiatry Res 1990; 35:115–136Google Scholar
37. Yue NC, Arnold AM, Longstreth WT Jr, Elster AD, Jungreis CA, O’Leary DH, Poirier VC, Bryan RN: Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the cardiovascular health study. Radiology 1997; 202:33–39Google Scholar
38. Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Charney DS, Keane TM: Clinician-Administered PTSD Scale for DSM–IV: Current and Lifetime Version. Boston, Boston VA Medical Center/Neurosciences Division, Behavioral Science Division; West Haven, Conn, West Haven VA Medical Center, 1997Google Scholar
39. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
40. Keane TM, Fairbank JA, Caddell JM, Zimering RT, et al: Clinical evaluation of a measure to assess combat exposure. Psychol Assess 1:53–55, 1989Google Scholar
41. Blake D, Weathers F, Nagy L, Kaloupek D, Klauminzer G, Charney D, Keane T, Buckley TC: Clinician-Administered PTSD Scale (CAPS) Instructional Manual. Boston, National Center for PTSD, 2000Google Scholar
42. Keane TM, Caddell JM, Taylor KL: Mississippi Scale for Combat-Related Posttraumatic Stress Disorder: three studies in reliability and validity. J Consult Clin Psychol 1988; 56:85–90Google Scholar
43. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Google Scholar
44. Selzer ML: The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry 1971; 127:1653–1658Google Scholar
45. Reiss AL, Hennessey JG, Rubin M, Beach L, Abrams MT, Warsofsky IS, Liu AM, Links JM: Reliability and validity of an algorithm for fuzzy tissue segmentation of MRI. J Comput Assist Tomogr 1998; 22:471–479Google Scholar
46. Kates WR, Warsofsky IS, Patwardhan A, Abrams MT, Liu AM, Naidu S, Kaufmann WE, Reiss AL: Automated Talairach atlas-based parcellation and measurement of cerebral lobes in children. Psychiatry Res 1999; 91:11–30Google Scholar
47. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988Google Scholar
48. Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL: Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res 1997; 75:31–48Google Scholar
49. Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA: Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 2000; 216:672–682Google Scholar
50. Arndt S, Cohen G, Alliger RJ, Swayze VW II, Andreasen NC: Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Res 1991; 40:79–89Google Scholar
51. Cochran WG: Analysis of covariance: its nature and uses. Biometrics 1957; 44:261–281Google Scholar
52. Miller GA, Chapman JP: Misunderstanding analysis of covariance. J Abnorm Psychol 2001; 110:40–48Google Scholar
53. Brewin CR, Andrews B, Valentine JD: Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol 2000; 68:748–766Google Scholar
54. Joy S, Fein D, Kaplan E, Freedman M: Speed and memory in WAIS-R-NI digit symbol performance among healthy older adults. J Int Neuropsychol Soc 2000; 6:770–780Google Scholar
55. Hedges LV, Olkin I: Statistical Methods for Meta-Analysis. New York, Academic Press, 1985Google Scholar
56. Drescher KD, Rosen CS, Burling TA, Foy DW: Causes of death among male veterans who received residential treatment for PTSD. J Trauma Stress 2003; 16:535–543Google Scholar
57. McLeod DS, Koenen KC, Meyer JM, Lyons MJ, Eisen S, True W, Goldberg J: Genetic and environmental influences on the relationship among combat exposure, posttraumatic stress disorder symptoms, and alcohol use. J Trauma Stress 2001; 14:259–275Google Scholar
58. Eisen S, True W, Goldberg J, Henderson W, Robinette CD: The Vietnam Era Twin (VET) Registry: method of construction. Acta Genet Med Gemellol (Roma) 1987; 36:61–66Google Scholar
59. Centers for Disease Control and Prevention: Clinical Growth Charts. Atlanta, U.S. Department of Health and Human Services, National Center for Health Statistics, 2000Google Scholar