White Matter in Schizophrenia Treatment Resistance
Abstract
Objective:
Failure of antipsychotic medications to resolve symptoms in patients with schizophrenia creates a clinical challenge that is known as treatment resistance. The causes of treatment resistance are unknown, but it is associated with earlier age at onset and more severe cognitive deficits. The authors tested the hypothesis that white matter deficits that are involved in both neurodevelopment and severity of cognitive deficits in schizophrenia are associated with a higher risk of treatment resistance.
Methods:
The study sample (N=122; mean age, 38.2 years) included schizophrenia patients at treatment initiation (N=45), patients whose symptoms were treatment responsive (N=40), and patients whose symptoms were treatment resistant (N=37), as well as healthy control subjects (N=78; mean age, 39.2 years). White matter regional vulnerability index (RVI) was tested as a predictor of treatment resistance and cognitive deficits. Higher RVI is indicative of better agreement between diffusion tensor imaging fractional anisotropy across the brain in an individual and the pattern identified by the largest-to-date meta-analysis of white matter deficits in schizophrenia.
Results:
Patients with treatment-resistant symptoms showed the highest white matter RVI (mean=0.38 [SD=0.2]), which was significantly higher than the RVI among patients with treatment-responsive symptoms (mean=0.30 [SD=0.02]). At the onset of treatment, schizophrenia patients showed significantly higher RVI than healthy control subjects (mean=0.18 [SD=0.03] and mean=0.13 [SD=0.02], respectively). RVIs were significantly correlated with performance on processing speed and negative symptoms.
Conclusions:
Schizophrenia affects white matter microstructure in specific regional patterns. Susceptibility to white matter regional deficits is associated with an increased likelihood of treatment resistance. Developments to overcome schizophrenia treatment resistance should consider white matter as an important target.
Modern antipsychotic medications fail to resolve clinical symptoms in approximately a third of people with schizophrenia, a condition known as treatment resistance (1). For several decades, this clinical challenge has motivated attempts to develop more effective next-generation antipsychotics (2–6). However, research has been hindered by insufficient understanding of the neurobiological mechanisms of and lack of valid brain biomarkers for treatment resistance. Current antipsychotic drugs share their neurotransmitter targets in dopaminergic systems. Their ineffectiveness in patients with treatment-resistant symptoms suggests that other mechanisms and neurotransmitter systems may be involved, although the evidence is still preliminary (7–10). Some structural neuroimaging studies suggest that treatment resistance is associated with reduced gray matter and cortical thickness (7, 11, 12). However, a meta-analysis found no replicated neuroimaging findings when comparing patients with treatment-resistant schizophrenia with patients whose clinical symptoms were treatment responsive (13).
Patients with treatment-resistant schizophrenia share two consistent features: earlier age at illness onset and more severe cognitive deficits (14–17); this suggests both neurodevelopmental and cognitive contributions to treatment resistance. Therefore, brain measures that track with neurodevelopmen and cognitive dysfunctions in schizophrenia may help identify biomarkers of treatment resistance. White matter shows promise as a research focus in schizophrenia, and the largest meta-analytic study of white matter deficits in schizophrenia to date, conducted by the Enhancing Neuro Imaging Genetics Through Meta-Analysis (ENIGMA) consortium, determined a region-specific pattern of white matter deficits (18). The pattern of regional differences found in the ENIGMA study confirmed findings from previous diffusion tensor imaging (DTI) studies that demonstrated significant deficits in the frontal associative white matter regions. This included the anterior corona radiata and the genu and body of the corpus callosum (19–23). The pattern indicated in the ENIGMA study was also in line with histological findings of reduced glial cell density and myelination in the frontal lobe in patients with schizophrenia (24–26). This regional white matter deficit pattern as identified in the ENIGMA study contributes to core cognitive dysfunctions, especially in processing speed deficits in schizophrenia (27–30). Interestingly, among all of the cognitive abnormalities associated with treatment resistance, the largest deficit is observed in processing speed (16). Both white matter and processing speed development follow an inverse-U neurodevelopmental trajectory, and the peak of myelination for associative white matter overlaps with the peak of processing speed abilities (31, 32). The origin of the white matter deficit pattern in schizophrenia is not fully understood, but the disorder-related alterations in development that prevents normal development of late-myelinating areas likely prevents the establishment of the normal pattern of interneuronal communication (33). This may lead to the observed contrast in regional deficits between late- and early-myelinating white matter regions in schizophrenia, as observed in the ENIGMA results and in other studies (18, 34, 35). Therefore, we hypothesized that the regional white matter deficit pattern may represent a neurobiological mechanism that leads to treatment resistance in schizophrenia. To test this hypothesis, we assessed whether white matter regional deficits index treatment resistance by comparing patients with treatment-resistant schizophrenia with patients whose symptoms were treatment responsive. In parallel, we compared regional deficits in patients who underwent imaging within 2 weeks of the initiation of antipsychotic treatment with healthy control subjects. We used this comparison to control for potential chronic antipsychotic medication effects on white matter deficits and to determine whether the identified white matter deficits were also associated with schizophrenia, independent of chronic disease and treatment courses. Evidence supporting this hypothesis would implicate white matter deficits as a contributing factor of treatment resistance.
Methods
Clinical Characteristics
Participants were 122 patients with schizophrenia (57 of them male; mean age, 38.2 years [SD=13.3]) and 78 healthy control subjects (37 of them male; mean age, 39.2 years [SD=14.0]) (Table 1). Patients with schizophrenia included individuals with treatment-resistant symptoms (17 of them male; mean age, 47.8 years [SD=8.9]) and, as a primary comparison group, patients with treatment-responsive symptoms (18 of them male; mean age, 46.3 years [SD=11.5]), who were frequency matched on age and sex (all p values >0.2). In addition, a third patient cohort comprising individuals who were within the first 2 weeks of treatment initiation (22 of them male; mean age, 28.6 years [SD=10.1]) was recruited to determine whether any identified white matter deficits were also associated with schizophrenia independently of chronic disease and treatment courses. This treatment-initiation group was necessarily younger; however, the three patient groups combined and the healthy control group were frequency matched on age and sex distribution (all p values >0.2). Data were collected in 2017 and 2018. The patients were recruited from Beijing Huilongguan Hospital. Healthy control subjects were recruited through local advertisements. All patients met DSM-IV criteria for schizophrenia. Participants were of a homogeneous Chinese ethnic background, which is considered advantageous for identifying treatment resistance biomarkers, because ethnicity may have significant effects on treatment resistance (36). Written informed consent was obtained from all study participants in accordance with the Helsinki Declaration, and the research protocol was approved by local ethics committees.
| Schizophrenia Patients | Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Healthy Control Subjects (N=78) | Treatment Initiation (N=45) | Treatment Responsive (N=40) | Treatment Resistant (N=37) | Four Groups | Treatment Resistant Versus Treatment Responsive | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | df | p | t | p | |
| Age (years) | 39.2 | 13.4 | 28.6 | 10.1 | 46.3 | 11.5 | 47.8 | 8.9 | 17.1 | 3, 196 | 1×10–10 | 0.6 | 0.5 |
| Positive and Negative Syndrome Scale (total score) | 74.7 | 13.0 | 49.6 | 12.2 | 69.0 | 19.5 | 11.8 | 1×10–19 | |||||
| Illness duration (years) | 2.5 | 1.7 | 23.4 | 5.0 | 22.9 | 6.3 | 0.4 | 0.7 | |||||
| Education (years) | 13.0 | 2.9 | 12.7 | 3.5 | 12.3 | 2.9 | 12.0 | 3.0 | 2.6 | 3, 196 | 0.11 | 0.5 | 0.6 |
| Antipsychotic dosage (chlorpromazine equivalents, mg/day) | 176 | 117 | 404 | 345 | 781 | 354 | 4.1 | 1×10–5 | |||||
| N | % | N | % | N | % | N | % | F | df | p | t | p | |
| Current smoker | 27 | 35 | 13 | 29 | 11 | 27 | 11 | 30 | 2.2 | 3, 196 | 0.15 | 0.7 | 0.5 |
TABLE 1. Demographic and clinical characteristics of patients with schizophrenia and healthy control subjects
The treatment-resistant and treatment-responsive groups were defined on the basis of consensus guidelines (1). Patients with treatment-resistant schizophrenia met the criteria of 1) little response to treatment with at least two different antipsychotic medications with a dosage equivalence of chlorpromazine ≥600 mg/day for ≥12 weeks, 2) a Brief Psychiatric Rating Scale (BPRS) score ≥45, and 3) a Clinical Global Impressions severity scale (CGI-S) score ≥4 during the current assessment. The treatment-responsive group was defined by periods of good clinical response to antipsychotic medications as indicated by a CGI-S score <3 over the ≥12-week duration. The treatment-resistant and treatment-responsive groups were frequency matched on age, sex, years of education, and duration of treatment. Patients who did not meet criteria for either group were excluded.
Participants in the treatment initiation group had no antipsychotic medication exposure until study enrollment and were included in order to identify whether treatment resistance biomarkers, if found, were present at the onset of illness with minimal antipsychotic exposure. These patients were treated on admission into the study without delay, and treatment helped stabilize them for MRI scanning. Imaging data were collected within 2 weeks of treatment initiation.
All participants had no current or past neurological conditions, unstable major medical conditions, or current or previous substance dependence (although nicotine dependence was permitted). The study participants’ demographic and clinical characteristics are summarized in Table 1.
Six patients were medication free (N=4, N=2, and N=0 for the treatment-initiation group, treatment-responsive group, and treatment-resistant group, respectively). Seven patients were taking first-generation antipsychotic medications (N=4, N=3, and N=0 for the three groups, respectively). The remaining patients were taking the following second-generation antipsychotic medications: risperidone (N=17, N=11, and N=7 for the three groups, respectively), clozapine (N=0, N=9, and N=22 for the three groups, respectively), olanzapine (N=9, N=11, and N=7 for the three groups, respectively), aripiprazole (N=8, N=5, and N=2 for the three groups, respectively), paliperidone (N=2, N=0, and N=3 for the three groups, respectively), or amisulpride, iloperidone, lurasidone, or quetiapine (N=1, N=4, and N=4 for the three patient groups, respectively); of the patients taking second-generation antipsychotics, 45 were taking more than one antipsychotic medication (N=4, N=7, and N=34 for the three groups, respectively). Chlorpromazine equivalents were calculated (37), and the mean chlorpromazine-equivalent dosage for patients in the treatment-resistant group was nearly twice that for patients in the treatment-responsive group (p<0.001) (Table 1). Patients in the treatment-initiation group were taking much smaller daily doses and had been medicated for an average of 4.2 days (SD=2.3; range, 0–12 days).
Symptom and Neurocognitive Evaluations
Patients were evaluated with the Positive and Negative Syndrome Scale (PANSS), the BPRS, and the CGI-S by one of three attending psychiatrists; interrater reliability was maintained at >0.80. The BPRS and CGI-S were used for group definition only. The PANSS was used for symptom assessment. Cognitive function was assessed with the MATRICS Consensus Cognitive Battery (MCCB), covering seven cognitive domains and a composite score (38–40). Raw scores were converted to Chinese normative T scores (40).
DTI and Data Processing
Imaging data were collected with a 3-T Prisma MRI scanner (Siemens Medical Solutions, Erlangen, Germany), equipped with a 64-channel radio frequency head coil, at the imaging research center of the Beijing Huilongguan Hospital. DTI data were collected using a spin-echo, echo-planar imaging sequence with a spatial resolution of 1.7×1.7×1.7 mm (TE=87 ms, TR=8,000 ms, field of view=200 mm, axial slice orientation with 82 slices and no gaps, 98 isotropically distributed diffusion-weighted directions, two diffusion-weighting values [b=0 and 1,000 seconds/mm2] and five b=0 images). Participants’ head movements were minimized with restraining padding. DTI data were processed using the ENIGMA-DTI analysis pipeline (https://www.nitrc.org/projects/enigma_dti) (41). All data included in the analysis passed ENIGMA-DTI quality assurance and quality control. Regional white matter fractional anisotropy (FA) was generated for 21 major regions on the basis of the ENIGMA-DTI atlas, averaged across hemispheres.
This cohort is independent from the samples used in the ENIGMA study and in our previous study (18, 27), and an ancillary aim was to use the sample to replicate the association between the regional deficit pattern identified in the ENIGMA study and cognitive deficits in schizophrenia (27).
Statistical Analysis
The ENIGMA study provided a meta-analysis of the case/control effects associated with schizophrenia in 21 major white matter regions (Cohen’s d) (for further details, see Table S1 in the online supplement). We developed an index of regional impairment at the individual level, the regional vulnerability index (RVI), as a simple measure of agreement between an individual’s pattern of FA in these 21 regions and the expected pattern of schizophrenia across these regions as shown in the ENIGMA results. FA for each of the white matter regions was converted to z values by 1) calculating the residual values following the regression of effects of age and sex, and 2) for each individual, subtracting the average value for a region and dividing it by the standard deviation calculated from the healthy control group. This produced a vector of normalized z values (one for each region) for each individual in the study sample. The RVI was then calculated as the correlation coefficient (normalized dot product) between the vector of region-wise z values for the study subjects and the vector of regional schizophrenia-healthy control effect sizes in the ENIGMA study. We used the term “vulnerability” here to imply a narrow definition—whether the regional white matter deficit pattern identified in the ENIGMA study is associated with an increased likelihood of treatment resistance. Higher RVI values imply that the pattern of white matter regional values followed the regional vulnerability pattern for schizophrenia as determined by the ENIGMA meta-analysis. All group comparisons of imaging and cognitive measures were performed using the general linear model while controlling for age and sex. Bonferroni corrections were applied to correct for the number of regions examined. Associations of RVI and clinical measures were examined by bivariate correlation analyses while controlling for age and sex, and Bonferroni corrections were applied.
Results
Specific White Matter Region and Treatment Resistance
Schizophrenia patients had significantly lower whole-brain average FA compared with healthy control subjects (Cohen’s d=0.69, t=5.1, p=1×10−6), and the regional effect sizes were significant in four of the 21 regions after correcting for multiple (N=21) comparisons (see Table S1 in the online supplement). The average effect size in this sample was not significantly different from the effect size in the ENIGMA study (t=1.2, p=0.2). The effect sizes of the regional schizophrenia-healthy control differences were correlated with those in the ENIGMA study (r=0.85, p=1×10−5) (Figure 1A). This pattern was also observed when the three patient groups were compared individually with the healthy control group (Figure 1B–1D), with the strongest effect in the treatment-resistant group (r=0.92, p=1×10−8) (Figure 1B). Frontal associative tracts, such as the anterior corona radiata and the genu of the corpus callosum, showed the largest schizophrenia-healthy control effect sizes in the ENIGMA study, and they were also the regions that showed the largest FA reduction in the treatment-resistant group compared with the healthy control group (Figure 1B). However, there were no significant differences in the whole-brain average or regional FA measurements between the treatment-resistant and treatment-responsive groups (Table 2) or between the treatment-initiation and healthy control groups (Table 2).
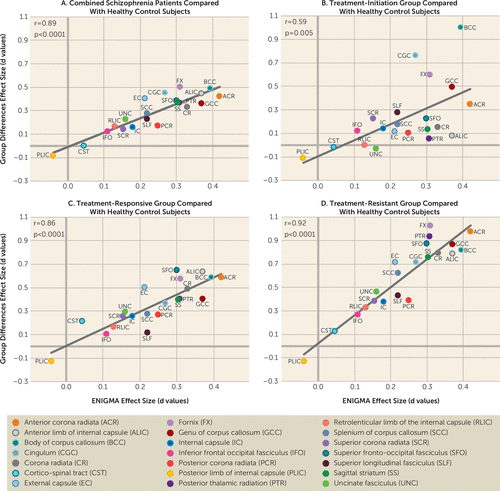
FIGURE 1. Effect sizes of group (schizophrenia groups compared with healthy control subjects) in 21 white matter regions, plotted against effect sizes in the ENIGMA studya
a The mean effect size of group (Cohen’s d) of the 21 white matter regions was plotted against the effect sizes from the region in the Enhancing Neuro Imaging Genetics Through Meta-Analysis (ENIGMA) meta-analysis for all study subjects (panel A) and for the three schizophrenia subgroups (panels B–D). The significant correlations indicate that the pattern of fractional anisotropy deficits in the Chinese sample were consistent with the pattern in the ENIGMA study. The data (means and standard deviations) of all the effect sizes are presented in Table S1 in the online supplement.
| MCCB Associations With Treatment Status | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schizophrenia-Related Cognitive Deficits | Treatment-Resistance Related Cognitive Deficits | MCCB Associations With RVI in Schizophrenia Patients | ||||||||||||||||
| Treatment Initiation (N=45) | Healthy Control (N=78) | Analysis | Treatment Resistant (N=37) | Treatment Responsive (N=40) | Analysis | Treatment Initiation (N=45) | Treatment Responsive (N=40) | Treatment Resistant (N=37) | ||||||||||
| Variable | Mean | SD | Mean | SD | t | p | Mean | SD | Mean | SD | t | p | r | p | r | p | r | p |
| Visual learning | 47.8 | 8.6 | 54.9 | 7.4 | 4.7 | 1×10–5b | 42.4 | 11.8 | 46.0 | 11.5 | 1.4 | 0.2 | 0.0 | 0.9 | –0.44 | 5×10–3c | –0.10 | 0.5 |
| Verbal learning | 50.5 | 10.2 | 54.5 | 8.7 | 2.3 | 0.02 | 43.3 | 13.0 | 51.0 | 11.7 | 2.4 | 0.01 | 0.0 | 0.9 | –0.32 | 0.03 | –0.11 | 0.4 |
| Social cognition | 48.4 | 9.3 | 53.1 | 10.0 | 2.4 | 0.02 | 42.1 | 12.4 | 46.3 | 10.5 | 1.6 | 0.1 | 0.0 | 0.9 | –0.08 | 0.6 | 0.0 | 0.9 |
| Reasoning and problem solving | 43.9 | 7.8 | 57.9 | 7.4 | 9.1, | 2×10–15b | 45.3 | 9.8 | 45.7 | 9.8 | 0.2 | 0.9 | –0.15 | 0.3 | –0.11 | 0.5 | –0.10 | 0.5 |
| Processing speed | 47.3 | 7.3 | 57.2 | 7.9 | 6.3 | 6×10–9b | 43.8 | 11.9 | 45.3 | 9.9 | 1.0 | 0.3 | –0.10 | 0.4 | –0.53 | 7×10–4b | –0.16 | 0.3 |
| Working memory | 47.0 | 9.8 | 47.0 | 6.9 | 7.1 | 1×10–10b | 39.3 | 14.1 | 46.8 | 10.7 | 2.9 | 0.004c | –0.05 | 0.6 | –0.24 | 0.12 | –0.12 | 0.4 |
| Total score | 46.8 | 7.9 | 58.9 | 6.8 | 8.2 | 1×10–12b | 40.0 | 11.1 | 45.2 | 11.5 | 2.5 | 0.1 | 0.0 | 0.9 | –0.42 | 5×10–3c | –0.10 | 0.5 |
TABLE 2. MATRICS Consensus Cognitive Battery (MCCB) cognitive domain and total scores and associations with treatment stages and regional vulnerability index (RVI)a
White Matter RVI and Treatment Resistance
The average RVI was significantly higher in the combined patient group (treatment initiation, treatment responsive, and treatment resistant) compared with the healthy control group (mean=0.29, SD=0.01, compared with mean=0.13, SD=0.02; t=6.4, df=198, p=1×10−9). The treatment-resistant group had the highest RVI (mean=0.38, SD=0.02), followed by the treatment-responsive group (mean=0.30, SD=0.03) and the treatment-initiation group (mean=0.18, SD=0.02), with all three patient groups showing significant differences compared with the healthy control group (mean=0.13, SD=0.02; all t values ≥2.7, p values ≤0.01) (Figure 2A).
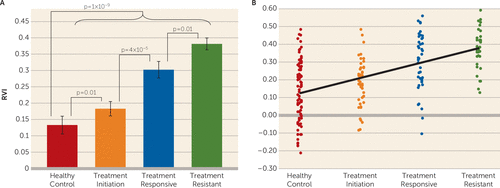
FIGURE 2. Regional vulnerability index (RVI) among healthy control subjects and schizophrenia patientsa
a Panel A shows the average coefficients of RVI plotted by group. Panel B shows a significant ordinal trend of elevation of RVI with respect to group assignment (p<0.001).
The treatment-resistant group had a significantly higher RVI than the treatment-responsive group (t=2.7, df=75, p=0.01), suggesting that a higher RVI was associated with treatment resistance. Patients in the treatment-initiation group had a significantly higher RVI than individuals in the healthy control group (t=2.8, df=121, p=0.007), suggesting that a higher RVI was unlikely to be primarily a result of chronic illness or chronic antipsychotic medication exposure. Chronic patients (the combined treatment-resistant and treatment-responsive cohort) had a significantly higher RVI compared with patients in the treatment-initiation group (t=4.2, df=153, p=4×10−5), suggesting perhaps additional disease progression or a chronic medication effect (Figure 2).
These relationships can be further illustrated by examining the ordinal trend of the group RVI in the following order: healthy control, treatment initiation, treatment responsive, and treatment resistant. We applied mid-rank scoring to assign scores for the group variable and performed general-linear regression analysis of the ordinal trend (42) and found that the trend was statistically significant (F=57.2, df=1, 198, p<0.001) (Figure 2B).
Cognition and White Matter Regional Vulnerability
The MCCB total score was most severely reduced in the treatment-resistant group (t=11.6, df=113, p=3×10−17), followed by the treatment-responsive group (t=8.4, df=116, p=7×10−13) and the treatment initiation group (t=8.2, df=121, p=2×10−12), compared with healthy control subjects (Table 2). Compared with patients in the treatment-responsive group, patients with treatment-resistant schizophrenia showed no significant impairment on MCCB total or domain scores after Bonferroni correction; however, working memory was nominally significantly different between these two groups (Table 2). There were significant differences in cognitive measures between the treatment-initiation and healthy control groups (Table 2). RVI was significantly associated with processing speed only for the treatment-responsive group (r=−0.53, p=7×10−4) after correcting for multiple comparisons (Table 1). We also explored MCCB correlations with the whole-brain average FA and 21 regional values. However, there were no significant regional FA-MCCB correlations in the combined or individual patient groups after correction for multiple comparisons, with the exception of significant correlations between whole-brain average FA and processing speed scores (r=0.31, r=5×10−4) in the combined patient sample after correcting for multiple comparisons.
Clinical Correlates
There were no significant associations between RVIs and the PANSS total, positive, or general symptom scores (all r values <0.1, all p values >0.2), but there was a significant correlation with negative symptoms in the combined patient group (r=0.26, p=0.002). However, no regional FA value was significantly correlated with negative symptoms after Bonferroni correction (all r values <0.27, all p values >0.003). No regional FA values were significant in any patient subgroup (all p values >0.1). RVIs were not significantly correlated with illness duration (all p values >0.20), chlorpromazine-equivalent dosage (all p values >0.41), or smoking status (all p values >0.15) in any combined or individual patient group. More than half the patients in the treatment-resistant group were taking clozapine (N=22, compared with N=15 for those not taking clozapine), but RVI values did not differ between patients who were taking clozapine and those who were not (t=0.2, df=35, p=0.8).
Replications for the Association Between Regional Deficit Pattern and Processing Speed
Regional FA effect sizes in the ENIGMA study were significantly correlated with regional FA-processing speed correlation coefficients (r=0.90, p<0.001) (Figure 3A) and regional FA-working memory (r=0.84, p<0.001) correlation coefficients (Figure 3C), replicating findings from another sample (27). When controlling for working memory, the ENIGMA-based regional effect sizes remained significant in explaining regional FA-processing speed correlation coefficients (partial r=0.88, p<0.001) (Figure 3B), but the opposite was not significant (partial r=0.25, p=0.4) (Figure 3D). Permutation analysis showed that the difference in the partial correlation coefficients was significant (p=0.01), suggesting that the relationships between cognition measures and white matter were driven primarily by processing speed rather than working memory. Similar trends were observed in patients (Figure 3E–H) and control subjects (Figure 3I–L) separately. Our findings in this sample of Chinese patients (Figure 3) largely replicate findings in the U.S. sample (see Figure S1 in the online supplement).
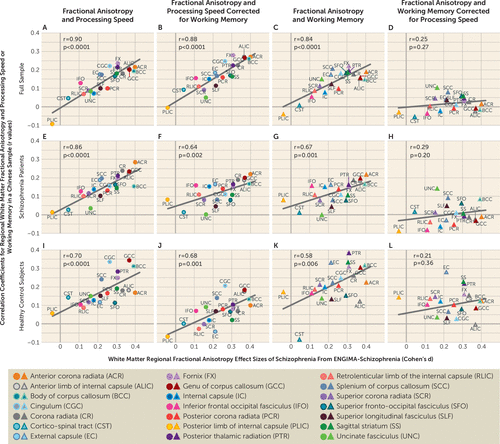
FIGURE 3. Replication of the relationship between cognition and white matter vulnerability to schizophrenia identified in the ENIGMA studya
a ENIGMA=Enhancing Neuro Imaging Genetics Through Meta-Analysis. The graphs show the replication of the relationship between cognition and white matter vulnerability to schizophrenia that was previously found by our research group (27) (see the figure of the original findings in the U.S. sample presented in Figure S1 in the online supplement). Effect sizes of the 21 major white matter regions in the ENIGMA study are also shown, with higher values indicating more severe impairment in schizophrenia patients compared with healthy control subjects in the ENIGMA meta-analysis (x-axis). In addition, the correlation coefficients between fractional anisotropy (FA) of each region and cognitive measures in processing speed or working memory in the Chinese sample in the present study are shown (y-axis). Panel A shows the relationship between correlation coefficients for regional FA-processing speed (y-axis) and regional FA effect sizes of schizophrenia from the ENIGMA project (x-axis, Cohen’s d). Panel B shows partial correlation coefficients in panel A after correcting for working memory. Panel C shows the relationship between correlation coefficients for regional FA values and working memory (y-axis) and regional FA effect sizes of schizophrenia (x-axis). Panel D shows partial correlation coefficients in panel C after correcting for processing speed. This was examined in the full sample (panels A–D) and then in the three patient groups (panels E–H) and the healthy control group (panels I–L) separately.
Discussion
We found that the white matter regional deficit pattern in schizophrenia was significantly associated with treatment resistance. The white matter RVI significantly differentiated patients with treatment-resistant schizophrenia from patients with treatment-responsive schizophrenia, even with matched treatment duration and in the absence of global FA differences. The RVI measure further significantly differentiated patients at treatment initiation from healthy control subjects. These results suggest that the extent of regional white matter vulnerability, as defined by the RVI, can be observed in schizophrenia at initial diagnosis and treatment and may mark the liability for treatment resistance to the currently available antipsychotic medications. Follow-up longitudinal studies will be required to test whether higher RVI at the onset would track with the development of treatment resistance.
Treatment resistance has been linked to cognitive deficits, especially in processing speed (15, 16) and negative symptoms (14), although the underlying mechanisms remain unknown (28–30). Data from this study suggest that white matter may represent a shared underlying neurobiology, because the regional vulnerability pattern appears to be associated with treatment resistance and with more severe negative symptoms. We also replicated findings from a previous study in which the schizophrenia pattern in the ENIGMA meta-analysis predicted an association between FA and processing speed and working memory (27) (Figure 3; see also Figure S1 in the online supplement). That study employed only two cognitive tasks, which was considered a limitation (27). In the present study, we used the MCCB to replicate these findings, and the similar pattern across two diverse (U.S. and Chinese) samples further validates white matter regional vulnerability in indexing core cognitive deficits in schizophrenia.
A white matter association with treatment resistance was initially suggested by white matter volume reduction findings (43), but opposite results have also been reported (11). Several DTI studies further suggested a white matter effect in patients with treatment-resistant schizophrenia (44, 45). However, without a direct comparison using sufficient sample sizes with age-, sex-, and treatment duration-matched patients who are treatment responsive, it is difficult to interpret whether these previous findings were related to schizophrenia in general or were specific to treatment-resistant schizophrenia. We showed that multiple white matter regions had robust and significant reductions in FA in schizophrenia patients compared with healthy control subjects (see Table S1 in the online supplement), which is consistent with many previous studies (19–23, 27, 46, 47). No individual white matter region could consistently differentiate patients with treatment-resistant schizophrenia from patients with treatment-responsive schizophrenia after correction for multiple comparisons. Why the RVI but not the individual regional measures captured treatment resistance (by comparison with treatment response) is not immediately clear. RVI is a readily obtainable index that correlates normalized regional FA of each person to the schizophrenia effect sizes in the ENIGMA study and is assumed to reflect the contrast between the high vulnerability to schizophrenia of late-developing associative white matter regions and the lower vulnerability of early-developing regions (34, 48, 49). We speculate that by taking into account white matter across the whole brain, the RVI may have reduced nonspecific effects that affect all white matter regions and further accentuated the regional effects specific to schizophrenia (Figure 1A–1C) and treatment resistance (Figure 1D). Therefore, a higher RVI in the patient groups may have identified individuals with more severe patterns of neurodevelopmental white matter impairment, who in turn may be more vulnerable to treatment resistance.
Patients with treatment-resistant schizophrenia are invariably given higher doses of medications, including multiple medications, and are more likely to receive prescriptions for clozapine, compared with patients with treatment-responsive schizophrenia. These factors could confound the discovery of the neurobiology of treatment resistance by hindering the isolation of biomarkers for treatment resistance from the medication effects. To mitigate this concern, we analyzed a group of patients who were assessed within 2 weeks of initiating antipsychotic treatment. We observed a significant heterogeneity in the RVI values within this patient group. However, the candidate treatment-resistant biomarker was present in patients with minimal antipsychotic medication exposure, ruling out the possibility that higher RVI is a chronic disease effect but rather is associated with schizophrenia even at the onset of treatment. However, this study is limited by its cross-sectional nature, and follow-up studies are needed to test whether RVI observed at this stage would predict treatment resistance compared with treatment response among patients initiating treatment.
The white matter pathways most strongly associated with treatment resistance—for example, the fornix (the main white matter bundle of axonal fibers inside and outside of the hippocampus) and the anterior corona radiata (the main white matter connecting ipsilateral prefrontal cortices) (effect sizes around 1.0) (Figure 1D)—are well-known associative pathways for supporting cognitive functions (50, 51). In patients with treatment-responsive schizophrenia, the effect sizes of the same tracts were weaker (e.g., range for the fornix and anterior corona radiate, 0.5–0.6) (Figure 1C), suggesting that impairments in associative tracts that support cognition may play a larger role in treatment resistance. The agreement between white matter FA regional effect sizes in patients with treatment-resistant schizophrenia in this study and patients in the ENIGMA study (r=0.92) also provides a novel perspective on the ENIGMA results. Currently available antipsychotic medications have failed to attenuate symptoms in treatment-resistant schizophrenia, having only limited effects on cognitive deficits (52, 53). The ENIGMA-DTI sample was global, and the meta-analytical aggregation likely removed specific local medication and environmental variables, yielding schizophrenia-related neurobiology that was untreated and shared across sites. The remarkable alignment between patients in the ENIGMA study and the treatment-resistant group in this study indicates that the white matter regional effect size pattern identified in the ENIGMA study may underlie the critical unmet treatment targets (i.e., treatment resistance, in addition to cognitive deficits) (27) in schizophrenia. It may also suggest that the white matter regional effect size pattern identified in the ENIGMA meta-analysis has limitations, because it is less strongly associated with schizophrenia during treatment initiation (r=0.58) and more strongly associated with the treatment-resistant aspect of schizophrenia.
Another potential limitation is that patients with treatment-resistant schizophrenia are treated with clozapine as the treatment of choice. Post hoc analyses did not show an association between the RVI and clozapine, and an elevated RVI was already present in the treatment-initiation group. This study focused on differences between treatment-resistant and treatment-responsive groups that were age- and sex-matched. The treatment-initiation group had a significantly lower mean age than the treatment-resistant and treatment-responsive groups, which is a potential limitation. However, the RVI was developed to be independent of age and sex, and all analyses were performed while controlling for age and sex. Finally, only FA was used to index white matter abnormalities, and other diffusion parameters (axial, radial, and mean diffusivities) were not explored. We chose FA because it showed higher sensitivity to schizophrenia deficits compared with these other parameters (18). Future studies should reexamine these findings using more advanced DTI parameters (54).
Conclusions
Part of the etiopathological origins of treatment resistance in schizophrenia may lie in white matter deficits. Patients showing a pattern of regional white matter impairment in the late-developing frontal associative fibers and no or limited impairment in the early-developing sensory and motor fibers were more likely to have symptoms that were resistant to contemporary antipsychotic medications. Development of new treatments and therapies to overcome schizophrenia treatment resistance should more strongly consider strategies that target white matter-related mechanisms.
1 : Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am J Psychiatry 2017; 174:216–229Link, Google Scholar
2 : Efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia: a network meta-analysis. JAMA Psychiatry 2016; 73:199–210Crossref, Medline, Google Scholar
3 : Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol Psychiatry 2015; 20:695–702Crossref, Medline, Google Scholar
4 : Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry 2003; 60:49–56Crossref, Medline, Google Scholar
5 : Placebo-controlled trial of d-cycloserine added to conventional neuroleptics, olanzapine, or risperidone in schizophrenia. Am J Psychiatry 2002; 159:480–482Link, Google Scholar
6 : Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796Crossref, Medline, Google Scholar
7 : Treatment-resistant schizophrenia patients show elevated anterior cingulate cortex glutamate compared to treatment-responsive. Schizophr Bull 2016; 42:744–752Crossref, Medline, Google Scholar
8 : Glutamatergic neurometabolites in clozapine-responsive and -resistant schizophrenia. Int J Neuropsychopharmacol 2015; 18:18(6) pii: pyu117. doi: 10.1093/ijnp/pyu117Crossref, Google Scholar
9 : Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry 2014; 75:e11–e13Crossref, Medline, Google Scholar
10 : Searching human brain for mechanisms of psychiatric disorders. Implications for studies on schizophrenia. Schizophr Res 2015; 167:91–97Crossref, Medline, Google Scholar
11 : Extensive gray matter volume reduction in treatment-resistant schizophrenia. Int J Neuropsychopharmacol 2015; 18:
12 : Dysfunctional striatal systems in treatment-resistant schizophrenia. Neuropsychopharmacology 2016; 41:1274–1285Crossref, Medline, Google Scholar
13 : Neuroimaging findings in treatment-resistant schizophrenia: a systematic review: lack of neuroimaging correlates of treatment-resistant schizophrenia. Schizophr Res 2015; 164:164–175Crossref, Medline, Google Scholar
14 : Formal thought disorder in people at ultra-high risk of psychosis. BJPsych Open 2017; 3:165–170Crossref, Medline, Google Scholar
15 : Differential cognitive performances between schizophrenic responders and non-responders to antipsychotics: correlation with course of the illness, psychopathology, attitude to the treatment and antipsychotics doses. Psychiatry Res 2013; 210:387–395Crossref, Medline, Google Scholar
16 : Profiling cognitive impairment in treatment-resistant schizophrenia patients. Psychiatry Res 2016; 235:133–138Crossref, Medline, Google Scholar
17 : Treatment-resistant schizophrenia. Psychiatr Clin North Am 2016; 39:239–265Crossref, Medline, Google Scholar
18 : Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry 2018; 23:1261–1269Crossref, Medline, Google Scholar
19 : Alterations of superficial white matter in schizophrenia and relationship to cognitive performance. Neuropsychopharmacology 2013; 38:1954–1962Crossref, Medline, Google Scholar
20 : Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry 2008; 165:1024–1032Link, Google Scholar
21 : What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front Integr Nuerosci 2013; 7:9Crossref, Medline, Google Scholar
22 : A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res 2007; 41:15–30Crossref, Medline, Google Scholar
23 : Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 2009; 108:3–10Crossref, Medline, Google Scholar
24 : White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry 2003; 60:443–456Crossref, Medline, Google Scholar
25 : Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia 2003;53:1075–1085Google Scholar
26 : Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res 2004; 67:269–275Crossref, Medline, Google Scholar
27 : Association of white matter with core cognitive deficits in patients with schizophrenia. JAMA Psychiatry 2017; 74:958–966Crossref, Medline, Google Scholar
28 : Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry 2010; 167:828–835Link, Google Scholar
29 : Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry 2007; 64:532–542Crossref, Medline, Google Scholar
30 : Defining a cognitive function decrement in schizophrenia. Biol Psychiatry 2005; 57:688–691Crossref, Medline, Google Scholar
31 : Neuroglialpharmacology: myelination as a shared mechanism of action of psychotropic treatments. Neuropharmacology 2012; 62:2137–2153Crossref, Medline, Google Scholar
32 : Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging 2010; 31:1554–1562Crossref, Medline, Google Scholar
33 : Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 2014; 344:
34 : Heterochronicity of white matter development and aging explains regional patient control differences in schizophrenia. Hum Brain Mapp 2016; 37:4673–4688Crossref, Medline, Google Scholar
35 : Abnormal trajectory of intracortical myelination in schizophrenia implicates white matter in disease pathophysiology and the therapeutic mechanism of action of antipsychotics. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3:454–462Crossref, Medline, Google Scholar
36 : Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol Med 2016; 46:3231–3240Crossref, Medline, Google Scholar
37 : Antipsychotic treatment response in schizophrenia. Am J Health Syst Pharm 2012; 69:1872–1879Crossref, Medline, Google Scholar
38 : The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry 2008; 165:214–220Link, Google Scholar
39 : The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 2008; 165:203–213Link, Google Scholar
40 : Clinical reliability and validity of the version of Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery. Chin J Psychiatry. 2009; 42:29–33Google Scholar
41 : Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage 2013; 81:455–469. doi:
42 : Categorical Data Analysis, 3rd ed, Hoboken, NJ, Wiley and Sons, 2013Google Scholar
43 : Increase in gray matter and decrease in white matter volumes in the cortex during treatment with atypical neuroleptics in schizophrenia. Schizophr Res 2005; 80:61–71Crossref, Medline, Google Scholar
44 : White matter changes in treatment refractory schizophrenia: Does cognitive control and myelination matter? Neuroimage Clin 2018; 18:186–191Crossref, Medline, Google Scholar
45 : Altered interhemispheric and temporal lobe white matter microstructural organization in severe chronic schizophrenia. Neuropsychopharmacology 2014; 39:944–954Crossref, Medline, Google Scholar
46 : White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry 2010; 167:451–458Link, Google Scholar
47 : Genetic contributions to the midsagittal area of the corpus callosum. Twin Res Hum Genet 2012; 15:315–323Crossref, Medline, Google Scholar
48 : On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology 1996; 14(Suppl):1S–11SCrossref, Medline, Google Scholar
49 : Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophr Res 1995; 16:87–110Crossref, Medline, Google Scholar
50 : Alterations of white matter tracts following neurotoxic hippocampal lesions in macaque monkeys: a diffusion tensor imaging study. Hippocampus 2010; 20:906–910Medline, Google Scholar
51 : The longitudinal decline of white matter microstructural integrity in behavioral variant frontotemporal dementia and its association with executive function. Neurobiol Aging 2019; 76:62–70Crossref, Medline, Google Scholar
52 : The CATIE schizophrenia trial: results, impact, controversy. Harv Rev Psychiatry 2007; 15:245–258Crossref, Medline, Google Scholar
53 : A meta-analysis and critical review of the effects of conventional neuroleptic treatment on cognition in schizophrenia: opening a closed book. Biol Psychiatry 2004; 55:1013–1022Crossref, Medline, Google Scholar
54 : Diffusion-weighted imaging uncovers likely sources of processing-speed deficits in schizophrenia. Proc Natl Acad Sci USA 2016; 113:13504–13509Crossref, Medline, Google Scholar



