Mapping Subcortical Brain Alterations in 22q11.2 Deletion Syndrome: Effects of Deletion Size and Convergence With Idiopathic Neuropsychiatric Illness
Abstract
Objective:
22q11.2 deletion syndrome (22q11DS) is among the strongest known genetic risk factors for schizophrenia. Previous studies have reported variable alterations in subcortical brain structures in 22q11DS. To better characterize subcortical alterations in 22q11DS, including modulating effects of clinical and genetic heterogeneity, the authors studied a large multicenter neuroimaging cohort from the ENIGMA 22q11.2 Deletion Syndrome Working Group.
Methods:
Subcortical structures were measured using harmonized protocols for gross volume and subcortical shape morphometry in 533 individuals with 22q11DS and 330 matched healthy control subjects (age range, 6–56 years; 49% female).
Results:
Compared with the control group, the 22q11DS group showed lower intracranial volume (ICV) and thalamus, putamen, hippocampus, and amygdala volumes and greater lateral ventricle, caudate, and accumbens volumes (Cohen’s d values, −0.90 to 0.93). Shape analysis revealed complex differences in the 22q11DS group across all structures. The larger A-D deletion was associated with more extensive shape alterations compared with the smaller A-B deletion. Participants with 22q11DS with psychosis showed lower ICV and hippocampus, amygdala, and thalamus volumes (Cohen’s d values, −0.91 to 0.53) compared with participants with 22q11DS without psychosis. Shape analysis revealed lower thickness and surface area across subregions of these structures. Compared with subcortical findings from other neuropsychiatric disorders studied by the ENIGMA consortium, significant convergence was observed between participants with 22q11DS with psychosis and participants with schizophrenia, bipolar disorder, major depressive disorder, and obsessive-compulsive disorder.
Conclusions:
In the largest neuroimaging study of 22q11DS to date, the authors found widespread alterations to subcortical brain structures, which were affected by deletion size and psychotic illness. Findings indicate significant overlap between 22q11DS-associated psychosis, idiopathic schizophrenia, and other severe neuropsychiatric illnesses.
22q11.2 deletion syndrome (22q11DS), also known as DiGeorge or velocardiofacial syndrome, is a multisystem disorder resulting from a hemizygous microdeletion on the long arm of chromosome 22, affecting multiple genes involved in neurodevelopment. 22q11DS results in craniofacial, cardiac, and immune system abnormalities as well as neurocognitive deficits (1). Up to 1 in 4 individuals with 22q11DS develop psychotic illness in adolescence or early adulthood, making it one of the strongest known genetic risk factors for schizophrenia (2). There is also considerable psychiatric comorbidity in 22q11DS, as elevated rates of attention deficit hyperactivity disorder (ADHD), anxiety and mood disorders, and autism spectrum disorder (ASD) are also observed (2–4). 22q11DS thus offers a genetically homogeneous framework to study how highly penetrant genetic variants disrupt neurobiological pathways contributing to developmental neuropsychiatric disorders. This genetics-first approach may result in greater power to detect biomarkers of psychiatric illness by providing larger effect sizes than those associated with common genetic variation.
22q11DS-associated psychotic disorder has a clinical presentation similar to that of idiopathic schizophrenia (5). In the largest coordinated analysis of subcortical brain structure in schizophrenia to date, smaller hippocampus volume was the strongest observed effect (6). However, the extent to which variations in underlying subcortical structures overlap between 22q11DS and idiopathic schizophrenia is not well understood, largely because of the lack of large, well-characterized cohorts. Elucidating concordant or divergent aspects of subcortical morphometry between 22q11DS-associated psychosis and idiopathic schizophrenia can shed light on brain mechanisms underlying expression of psychotic illness. Moreover, it will provide information on whether such subcortical alterations reflect a specific neuroanatomic signature of psychosis or are characteristic of other neuropsychiatric disorders.
Mouse models of the 22q11.2 deletion have shown disrupted neurogenesis (7) and altered brain development along the anterior-posterior axis (8). Consistent with this finding, subcortical volume reductions have been reported in human 22q11.2 deletion carriers (9–11), with greater volumetric reductions in more posterior brain regions (12) and thinning in midline structures (13). Even so, most studies have examined small samples, typically ascertained from a single site, limiting power to detect subtle brain abnormalities and determine brain signature consistency across cohorts. Addressing these questions requires larger samples employing similar neuroimaging processing and analysis techniques.
While most neuroimaging studies examine regional volumes, haploinsufficiency for 22q11.2 genes may differentially affect subregions or subfields of subcortical structures (11). High-resolution shape analysis has been used to map fine-grained subcortical alterations in Alzheimer’s disease (14) and in multiple neuropsychiatric disorders (15, 16), offering insights into differential impact on subcompartments or subfields with known structural and functional connectivity patterns. Our recent study of 22q11DS cortical structure (17) revealed distinct disruptions of cortical thickness and surface area, measures linked to known and dissociable developmental determinants (18, 19).
The size of the 22q11.2 microdeletion may also be a source of heterogeneity. Recently, we found that smaller deletions were associated with higher IQ and increased cortical surface area in 22q11DS (17, 20), but whether these effects extend to subcortical brain morphometry is unknown.
To address the limitations of smaller, single-site studies of 22q11DS, we performed a coordinated analysis of the largest MRI data set to date, ascertained by the ENIGMA 22q11.2 Deletion Syndrome Working Group. To map abnormalities on a finer scale than is possible with regional volumetry, we used a surface-based mapping approach, which is sensitive to subtle variations in subcortical morphometry, thus offering insight into the disruption of known subcompartments or subfields (21, 22).
We assessed overall subcortical brain volumes and pointwise shape differences across the entire surface of each structure to answer three main questions:
What is the spatial distribution of subcortical differences between individuals with 22q11DS and healthy control subjects?
Do differences in subcortical structure in individuals with 22q11DS depend on deletion size?
Do subcortical differences exist between individuals with 22q11DS with and without a history of psychosis? And do the subcortical patterns associated with 22q11DS with psychosis overlap with those found in idiopathic schizophrenia and other neuropsychiatric disorders?
Methods
Data Sample
A total of 863 unrelated individuals (22q11DS group, N=533; healthy control group, N=330) from 11 study sites were included. Participants’ demographic characteristics are listed in Table 1 (for demographic characteristics listed by site, see Table S1 in the online supplement). All participating research studies obtained approval from their local ethics committees or institutional review boards, and written informed consent (and/or assent for minors) was obtained for all participants. Comparison of the two most common deletion subtypes (A-D and A-B) was conducted on matched samples: 106 individuals with 22q11DS with A-D deletions, 23 with A-B deletions, and 86 control subjects (see the Supplemental Methods section and Table S2 in the online supplement). Psychosis diagnosis was assessed by structured clinical interview at each study site, with diagnoses validated across sites using a consensus procedure (23). Sixty-four participants with 22q11DS with a psychotic disorder diagnosis were compared with 64 participants with 22q11DS with no history of psychosis by matching participants with and without psychosis within each site by sex and the nearest possible age (see Table S3 in the online supplement). Participant ascertainment and inclusion and exclusion criteria are further described in the Supplemental Methods section and Table S4 in the online supplement.
| Characteristic | Healthy Control Group (N=330) | 22q11DS Group (N=533) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 18.14 | 9.24 | 17.85 | 8.60 |
| IQ | 110.64 | 15.35 | 74.95 | 12.53 |
| N | % | N | % | |
| Female | 148 | 44.8 | 275 | 51.6 |
| Psychotic disorder | 0 | 0.0 | 73 | 13.8 |
| Deletion type | ||||
| A-B | 0 | 0.0 | 28 | 8.0 |
| A-D | 0 | 0.0 | 311 | 88.6 |
| Other | 0 | 0.0 | 12 | 3.5 |
| Current medication | ||||
| First-generation antipsychotic | 0 | 0.0 | 15 | 3.0 |
| Second-generation antipsychotic | 0 | 0.0 | 72 | 14.5 |
| Lithium | 0 | 0.0 | 4 | 0.8 |
| Anticonvulsant | 1 | 0.4 | 31 | 6.3 |
| Antidepressant | 5 | 1.8 | 95 | 19.2 |
| Psychostimulant | 5 | 1.8 | 65 | 13.1 |
TABLE 1. Demographic characteristics and use of psychotropic medication for all participants in the study, and frequency of psychosis and deletion subtypes among those with 22q11.2 deletion syndrome (22q11DS)a
Image Acquisition and Processing
T1-weighted brain MRI data were collected from 11 sites (for acquisition parameters, see Table S5 in the online supplement). Sites with more than one scanner or acquisition protocol were treated as separate sites in the analysis. UCLA, University of California Davis, and University of Toronto each acquired data on two scanners, yielding a total of 14 scanning sites. MRI images were centralized on a secure server at the University of Southern California Imaging Genetics Center for processing and analysis.
Subcortical Segmentation
All T1-weighted scans were segmented using FreeSurfer, version 5.3.0 (24), to derive volumes for eight bilateral regions of interest: lateral ventricles, nucleus accumbens, amygdala, caudate, hippocampus, putamen, pallidum, and thalamus (16 structures per scan) along with intracranial volume (ICV).
Subcortical Shape Analysis
Because subtle and complex variations in local volume may be undetectable by gross volume measures, we applied a novel surface-based parametric mapping technique, the ENIGMA subcortical shape analysis pipeline (22), to investigate high-definition shape variation within the bilateral subcortical structures described above (14 regions of interest, excluding the lateral ventricles). We recently applied this technique in a single-site study of reciprocal 22q11.2 copy number variants (25).
Briefly, with the subcortical FreeSurfer segmentations as inputs, two measures of shape morphometry were derived across the surface of each region of interest. The first, “radial distance” (subsequently referred to as “thickness”), is the distance from each surface vertex to a medial curve and represents a measure of local thickness. The second, the logarithm of the Jacobian determinant (subsequently referred to as the Jacobian or surface area), is the surface dilation ratio between the template and the individual participant’s structure. The Jacobian can be interpreted as areal dilation or contraction of the surface of the region of interest, where higher Jacobian measures suggest larger local surface area.
Both thickness and Jacobian measures were calculated in native space for up to 2,502 homologous points across each of the 14 subcortical shape models to index detailed regional shape differences across participants (see the Supplemental Methods section in the online supplement).
Quality Control
Visual quality inspection was performed by a rater trained in neuroanatomy for all volumes and shape models using ENIGMA standardized quality control protocols (see the Supplemental Methods section in the online supplement).
Statistical Analysis
Primary analyses were conducted with multiple linear regression via the lm function in R, version 3.1.3 (https://stat.ethz.ch/R-manual/R-devel/library/stats/html/lm.html). The dependent variable was region-of-interest volume for gross volumetric analysis and either thickness or Jacobian for vertex-wise shape analysis. Primary analyses were run on left and right structures separately. The independent variable was the grouping variable of interest (e.g., diagnosis, deletion subtype, or history of psychosis), adjusted for appropriate covariates.
Basic covariate adjustments included those for age, age-squared, sex, and ICV. Age effects were modeled with both a linear and a quadratic term, based on model fit (see Tables S6 and S7 in the online supplement). Sex and ICV were included as covariates, as they were associated with region-of-interest volume (see Tables S8 and S9 and Figures S1 and S2 in the online supplement). No age-by-sex interactions on region-of-interest volume were detected (see Table S10 in the online supplement). Handedness was largely not associated with region-of-interest volumes and therefore was not used as a covariate in follow-up models, in line with our previous large-scale studies of handedness and brain laterality (26) (see Table S11 in the online supplement). Because IQ and related measures are consistently found to be associated with brain volume (see Table S12 in the online supplement), IQ was included in secondary analyses.
Medications that have been found to have significant associations with subcortical volume, including first- and second-generation antipsychotics, anticonvulsants, and antidepressants, were added as covariates in secondary analyses (see the Supplemental Methods section and Table S13 in the online supplement).
For gross volumetric analyses, Cohen’s d effect size estimates were computed from the t-statistic of the group variable from the regression models (27). To correct for multiple comparisons, a standard false discovery rate (FDR) correction was applied across all regions of interest of the five main comparisons (22q11DS compared with controls, A-D compared with controls, A-B compared with controls, A-B compared with A-D, and 22q11DS with psychosis compared with 22q11DS without psychosis) at the conventionally accepted level of 5% (q=0.05) (28). FDR-corrected p values <0.05 were considered significant. Gross volume results surviving Bonferroni correction (0.05/85 total tests across all five main analysis contrasts, p<0.00058) are reported in Table S14 in the online supplement.
For vertex-wise Jacobian and thickness analyses, the multiple linear regression model was fitted at each point across the surface. Because these values were calculated in native space (i.e., without scaling the image), ICV was used to adjust for effects of head size. A modified searchlight FDR procedure was applied globally across all structures for each statistical model, with FDR-corrected p values <0.05 considered significant (see the Supplemental Methods section in the online supplement).
Additional details regarding the main analyses (22q11DS compared with controls, effects of deletion size, 22q11DS with psychosis compared with 22q11DS without psychosis) are provided in the Supplemental Methods section.
Cross-Disorder Comparison: 22q11DS With Psychosis, Idiopathic Schizophrenia, and Other Neuropsychiatric Disorders
To compare the pattern of 22q11DS with psychosis with that of other neuropsychiatric disorders, Spearman rank correlations were used to correlate Cohen’s d effect size estimates from the 22q11DS with and without psychosis analyses with comparable case-control analyses from the ENIGMA schizophrenia (6), major depressive disorder (29), bipolar disorder (30), obsessive-compulsive disorder (OCD) (31), ASD (32), and ADHD (33) working group studies. Each of these studies constitutes the largest investigation of subcortical structure to date and used harmonized processing and quality control protocols (see the Supplemental Methods section for details).
Results
22q11DS Group Compared With Healthy Control Group
Gross volumetric analysis revealed significant group differences across the majority of regions of interest (14 of 17), with moderate to large effects (Cohen’s d values, −0.90 to 0.93) (Figure 1; see also Table S14 in the online supplement). The pattern of effects included significantly lower volumes in the 22q11DS group relative to the control group for total ICV and thalamus, putamen, hippocampus, and amygdala volumes. In contrast, the 22q11DS group had greater lateral ventricle, caudate, and accumbens volumes. Effects were greatest for the lateral ventricles (54.05%−60.02% weighted mean difference, larger in the 22q11DS group) and the hippocampus (10.75%−11.85% weighted mean difference, smaller in the 22q11DS group). These results (in terms of both pattern and effect size) essentially remained when adjusted for medication, when adjusted for IQ, and when scanning site was treated as a random effect (see Tables S15, S16, and S17 in the online supplement). In addition, a significant group-by-age interaction was detected for the left and right caudate and pallidum and the left thalamus. Whereas the left thalamus and left and right caudate volumes tended to be lower in the 22q11DS group with increasing age, the pattern was reversed for the pallidum (i.e., greater age-associated decrease in pallidum volume in the control group relative to the 22q11DS group; see Table S18 and Figure S3 in the online supplement). No sex-by-diagnosis interactions were detected for any region of interest (see Table S19 in the online supplement).
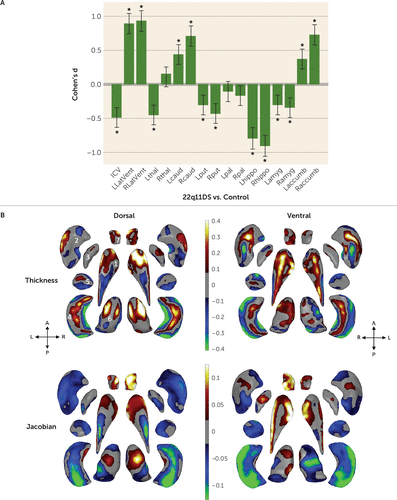
FIGURE 1. Participants with 22q11.2 deletion syndrome compared with healthy control subjects: gross volume and shape analysis of brain regions of interesta
a In panel A, effect sizes (Cohen’s d) with 95% confidence intervals are plotted for major pairwise gross volumetric comparisons. An asterisk (*) indicates a significant group difference after correction for multiple comparisons for the 22q11.2 deletion syndrome (22q11DS) group compared with the control group (false discovery rate [FDR] adjusted q<0.05). Positive effect sizes indicate 22q11DS group > control group; negative effect sizes indicate control group > 22q11DS group. Models were adjusted for age, age-squared, sex, ICV, and scan site. (Full statistical model outputs including standard error, coefficient estimates, p values, and percent difference are provided in Table S14 in the online supplement.) accumb=accumbens; amyg=amygdala; caud=caudate; hippo=hippocampus; ICV=intracranial volume; L=left; LatVent=lateral ventricle; put=putamen; pal=pallidum; R=right; thal=thalamus. Panel B presents the shape analysis, with regression coefficients plotted in regions that met the threshold for correction for multiple comparisons (FDR q<0.05). Blue/green colors indicate negative coefficients, or regions of lower thickness (local radial distance) or Jacobian (local surface area contraction) measures in the 22q11DS group compared with the control group. Red/yellow colors indicate positive coefficients, or regions of greater thickness or Jacobian values in the 22q11DS group compared with the control group. Gray regions indicate areas of no significant difference after correction for multiple comparisons. Dorsal and ventral views of the structures are provided: A=anterior; P=posterior; L=left; R=right. The numbering in the upper left subpanel indicates structures as follows: 1=caudate; 2=putamen; 3=globus pallidus; 4=hippocampus; 5=amygdala; 6=thalamus; 7=nucleus accumbens.
Subcortical shape analysis revealed complex group differences, involving subregions with both higher and lower thickness and Jacobian values in the 22q11DS relative to the control group (Figure 1). In particular, greater local thickness was observed in the head of the caudate, the thalamus, and dorsal and ventral hippocampal regions, while the caudate body and lateral and medial hippocampal subregions were thinner. The Jacobian analysis revealed surface area contraction across large portions of the putamen, amygdala, and hippocampus and dilation across anterior and lateral regions of the caudate and most of the nucleus accumbens. These effects were robust to adjustments for medication and region-of-interest volume (see Figure S4 in the online supplement).
Effects of Deletion Size
Analysis of covariance results indicated significant differences between gross volumes across the A-D, A-B, and control group matched samples (see Table S20 in the online supplement). While no gross volume differences between participants with 22q11DS with A-B compared with A-D deletions surpassed correction for multiple comparisons (Figure 2; see also Table S14 in the online supplement), shape analysis revealed that participants with A-B deletions had higher local surface area (higher Jacobian measures) in the hippocampus, thalamus, and putamen and lower caudate and accumbens thickness/Jacobian measures compared with those with A-D deletions (Figure 2). The hippocampus showed complex subregional thickness effects, with thicker medial and lateral aspects and thinner dorsal and ventral regions in the A-B deletion group compared with the A-D deletion group. These results remained stable when adjusted for region of interest and medication (see Figure S7 in the online supplement). Comparisons of both deletion groups relative to control subjects are detailed in Figure 2 and in the Supplemental Results section (Figures S5 and S6) and Discussion sections A and B in the online supplement.
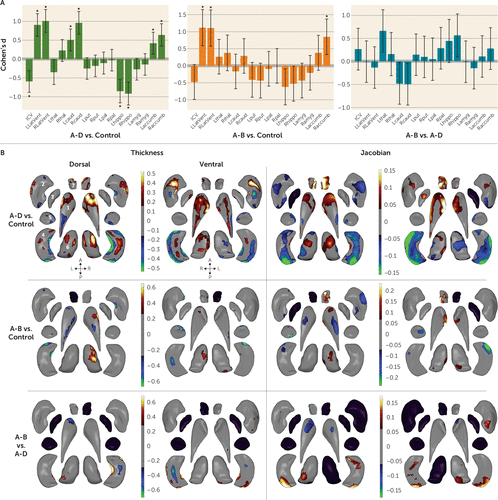
FIGURE 2. Effects of deletion size in participants with 22q11.2 deletion syndrome compared with healthy control subjects: gross volume and shape analysis of brain regions of interesta
a In panel A, effect sizes (Cohen’s d) with 95% confidence intervals are plotted for major pairwise gross volumetric comparisons. An asterisk (*) indicates a significant group difference after correction for multiple comparisons (false discovery rate [FDR] adjusted q<0.05). FDR-corrected p values <0.05 were considered significant. All models were adjusted for age, age-squared, sex, ICV, and scan site. (Full statistical model outputs including standard error, coefficient estimates, p values, and percent difference are provided in Table S14 in the online supplement.) accumb=accumbens; amyg=amygdala; caud=caudate; hippo=hippocampus; ICV=intracranial volume; L=left; LatVent=lateral ventricle; put=putamen; pal=pallidum; R=right; thal=thalamus. Panel B presents the shape analysis, with regression coefficients plotted in regions that met the threshold for correction for multiple comparisons (FDR q<0.05). Blue/green colors indicate negative coefficients, or regions of lower thickness or Jacobian measures in the case group compared with the control group (the group listed first is the case group, and the group listed second is the control group). Red/yellow colors indicate positive coefficients, or regions of greater thickness or Jacobian values in the case group compared with the control group. Thickness represents local radial distance and Jacobian represents local surface area dilation/contraction. Gray regions indicate areas of no significant difference after correction for multiple comparisons. Black structures are those for which no vertex-wise test was significant after correction for multiple comparisons. Dorsal and ventral views of the structures are provided: A=anterior; P=posterior; L=left; R=right. The numbering in the upper left subpanel indicates structures as follows: 1=caudate; 2=putamen; 3=globus pallidus; 4=hippocampus; 5=amygdala; 6=thalamus; 7=nucleus accumbens.
Effects of Psychotic Disorder in 22q11DS
The groups of participants with 22q11DS with and without psychosis were well matched demographically. However, as expected, the group with psychosis had higher rates of treatment with antipsychotics and anticonvulsants and lower IQ compared with the group without psychosis (see Table S3 in the online supplement). A significant psychosis-by-age interaction was observed for the left and right caudate, in which the group with psychosis had relatively larger caudate volumes with increased age compared with the group without psychosis (see Table S23 in the online supplement).
The 22q11DS group with psychosis showed significantly smaller hippocampus, amygdala, and right thalamus volumes and ICV compared with the matched group with 22q11DS without psychosis (Figure 3; see also Table S14 in the online supplement). These effects were similar when the analyses were adjusted for medication and IQ (see Tables S24 and S25 in the online supplement). However, when the analyses were additionally adjusted for ICV, no group differences survived correction for multiple comparisons, likely because of significantly lower overall ICV volumes in the group with psychosis (see Table S26 in the online supplement).
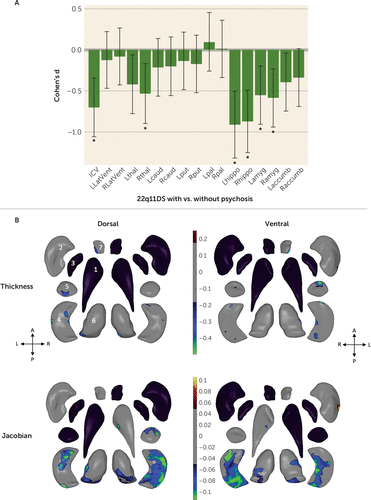
FIGURE 3. Effects of psychotic illness in participants with 22q11.2 deletion syndrome: gross volume and shape analysis of brain regions of interesta
a In panel A, Cohen’s d effect sizes (with 95% confidence intervals) are plotted for major pairwise gross volumetric comparisons. An asterisk (*) indicates significant group difference after correction for multiple comparisons (false discovery rate [FDR] adjusted q<0.05) for participants with 22q11.2 deletion syndrome (22q11DS) with psychosis compared with those without psychosis. Models were adjusted for age, age-squared, sex, and scan site. (Full statistical model outputs including standard error, coefficient estimates, p values, and percent difference are provided in Table S14 in the online supplement.) accumb=accumbens; amyg=amygdala; caud=caudate; hippo=hippocampus; ICV=intracranial volume; L=left; LatVent=lateral ventricle; put=putamen; pal=pallidum; R=right; thal=thalamus. Panel B presents the shape analysis comparing the 22q11DS groups with and without psychosis, with regression coefficient values plotted in regions that met the threshold for correction for multiple comparisons (q<0.05). Blue/green colors indicate negative coefficients, or regions of lower thickness (local radial distance) or Jacobian (local surface area contraction) measures in the 22q11DS with psychosis group compared with the 22q11DS without psychosis group. Red/yellow colors indicate positive coefficients, or regions of greater thickness or Jacobian values in the 22q11DS with psychosis group compared with the 22q11DS without psychosis group. Gray regions indicate areas of no significant difference after correction for multiple comparisons. Black structures are those for which no vertex-wise test was significant after correction for multiple comparisons. Dorsal and ventral views of the structures are provided: A=anterior; P=posterior; L=left; R=right. The numbering in the upper left subpanel indicates structures as follows: 1=caudate; 2=putamen; 3=globus pallidus; 4=hippocampus; 5=amygdala; 6=thalamus; 7=nucleus accumbens.
Subcortical shape analysis revealed lower thalamus, hippocampus, amygdala, and nucleus accumbens thickness and Jacobian measures in participants with 22q11DS with psychosis compared with those without psychosis, with particularly prominent reductions in the hippocampus. There was one region along the left dorsal putamen where the reverse pattern was observed (higher surface area in the 22q11DS group with psychosis) (Figure 3). After adjustment for medication, the effects were diminished but exhibited a similar pattern (see Figure S8 in the online supplement). When ICV was also adjusted for, two clusters survived correction for multiple comparisons: a region of higher surface area in the left putamen and lower surface area in the right hippocampus (see Figure S9 in the online supplement). When the analyses adjusted for both ICV and medication, no shape differences survived correction for multiple comparisons.
22q11DS Psychosis Cross-Disorder Comparisons
Effect sizes for subcortical region-of-interest volumes when comparing the 22q11DS group with psychosis and the group without (see Tables S27 and S28 in the online supplement) were significantly correlated with those from the ENIGMA schizophrenia, major depressive disorder, bipolar disorder, and OCD case-control studies. However, effect sizes for the 22q11DS group with psychosis were not significantly correlated with those from the ENIGMA ASD and ADHD case-control studies (Figure 4). In contrast, effect sizes for the comparison between the 22q11DS group overall and the control group were not significantly correlated with those observed in any other disorder (see Figure S10 in the online supplement).
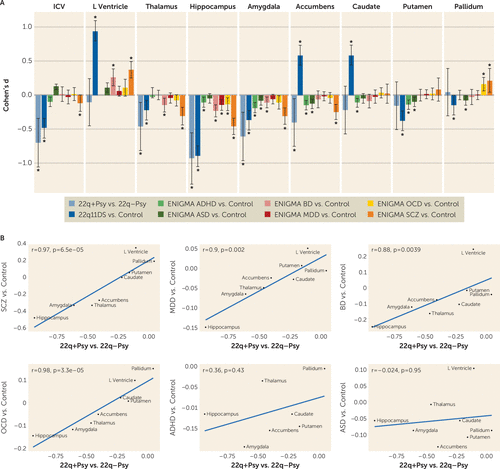
FIGURE 4. Cross-disorder comparisons from the ENIGMA psychiatric working group subcortical studiesa
a In panel A, case-control effect size estimates (Cohen’s d) are plotted from the ENIGMA schizophrenia (6), major depression (29), bipolar disorder (30), obsessive-compulsive disorder (31), autism spectrum disorder (32), and attention deficit hyperactivity disorder (33) working group studies. An asterisk (*) indicates significant group difference, including 95% confidence intervals from original study publication. Note that the ENIGMA ADHD group did not assess lateral ventricle volume in their subcortical study. Panel B presents Spearman rank correlations between 22q11.2 deletion syndrome (22q11DS) with psychosis (22q+Psy) compared with 22q11.2 deletion syndrome without psychosis (22q–Psy) effect size estimates and those from the other ENIGMA psychiatric working groups (eight regions of interest: lateral ventricle, amygdala, hippocampus, thalamus, caudate, putamen, pallidum, and nucleus accumbens). Significant correlations were found between 22q11DS with psychosis and the ENIGMA schizophrenia, major depressive disorder, bipolar disorder, and obsessive-compulsive disorder working group studies. ADHD=attention deficit hyperactivity disorder; ASD=autism spectrum disorder; BD=bipolar disorder; L ventricle=lateral ventricle; MDD=major depressive disorder; OCD=obsessive-compulsive disorder; SCZ=schizophrenia.
Discussion
This study represents the largest neuroimaging investigation to date of subcortical brain structure in 22q11DS and provides five key findings:
We detected robust group differences between participants with 22q11DS and healthy control subjects using conventional measures of gross volume. Even when accounting for overall smaller ICV, we found smaller left and right hippocampus, amygdala, and putamen and left thalamus volumes and larger left and right caudate, accumbens, and lateral ventricle volumes.
Subcortical shape analysis revealed complex local morphometric differences between participants with 22q11DS and healthy control subjects across most subcortical regions of interest, not discernible by conventional gross volumetric analysis.
Shape analysis also revealed, for the first time, significant localized effects of deletion size on subregions of the hippocampus, thalamus, caudate, putamen, and accumbens, with less severe disruptions of subcortical morphometry in participants with smaller deletions.
Participants with 22q11DS with psychosis had lower ICV and thalamus, hippocampus, and amygdala volumes compared with participants with 22q11DS without a history of psychosis, with effects driven largely by contracted surface area across subregions of these structures.
Specifically, subcortical alterations in the 22q11DS psychosis group significantly overlapped with effects observed in the largest studies of subcortical structure in schizophrenia, bipolar disorder, major depressive disorder, and OCD, but not with those seen in ASD and ADHD. Effect sizes for the 22q11DS group overall and the 22q11DS with psychosis group were generally greater than those found in other ENIGMA studies of idiopathic neuropsychiatric disorders.
Our large multisite cohort study revealed overlapping but more extensive group differences than previously detected in our single-site study (25), with significant differences in gross volume observed across 14 of 17 regions, all with moderate to large effect sizes and with consistent results across sites. Typically developing control subjects showed generally expected age effects on subcortical volumes, with age-by-diagnosis interactions in gross volume of the left and right caudate and pallidum and the left thalamus, indicating possible divergent developmental trajectories, which will be the focus of future longitudinal studies.
Shape analysis revealed subregional patterns of both higher and lower local thickness and surface area relative to control subjects, particularly in larger structures (caudate, putamen, hippocampus, and thalamus). Interestingly, these findings parallel our cortical analysis of 22q11DS, in which general patterns of lower cortical surface area and greater thickness were reversed in regions with extensive subcortical connections, such as the cingulate and parahippocampal gyri (17).
In the hippocampus, participants with 22q11DS showed thinning along the lateral and medial axis, likely corresponding to CA1 and subiculum subfields (16), with thickening in dorsal and ventral regions, which may correspond to CA2–CA4 subfields and parts of the subiculum. Jacobian maps indicate a more extensive pattern of contracted surface area, consistent with decreased density of dendritic spines and impaired dendritic growth in hippocampal neurons observed in mouse models of 22q11DS (34). Complex alterations in other structures, such as the thalamus and caudate, appear to overlap with underlying nuclei that project to cortical association areas serving higher-order cognitive functions (see the Supplemental Results section and Discussion section C in the online supplement).
While there were no significant differences in gross volume, shape analysis revealed regions of lower surface area in the hippocampus, putamen, and thalamus as well as greater caudate surface area and thickness in participants with the large A-D deletion compared with those with the A-B deletion. This pattern again parallels our cortical findings, where the larger A-D deletion was associated with lower cortical surface area compared with the A-B deletion (17). While significant, the observed effects of deletion size on subcortical morphometry warrant replication in larger samples, given the limited number of participants in our sample with smaller (A-B) deletions.
Consistent with findings in the ENIGMA schizophrenia cohort (6), psychosis in 22q11DS was associated with lower ICV and hippocampus, amygdala, and right thalamus volumes. Significant correlations between the pattern of subcortical disruptions in 22q11DS with psychosis and schizophrenia suggest concordance with idiopathic schizophrenia, also observed at the cortical level (17). These findings further support the genetics-first approach in providing valuable insight into mechanisms underlying the development of psychosis not only in 22q11DS but in the broader population. Here, shape analysis additionally revealed that lower gross volumes in the 22q11DS with psychosis group were driven primarily by contracted surface area across these structures, with several regional effects (lower hippocampus and higher putamen surface area) that exceeded global brain size effects after adjusting for ICV. Functionally, altered hippocampal-prefrontal connectivity has been associated with working memory impairments in 22q11DS mice (35); deficits in both spatial working memory and functional connectivity (36) are well documented in both 22q11DS and idiopathic schizophrenia. Overall smaller hippocampal volumes observed in individuals with 22q11DS, and particularly in those with psychosis, may underlie connectivity defects.
Interestingly, subcortical effect sizes in the 22q11DS with psychosis group were also correlated with those from ENIGMA studies of bipolar disorder, major depressive disorder, and OCD, suggesting globally similar profiles of subcortical alterations across this set of neuropsychiatric disorders. Although elevated rates of bipolar disorder have not been reported in large studies of 22q11DS (2, 37), the correlation between subcortical patterns in 22q11DS with psychosis and other disorders may reflect the underlying genetic overlap between schizophrenia, bipolar disorder, major depression, and other psychiatric illnesses (38). Common subcortical structural abnormalities across disorders further motivates the use of shape analysis techniques to define more localized effects across subcompartments with known structural and functional connectivity patterns.
In contrast, the patterns we found in 22q11DS with psychosis diverged from those seen in ASD and ADHD, suggesting distinct subcortical disruptions in these earlier-onset disorders. Although these are common comorbidities in 22q11DS, subcortical disruptions in the 22q11DS group overall did not significantly overlap with those seen in the corresponding idiopathic disorders investigated (see Figure S10 in the online supplement).
The large sample size (the largest ever studied in 22q11DS) and centralized processing and analysis of raw neuroimaging data were key strengths of our study. However, certain limitations must be noted. First, the relationship between subregional shape measures and underlying cytoarchitecture is not well understood. The correspondence of such shape variations to changes in underlying subfields and gene expression is a focus of ongoing work. Second, we cannot rule out the possibility that some nonpsychotic individuals with 22q11DS may later develop a psychotic disorder, which would likely attenuate the group differences reported here. Further investigation of 22q11DS medical comorbidities (e.g., cardiovascular abnormalities) and comorbid psychiatric diagnoses (e.g., ASD) were outside the scope of this study but will be pursued in future work, as they may also contribute to variability in brain structure. Longitudinal studies are under way to investigate the developmental trajectories of psychotic symptom emergence and other psychiatric disorders, which will greatly improve our understanding of both the heterogeneity and the developmental effects of 22q11DS.
While our cross-disorder analysis is strengthened by harmonized processing protocols applied across the largest existing neuroimaging studies of their kind, these studies included individuals with different age ranges and demographic profiles, which could affect such relationships. Future work directly comparing harmonized measures across demographically matched samples will help address such limitations.
In summary, we have shown robust differences in subcortical structure between individuals with 22q11DS and demographically comparable healthy control subjects, with more extreme alterations in participants with 22q11DS with larger deletions or psychosis. Subcortical alterations in 22q11DS-associated psychosis overlapped with those from the largest studies to date of subcortical structure in idiopathic schizophrenia and other serious mental illnesses. This finding further supports 22q11DS as a biologically applicable framework for understanding brain mechanisms that underlie the development of these disorders, and it will be the focus of future ENIGMA cross-disorder and genetic analyses.
1 : 22q11.2 deletion syndrome. Nat Rev Dis Primers 2015; 1:15071Crossref, Medline, Google Scholar
2 : Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry 2014; 171:627–639Link, Google Scholar
3 : The 22q11.2 deletion syndrome as a window into complex neuropsychiatric disorders over the lifespan. Biol Psychiatry 2014; 75:351–360Crossref, Medline, Google Scholar
4 : Copy number variation at 22q11.2: from rare variants to common mechanisms of developmental neuropsychiatric disorders. Mol Psychiatry 2013; 18:1153–1165Crossref, Medline, Google Scholar
5 : The schizophrenia phenotype in 22q11 deletion syndrome. Am J Psychiatry 2003; 160:1580–1586Link, Google Scholar
6 : Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 2016; 21:585Crossref, Medline, Google Scholar
7 : Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proc Natl Acad Sci USA 2009; 106:16434–16445Crossref, Medline, Google Scholar
8 : FGF8 acts as a classic diffusible morphogen to pattern the neocortex. Development 2010; 137:3439–3448Crossref, Medline, Google Scholar
9 : Frontal and caudate alterations in velocardiofacial syndrome (deletion at chromosome 22q11.2). J Child Neurol 2004; 19:337–342Crossref, Medline, Google Scholar
10 : Thalamic reductions in children with chromosome 22q11.2 deletion syndrome. Neuroreport 2004; 15:1413–1415Crossref, Medline, Google Scholar
11 : Hippocampal volume reduction in chromosome 22q11.2 deletion syndrome (22q11.2DS): a longitudinal study of morphometry and symptomatology. Psychiatry Res 2012; 203:1–5Crossref, Medline, Google Scholar
12 : An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development 2002; 129:4591–4603Medline, Google Scholar
13 : Alterations in midline cortical thickness and gyrification patterns mapped in children with 22q11.2 deletions. Cereb Cortex 2009; 19:115–126Crossref, Medline, Google Scholar
14 : Maximizing power to track Alzheimer’s disease and MCI progression by LDA-based weighting of longitudinal ventricular surface features. Neuroimage 2013; 70:386–401Crossref, Medline, Google Scholar
15 : Progressive deformation of deep brain nuclei and hippocampal-amygdala formation in schizophrenia. Biol Psychiatry 2008; 64:1060–1068Crossref, Medline, Google Scholar
16 : Subcortical neuromorphometry in schizophrenia spectrum and bipolar disorders. Neuroimage Clin 2016; 11:276–286Crossref, Medline, Google Scholar
17 : Large-scale mapping of cortical alterations in 22q11.2 deletion syndrome: convergence with idiopathic psychosis and effects of deletion size. Mol Psychiatry (Epub ahead of print, June 13, 2018)Google Scholar
18 : How does your cortex grow? J Neurosci 2011; 31:7174–7177Crossref, Medline, Google Scholar
19 : Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci 2017; 37:3402–3412Crossref, Medline, Google Scholar
20 : Variance of IQ is partially dependent on deletion type among 1,427 22q11.2 deletion syndrome subjects. Am J Med Genet A 2018; 176:2172–2181Crossref, Medline, Google Scholar
21 : Heritability of the shape of subcortical brain structures in the general population. Nat Commun 2016; 7:13738Crossref, Medline, Google Scholar
22 : A Riemannian framework for intrinsic comparison of closed genus-zero shapes. Inf Process Med Imaging 2015; 24:205–218Medline, Google Scholar
23 : A neurogenetic model for the study of schizophrenia spectrum disorders: the International 22q11.2 Deletion Syndrome Brain Behavior Consortium. Mol Psychiatry 2017; 22:1664–1672Crossref, Medline, Google Scholar
24 : Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355Crossref, Medline, Google Scholar
25 : Mapping 22q11.2 gene dosage effects on brain morphometry. J Neurosci 2017; 37:6183–6199Crossref, Medline, Google Scholar
26 : Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the ENIGMA consortium. Proc Natl Acad Sci USA 2018; 115:E5154–E5163Crossref, Medline, Google Scholar
27 : Effect size, confidence interval, and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc 2007; 82:591–605Crossref, Medline, Google Scholar
28 : Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995; 57:289–300Google Scholar
29 : Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 2016; 21:806–812Crossref, Medline, Google Scholar
30 : Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry 2016; 21:1710–1716Crossref, Medline, Google Scholar
31 : Distinct subcortical volume alterations in pediatric and adult OCD: a worldwide meta- and mega-analysis. Am J Psychiatry 2017; 174:60–69Link, Google Scholar
32 : Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: results from the ENIGMA ASD working group. Am J Psychiatry 2018; 175:359–369Link, Google Scholar
33 : Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry 2017; 4:310–319Crossref, Medline, Google Scholar
34 : Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci 2008; 11:1302–1310Crossref, Medline, Google Scholar
35 : Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature 2010; 464:763–767Crossref, Medline, Google Scholar
36 : Molecular substrates of altered axonal growth and brain connectivity in a mouse model of schizophrenia. Neuron 2015; 86:680–695Crossref, Medline, Google Scholar
37 : High frequencies of de novo CNVs in bipolar disorder and schizophrenia. Neuron 2011; 72:951–963Crossref, Medline, Google Scholar
38 : Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet 2013; 45:984–994Crossref, Medline, Google Scholar



