Hippocampal Volume Reduction in Major Depression
Abstract
OBJECTIVE: Elevated levels of glucocorticoids in depression have been hypothesized to be associated with damage to the hippocampus, a brain area involved in learning and memory. The purpose of this study was to measure hippocampal volume in patients with depression. METHOD: Magnetic resonance imaging was used to measure the volume of the hippocampus in 16 patients with major depression in remission and 16 case-matched nondepressed comparison subjects. RESULTS: Patients with depression had a statistically significant 19% smaller left hippocampal volume than comparison subjects, without smaller volumes of comparison regions (amygdala, caudate, frontal lobe, and temporal lobe) or whole brain volume. The findings were significant after brain size, alcohol exposure, age, and education were controlled for. CONCLUSIONS: These findings are consistent with smaller left hippocampal volume in depression.
Major depression is a common psychiatric disorder that is associated with considerable morbidity. Depressive episodes are associated with high levels of cortisol in about 40%–50% of patients (1). Elevated levels of glucocorticoids seen in stress have been associated with damage to hippocampal neurons, and the diagnosis of posttraumatic stress disorder (PTSD) has been associated with hippocampal volume reduction and deficits in declarative memory function (2, 3). Hippocampal dysfunction may contribute to verbal declarative memory deficits in depression (4, 5). Few studies, however, have examined hippocampal volume in patients with depression. Neuroimaging findings in affective disorders include ventricular enlargement, widening of the cortical sulci (6), and smaller volumes in the right hippocampus (7), left amygdala (8), and temporal lobe (9) in bipolar disorder and increased subcortical white matter areas of increased signal intensity (10), smaller caudate and putamen volumes (11), alterations in hippocampal T1 relaxation time (12), and reductions in gray matter in the left temporal lobe (13) in unipolar depression. Studies of hippocampal volume in unipolar depression have had conflicting findings; some studies of amygdala/hippocampal volume (combined) found no difference in comparison with control subjects (14), and others found a reduction (15). The purpose of the current study was to compare hippocampal volume of patients with treated unipolar depression and nondepressed subjects.
METHOD
The study group consisted of 16 patients with a history of depression based on the Structured Interview for DSM-IV currently treated on an outpatient basis with antidepressant medication (paroxetine, fluoxetine, or desipramine). Ten of the patients were men, and six were women. Patients were excluded according to criteria described elsewhere (3); in addition, patients with a history of PTSD or current medication use other than antidepressant were excluded. Depressed patients had a mean of three inpatient hospitalizations (SD=4, range=0–10), they had been in remission from depression for a mean of 31 weeks (SD=33, range=6–120), and they had experienced an average of two previous episodes of depression (SD=3, range=0–10). Comparison subjects (N=16) were case-matched to patients for sex and handedness. There were no differences between patients and comparison subjects in age (mean=43 years, SD=8, versus mean=45 years, SD=10), years of alcohol abuse (mean=4, SD=7, versus mean=3, SD=5), or years of education (mean=14, SD=2, versus mean=15, SD=2). One of the patients had a past history of alcohol dependence, three had a past history of alcohol abuse, and two had a past history of polysubstance abuse. One of the patients had current panic disorder without agoraphobia. All patients and comparison subjects gave written informed consent before participating in the study.
Magnetic resonance images were obtained and analyzed by using methods described in detail elsewhere (3), including measurements of a mid-hippocampal segment performed by two blind raters. Volumetric assessments were also made of other regions (table 1) for purposes of comparison and to determine if volume changes were specific to the hippocampus as previously reported (3). Frontal lobe measurements were performed by measuring the frontal lobe on all coronal slices anterior to the anterior commissure. There was a high level of interrater reliability for hippocampal volume measurements between the two independent raters for the left hippocampus (r=0.93, df=31, p<0.05) and the right hippocampus (r=0.94, df=31, p<0.05).
Repeated measures analysis of variance (ANOVA) with side (left versus right hemisphere) as the repeated factor was performed to compare left and right hippocampal volume (as well as comparison regions) between patients and nondepressed subjects. Univariate statistics were used to compare left and right hippocampal volume; a level of significance of p<0.03 was applied to correct for multiple comparisons. Multiple linear regression was used to examine the relationship between hippocampal volume and diagnosis while controlling for potential confounders.
RESULTS
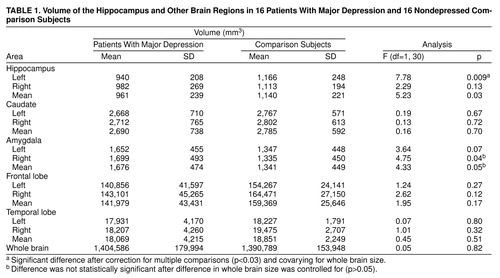
Repeated measures ANOVA with side (left versus right hemisphere) as the repeated factor showed a significant main effect for diagnosis (table 1) and no significant main effect for side or side-by-diagnosis interaction. Univariate analyses showed a statistically significant 19% smaller volume of the left hippocampus in the depressed patients than the comparison subjects; the difference remained significant after differences in whole brain volume were controlled for, but a 12% difference in right hippocampal volume between the groups was not significant (table 1). There were no differences in volume in the comparison regions with the exception of the right amygdala, which was related to a larger volume of the right amygdala in depression (table 1). This effect was not significant, however, after differences in whole brain volume were controlled for.
There was no correlation between left hippocampal volume and clinical variables, including number of weeks in remission, number of previous episodes of depression, or number of hospitalizations for depression. Multiple linear regression to control for possible confounds that may affect hippocampal volume, including age, years of education, and years of alcohol abuse, continued to show differences in left hippocampal volume between patients and comparison subjects when these potential confounders were entered in the model.
DISCUSSION
Patients with remitted major depression in this study had a 19% smaller volume of the left hippocampus than matched comparison subjects. There were no differences in volumes of comparison regions between the groups in the temporal lobe, caudate, frontal lobe, or whole brain.
There are several possible explanations for our findings of hippocampal volume reduction in depression. Elevated levels of glucocorticoids during depressive episodes could cause hippocampal damage, leading to a reduction in volume. In this model, chronic repeated episodes of depression may lead to progressive hippocampal atrophy over time, possibly increasing the risk for subsequent depressive relapse. Other factors, such as reductions in neurotrophins, could also be responsible for hippocampal volume reduction. However, the current study did not include measures of cortisol during depressive episodes, and future studies are needed to evaluate the relationship between cortisol and hippocampal volume in depression. It is also possible that smaller hippocampal volume from birth, or secondary to some premorbid environmental factor, is a risk factor for depression.
Amygdala volume was larger in depression but not significantly larger after we controlled for brain size. The meaning of these findings is unclear and should be replicated. It may account for the finding of Axelson et al. (14) of no difference in combined amygdala/hippocampal volume in patients with unipolar depression.
Received Nov. 13, 1998; revision received June 4, 1999; accepted June 8, 1999. From the Departments of Diagnostic Radiology and Psychiatry, Yale University School of Medicine, New Haven, Conn.; the National Center for PTSD, West Haven, Conn.; the VA Connecticut Healthcare System; and the Yale Psychiatric Institute, New Haven, Conn. Address reprint requests to Dr. Bremner, VA Connecticut Healthcare System (115a), 950 Campbell Ave., West Haven, CT 06516. This study was supported by a grant from the National Center for PTSD and a VA Career Development Award grant to Dr. Bremner. The authors thank Kathy Colonese, R.N., Jacque Piscitelli, M.S., and Lisa Roach, B.S., for assistance in patient assessments and data management, and Hedy Sarofin for expert assistance in magnetic resonance image acquisition.
 |
1. Carroll BJ, Curtis GC, Davies BM, Mendels J, Sugarman AA: Urinary free cortisol excretion in depression. J Psychol Med 1976; 6:43–50Crossref, Medline, Google Scholar
2. Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB: MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995; 152:973–981Link, Google Scholar
3. Bremner JD: Does stress damage the brain? Biol Psychiatry 1999; 45:797–805Google Scholar
4. Sass KJ, Spencer DD, Kim JH, Westerveld M, Novelly RA, Lencz T: Verbal memory impairment correlates with hippocampal pyramidal cell density. Neurology 1990; 40:1694–1697Google Scholar
5. Burt DB, Zembar MJ, Niederehe G: Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull 1995; 117:285–305Crossref, Medline, Google Scholar
6. Andreasen NC, Swayze VW II, Flaum M, Alliger R, Cohen G: Ventricular abnormalities in affective disorder: clinical and demographic correlates. Am J Psychiatry 1990; 147:893–900Link, Google Scholar
7. Swayze VW II, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC: Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry 1992; 31:221–240Crossref, Medline, Google Scholar
8. Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, Federman EB, Chase GA, Petty RG, Tien AY: Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry 1997; 41:1–14Crossref, Medline, Google Scholar
9. Altshuler LL, Conrad A, Hauser P, Li X, Guze BH, Denikoff K, Tourtellotte W, Post R: Reduction of temporal lobe volume in bipolar disorder: a preliminary report of magnetic resonance imaging (letter). Arch Gen Psychiatry 1991; 48:482–483Crossref, Medline, Google Scholar
10. Krishnan KRR, Goli V, Ellinwood EH, France RD, Blazer DG, Nemeroff CB: Leukoencephalopathy in patients diagnosed as major depressives. Biol Psychiatry 1988; 23:519–522Crossref, Medline, Google Scholar
11. Krishnan KRR, McDonald WM, Escalona PR, Doraiswamy PM, Na C, Husain MM, Figiel GS, Boyko OB, Ellinwood EH, Nemeroff CB: Magnetic resonance imaging of the caudate nuclei in depression. Arch Gen Psychiatry 1992; 49:553–558Crossref, Medline, Google Scholar
12. Krishnan KRR, Doraiswamy PM, Figiel GS, Husain MM, Shaw SA, Na C, Boyko OB, McDonald WM, Nemeroff CB, Ellinwood EH Jr: Hippocampal abnormalities in depression. J Neuropsychiatry Clin Neurosci 1991; 3:387–391Crossref, Medline, Google Scholar
13. Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM: Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression: controlled magnetic resonance imaging study. Br J Psychiatry 1998; 172:527–532Crossref, Medline, Google Scholar
14. Axelson DA, Doraiswamy PM, McDonald WM, Boyko OB, Tupler LA, Patterson LJ, Nemeroff CB, Ellinwood EH Jr, Krishnan KRR: Hypercortisolemia and hippocampal changes in depression. Psychiatry Res 1993; 47:163–173Crossref, Medline, Google Scholar
15. Sheline Y, Wang P, Gado M, Csernansky J, Vannier M: Hippocampal atrophy in major depression. Proc Natl Acad Sci USA 1996; 93:3908–3913Google Scholar