Identifying Novel Types of Irritability Using a Developmental Genetic Approach
Abstract
Objective:
Irritability, which is strongly associated with impairment and negative outcomes, is a common reason for referral to mental health services but is a nosological and treatment challenge. A major issue is how irritability should be conceptualized. The authors used a developmental approach to test the hypothesis that there are several forms of irritability, including a “neurodevelopmental/ADHD-like” type, with onset in childhood, and a “depression/mood” type, with onset in adolescence.
Methods:
Data were analyzed from the Avon Longitudinal Study of Parents and Children, a prospective U.K. population-based cohort. Irritability trajectory classes were estimated for 7,924 individuals with data at multiple time points across childhood and adolescence (four possible time points from approximately ages 7 to 15). Psychiatric diagnoses were assessed at approximately ages 7 and 15. Psychiatric genetic risk was indexed by polygenic risk scores (PRSs) for attention deficit hyperactivity disorder (ADHD) and depression, derived using large genome-wide association study results.
Results:
Five irritability trajectory classes were identified: low (81.2%), decreasing (5.6%), increasing (5.5%), late-childhood limited (5.2%), and high-persistent (2.4%). The early-onset high-persistent trajectory was associated with male preponderance, childhood ADHD (odds ratio=108.64, 95% CI=57.45–204.41), and ADHD PRS (odds ratio=1.31, 95% CI=1.09–1.58). The adolescent-onset increasing trajectory was associated with female preponderance, adolescent depression (odds ratio=5.14, 95% CI=2.47–10.73), and depression PRS (odds ratio=1.20, 95% CI=1.05–1.38). Both the early-onset high-persistent and adolescent-onset increasing trajectory classes were associated with adolescent depression diagnosis and ADHD PRS.
Conclusions:
The developmental context of irritability may be important in its conceptualization: early-onset persistent irritability may be more neurodevelopmental/ADHD-like and later-onset irritability more depression/mood-like. These findings have implications for treatment as well as nosology.
Irritability—a heightened propensity to anger, relative to peers—is a common reason for referral to mental health services and is strongly associated with impairment and long-term adverse outcomes (1–6), yet it remains a nosological and treatment challenge (1, 2). Currently, it is treated as a homogeneous construct; however, it is a core or accompanying feature of several psychiatric disorders, and such differential associations suggest that subtyping may be necessary. The purpose of this study was to examine the possibility that there are multiple forms of irritability, including a “neurodevelopmental/ADHD-like” type, with onset in childhood, and a “depression/mood” type, with onset in adolescence.
Childhood irritability has typically been considered a feature of oppositional defiant disorder (7)—in the forthcoming ICD-11, it is likely to be considered a specifier of oppositional defiant disorder. However, irritability has been shown to be distinct from other oppositional defiant disorder dimensions (headstrong, hurtful) in that it shows phenotypic and genetic associations with unipolar depression (5, 8). In DSM-5, severe, chronic childhood irritability is categorized as disruptive mood dysregulation disorder and grouped with the mood disorders (9). ICD-11 and DSM-5 also include irritability as a diagnostic symptom of depression in children and adolescents (specifically dysthymic disorder in ICD-11).
Yet irritability—and, more broadly, emotional dysregulation—is an especially common feature of attention deficit hyperactivity disorder (ADHD), which is categorized as a neurodevelopmental disorder in DSM-5. Irritability prevalence rates as high as 91% have been reported in children with the disorder (10). Evidence of clinical overlap between irritability and ADHD (11, 12), genetic overlap with ADHD, and features such as its manifestation in early development and male preponderance led recently to the suggestion that irritability should perhaps be conceptualized as a neurodevelopmental/ADHD-like problem, rather than a mood problem (12).
Two differentiating factors between neurodevelopmental and mood problems are developmental course and sex preponderance. Neurodevelopmental problems typically have an early onset, decline across childhood and adolescence, and are more common in males, whereas mood problems tend to start in adolescence and are more common in females (13). A developmental approach may therefore help to better establish whether irritability is more appropriately conceptualized as a mood or a neurodevelopmental problem.
One possibility is that irritability is a heterogeneous construct: there may be different “types” of irritability, including a neurodevelopmental/ADHD-like irritability and a depression/mood-like irritability. Consistent with this premise, a recent population-based cross-sectional investigation of irritability symptoms found different developmental patterns among males and females: irritability was more common in boys during childhood (and levels tended to decrease with age) but more common in girls in adolescence (and levels tended to increase with age) (12). These findings are consistent with there being two types of irritability: one type that has an early onset and is more common among boys (a pattern typical of neurodevelopmental problems) and another type that starts in adolescence and is more common in girls (a pattern typical of mood problems). To our knowledge, age at onset in childhood compared with adolescence has not previously been investigated as a possible source of heterogeneity in irritability.
Our aim in this study was to take advantage of a longitudinal population-based cohort and use a developmental approach to test the hypothesis that there are at least two forms of irritability: one neurodevelopmental/ADHD-like type with onset in childhood and one depression/mood type with onset in adolescence. Specifically, we used a latent growth-modeling approach to test the following hypotheses suggested by this formulation of irritability: 1) an irritability trajectory defined by an early age at onset would be associated with male sex, ADHD genetic liability as indexed by ADHD genetic risk scores (polygenic risk scores [PRSs]), and a higher rate of diagnosis of ADHD in childhood; and 2) an irritability trajectory defined by an age at onset around early to mid-adolescence would be associated with female sex, depression genetic liability as indexed by depression genetic risk scores, and a diagnosis of depression in adolescence.
Methods
Participants
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a well-established prospective longitudinal birth cohort study. The enrolled core sample comprised 14,541 mothers living in Avon, England, who had expected delivery dates between April 1, 1991, and December 31, 1992. Of these pregnancies, 13,988 offspring were alive at 1 year of age. When the oldest children were approximately 7 years old, the sample was augmented with eligible children who had not joined the study originally, resulting in enrollment of 713 additional children. The resulting total sample size of children who were alive at 1 year was 14,701. Genotype data were available for 8,365 children after quality control. Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the local research ethics committees. Full details of the study design, measures, and sample have been published elsewhere (14, 15). The study web site contains details of all data available via a fully searchable data dictionary (http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary). For families with multiple births, we included the oldest sibling. In the analyses, participants with at least two time points of irritability data (N=7,924) were included. For further details of sample sizes for available data, see Figure S1 in the online supplement.
Irritability
In line with previous research (7), irritability was defined using parent-reported data from the oppositional defiant disorder section of the Development and Well-Being Assessment (16)—a structured research diagnostic interview—at ages 7 years 7 months, 10 years 8 months, 13 years 10 months, and 15 years 6 months. For the first three assessments, the parent version of the Development and Well-Being Assessment was sent to mothers in a package of measures. For the final assessment, mothers independently completed the same version of the assessment at the ALSPAC clinic. Irritability during the past 6 months was measured by three items (severe temper tantrums, touchy and easily annoyed, and angry and resentful) rated on a 3-point scale (0 to 2, indicating no more than others, a little more than others, a lot more than others) and summed to yield a total score (0–6). Distributions and descriptive statistics are summarized in Table S1 in the online supplement.
Diagnoses
The Development and Well-Being Assessment was also used to assess ADHD, oppositional defiant disorder (which included the irritability items), conduct disorder, generalized anxiety disorder, and depression. In childhood (age 7 years 7 months), parent reports were used to assess all diagnoses. In adolescence (age 15 years 6 months), parent reports were used to assess ADHD, oppositional defiant disorder, and conduct disorder, and participant self-reports were used to assess generalized anxiety disorder and depression. DSM-IV diagnoses were generated through computer algorithms (17). No diagnoses were mutually exclusive.
Genetic Liability
PRSs were used to capture common variant genetic liability for two disorders: depression and ADHD. For each disorder, PRSs were generated as the weighted mean number of disorder risk alleles in approximate linkage equilibrium (r2<0.1), derived from imputed autosomal single-nucleotide polymorphisms using PRSice (18) (genetic data were available for 5,559 ALSPAC participants included in this study [for further details, see Figure S1 in the online supplement]). Sensitivity analyses were conducted using inverse probability weighting (19) to assess the impact of missing data (see the online supplement). Risk alleles were defined as those associated with case status from the latest genome-wide association studies (GWAS) of depression (N=135,458 case subjects and N=344,901 control subjects) (20) and ADHD (N=19,099 case subjects and N=34,194 control subjects) (21). The depression GWAS sample consisted of adults with depression, although affected individuals had varying ages at onset (e.g., see reference 22). Individuals in the ADHD GWAS sample all had ADHD, which by definition has onset in childhood. Primary analyses defined risk alleles as those associated at a p value <0.05; associations across a range of p-value thresholds are presented in Figure S2 in the online supplement. Scores were standardized using Z-score transformation. Genotyping details, as well as full methods for generating the PRSs, are summarized in the online supplement.
Analyses
Growth mixture modeling was conducted to identify developmental trajectories of irritability across ages 7–15 in Mplus (23). Growth mixture modeling aims to group individuals into categories (trajectories) based on patterns of change across multiple time points, with individuals within each category assumed to have the same growth curve (24). Thus, differing levels of irritability are captured on the basis of observed differences (i.e., data driven) rather than cut-points, in line with evidence supporting a continuous distribution for liability to psychiatric problems (25). Starting with a single k-class solution, k+1 solutions are fitted until the optimum solution is reached. Models were run using a robust maximum likelihood parameter estimator and full-information maximum likelihood estimation (23). As recommended, the optimal number of categories was determined by interpretability as well as model-fit indices (see the online supplement) (24). After identification of the irritability trajectories, to test associations with PRS, sex, and diagnosis and to estimate and compare prevalence rates, analyses were conducted in Mplus using a bias-free three-step approach that accounts for measurement error in class assignment (26); multinomial odds ratios are reported. Inverse probability weighting (19) was used to assess the impact of missing genetic data, which weights observations based on measures assessed in pregnancy that were predictive of variables in the analysis or inclusion in the subsample with genetic data (see the online supplement). Sensitivity analyses were conducted including sex as a covariate.
Results
Irritability Trajectories
We identified a five-class solution (see the online supplement), characterized by distinct irritability trajectory classes: low (81.2%), decreasing (5.6%), increasing (5.5%), late-childhood limited (5.2%), and high-persistent (2.4%) (Figure 1).
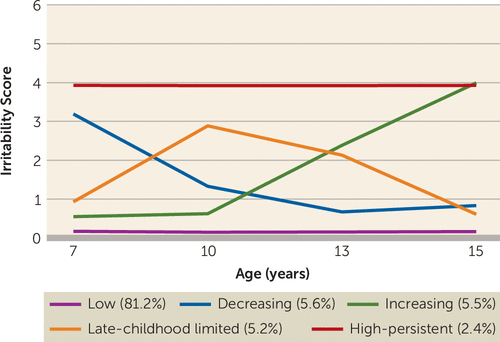
FIGURE 1. Irritability trajectories at ages 7 to 15, in a longitudinal cohort (N=7,924)
A male preponderance was observed for the decreasing, late-childhood limited, and high-persistent trajectory classes (male, 55.7%, 57.7%, and 63.7%, respectively) and a female preponderance for the increasing trajectory class (male, 40.5%). Accordingly, male sex was associated with an increased likelihood of being in the decreasing (odds ratio=1.27, 95% CI=1.01–1.59, p=0.038), late-childhood limited (odds ratio=1.37, 95% CI=1.09–1.73, p=0.007), or high-persistent (odds ratio=1.76, 95% CI=1.30–2.40, p<0.001) class and a decreased likelihood of being in the increasing trajectory class (odds ratio=0.68, 95% CI=0.54–0.87, p=0.002) compared with the low trajectory class (male, 49.8%).
Genetic Risk
Associations between irritability trajectory classes and ADHD PRS and depression PRS are presented in Table 1. Mean PRSs for each of the trajectory classes are shown in Figure 2. Compared with the low trajectory class, ADHD PRS was associated with an increased likelihood of being in both the high-persistent (odds ratio=1.31, 95% CI=1.09–1.58, p=0.005) and increasing (odds ratio=1.28, 95% CI=1.11–1.48, p=0.001) trajectory classes, with a similar risk of being in either trajectory (high-persistent compared with increasing trajectory class: odds ratio=1.02, 95% CI=0.81–1.29, p=0.854).
| ADHD PRS | Depression PRS | |||||
|---|---|---|---|---|---|---|
| Irritability Trajectorya | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p |
| Childhood-onset irritability | ||||||
| Decreasing | 1.06 | 0.92–1.22 | 0.425 | 1.12 | 0.97–1.29 | 0.126 |
| Late-childhood limited | 1.14 | 0.99–1.32 | 0.071 | 0.93 | 0.82–1.06 | 0.266 |
| High-persistent | 1.31 | 1.09–1.58 | 0.005 | 1.00 | 0.82–1.21 | 0.992 |
| Adolescent-onset irritability | ||||||
| Increasing | 1.28 | 1.11–1.48 | 0.001 | 1.20 | 1.05–1.38 | 0.009 |
TABLE 1. Association between attention deficit hyperactivity disorder (ADHD) and depression polygenic risk scores (PRSs) with irritability trajectories in a longitudinal cohort (N=7,924)
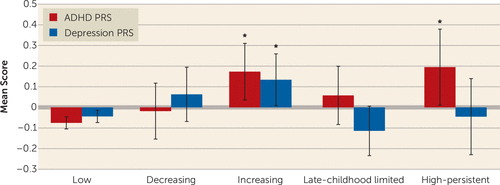
FIGURE 2. Attention deficit hyperactivity disorder (ADHD) and depression polygenic risk scores (PRSs), by irritability trajectory, in a longitudinal cohort (N=7,924)a
a Error bars indicate 95% confidence intervals.
*p<0.05.
Depression PRS was associated with an elevated likelihood of being in the increasing trajectory class compared with the low trajectory class (odds ratio=1.20, 95% CI=1.05–1.38, p=0.009), although evidence for an elevated likelihood of being in the increasing trajectory class compared with the high-persistent trajectory class was weaker (odds ratio=1.20, 95% CI=0.96–1.52, p=0.116).
Multivariable analyses including both PRSs in the same model revealed the same pattern of results (see Table S3 in the online supplement), as did sensitivity analyses using inverse probability weighting to assess the impact of missing genetic data (see Table S4 in the online supplement).
Diagnoses
Estimated prevalence rates of ADHD, depression, generalized anxiety disorder, oppositional defiant disorder, and conduct disorder in childhood and adolescence are presented in Tables 2 and 3. Rates of all diagnoses varied across the irritability trajectory classes, with the exception that there was not strong evidence of variation in adolescent generalized anxiety disorder across classes. At both developmental stages, rates of all diagnoses were generally highest in the high-persistent trajectory and were particularly high for oppositional defiant disorder.
| Irritability Trajectory | Attention Deficit Hyperactivity Disorder (N=7,043) | Depression (N=6,947) | Generalized Anxiety Disorder (N=7,029) | Oppositional Defiant Disorder (N=7,034) | Conduct Disorder (N=6,979) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | SE | % | SE | % | SE | % | SE | % | SE | |
| Low | 0.3 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 |
| Childhood-onset irritability | ||||||||||
| Decreasing | 9.3 | 1.6 | 5.7 | 1.2 | 1.1 | 0.6 | 28.3 | 2.5 | 4.0 | 1.1 |
| Late-childhood limited | 6.3 | 1.4 | 0.7 | 0.5 | 0.5 | 0.4 | 5.9 | 1.5 | 1.0 | 0.6 |
| High-persistent | 26.4 | 3.4 | 4.2 | 1.6 | 3.6 | 1.4 | 51.0 | 3.9 | 11.7 | 2.5 |
| Adolescent-onset irritability | ||||||||||
| Increasing | 1.9 | 0.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 0.7 | 0.2 | 0.3 |
| χ2 | p | χ2 | p | χ2 | p | χ2 | p | χ2 | p | |
| Comparison across trajectoriesa | 122.64 | <0.001 | 45.68 | <0.001 | 14.92 | 0.005 | 342.50 | <0.001 | 43.22 | <0.001 |
TABLE 2. Estimated prevalence of diagnoses in childhood across irritability trajectories in a longitudinal cohort
| Irritability Trajectory | Attention Deficit Hyperactivity (N=4,500) | Depression (N=4,900) | Generalized Anxiety Disorder (N=4,896) | Oppositional Defiant Disorder (N=4,490) | Conduct Disorder (N=4,489) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | SE | % | SE | % | SE | % | SE | % | SE | |
| Low | 0.2 | 0.1 | 1.1 | 0.1 | 0.4 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Childhood-onset irritability | ||||||||||
| Decreasing | 1.7 | 0.9 | 2.5 | 1.1 | 0.8 | 0.6 | 2.1 | 1.2 | 1.1 | 0.8 |
| Late-childhood limited | 0.6 | 0.8 | 2.1 | 1.1 | 0.8 | 0.7 | 1.1 | 1.5 | 0.7 | 1.0 |
| High-persistent | 14.2 | 3.4 | 7.3 | 2.6 | 3.1 | 1.8 | 60.9 | 5.1 | 17.4 | 3.8 |
| Adolescent-onset irritability | ||||||||||
| Increasing | 7.3 | 1.9 | 5.3 | 1.6 | 2.9 | 1.2 | 46.0 | 3.9 | 17.0 | 2.7 |
| χ2 | p | χ2 | p | χ2 | p | χ2 | p | χ2 | p | |
| Comparison across trajectoriesa | 43.83 | <0.001 | 15.34 | <0.001 | 7.51 | 0.111 | 325.23 | <0.001 | 76.93 | <0.001 |
TABLE 3. Estimated prevalence of diagnoses in adolescence across irritability trajectories in a longitudinal cohort
Childhood ADHD.
Childhood ADHD was associated with an increased likelihood of being in the decreasing (odds ratio=30.97, 95% CI=15.47–61.98, p<0.001), increasing (odds ratio=5.89, 95% CI=1.96–17.73, p=0.002), late-childhood limited (odds ratio=20.39, 95% CI=9.78–42.52, p<0.001), and high-persistent (odds ratio=108.64, 95% CI=57.45–204.41, p<0.001) trajectory classes compared with the low trajectory class.
Comparing these four irritability trajectories, childhood ADHD was associated with a decreased likelihood of being in the increasing trajectory class (compared with decreasing trajectory: odds ratio=0.19, 95% CI=0.07–0.52, p=0.001; late-childhood limited trajectory: odds ratio=0.29, 95% CI=0.10–0.84, p=0.023; high-persistent trajectory: odds ratio=0.05, 95% CI=0.02–0.15, p<0.001) and with the greatest likelihood of being associated with the high-persistent trajectory class (compared with decreasing trajectory: odds ratio=3.50, 95% CI=2.09–5.87, p<0.001; late-childhood limited trajectory: odds ratio=5.33, 95% CI=2.92–9.73, p<0.001).
Adolescent depression.
Compared with the low trajectory class, an increased likelihood of adolescent depression was found in the increasing (odds ratio=5.14, 95% CI=2.47–10.73, p<0.001) and high-persistent odds ratio=7.18, 95% CI=3.10–16.61, p<0.001) trajectory classes (decreasing trajectory: odds ratio=2.32, 95% CI=0.88–6.12, p=0.088; late-childhood limited trajectory: odds ratio=1.95, 95% CI=0.63–6.04, p=0.250). Likelihood of adolescent depression was similar for the high-persistent trajectory class compared with the increasing trajectory class (odds ratio=1.40, 95% CI=0.51–3.84, p=0.516).
Sensitivity Analyses
Controlling for sex revealed the same pattern of results for both PRS and diagnosis (see Tables S5 and S6 in the online supplement). Mean PRSs and estimated prevalence rates for diagnoses by sex are presented in Figures S3 and S4 in the online supplement.
Discussion
In this study, we investigated developmental trajectories of irritability across childhood and adolescence to test the hypothesis that there are different forms of neurodevelopmental/ADHD-like and depression/mood irritability. Specifically, we hypothesized that a neurodevelopmental/ADHD-like irritability trajectory would be defined by an early age at onset and a male preponderance and would be associated with an increased genetic liability to ADHD and ADHD diagnosis in childhood, whereas a depression/mood irritability trajectory would be defined by a later age at onset and a female preponderance and would be associated with increased genetic liability to depression and depression diagnosis in adolescence.
We identified five distinct developmental trajectory classes of irritability across childhood and adolescence. Four classes were characterized by elevated levels of irritability during at least some of this developmental period. Two irritability trajectory classes were early onset: one was defined by symptoms that decreased over time, and the other was defined by high symptoms that persisted (5.6% and 2.4% of the study sample, respectively). These two groups exhibited developmental patterns similar to patterns found in ADHD, with some individuals showing persistence over time and others remitting. An additional, unexpected trajectory with irritability onset in late childhood was defined by an increase in symptoms around age 10 and a subsequent decrease around age 13 (5.2% of the study sample). This is the age of high school transition in the United Kingdom, as well as the onset of puberty for many; however, we can only speculate as to the underlying mechanisms for this trajectory class, because this group has not been previously described. The final trajectory was defined by increasing symptoms with a later onset, around adolescence (5.5% of the study sample).
In line with our first hypothesis, the two irritability trajectories with early onset (decreasing and high persistent) were both associated with male sex and ADHD diagnosis in childhood, as was the late-childhood onset (late-childhood limited) class. The high-persistent trajectory was also associated with increased ADHD genetic risk scores, although the childhood-onset trajectories that did not have persistent symptoms (decreasing and late-childhood limited) were not. This is similar to findings on the developmental patterns of ADHD symptoms, that individuals with persistent compared with childhood-limited symptoms of ADHD have an increased genetic liability to ADHD (27). An association between irritability and ADHD PRS has been observed previously in the total sample, as well as in a clinical sample, and accords with an earlier twin study that observed shared genetic links between ADHD and emotional lability (11, 12). Interestingly, the later-onset (increasing) irritability trajectory was also associated with ADHD PRS, although rates of childhood ADHD diagnosis were low. It may be that the phenotypic expression of ADHD genetic liability in this predominantly female group manifests as mood problems (e.g., see reference 28), although further work would be needed to investigate this hypothesis. Our findings therefore support the suggestion of a neurodevelopmental/ADHD-like type of irritability, which has an early onset, has a male preponderance, and is associated with ADHD.
In line with our second hypothesis, the trajectory with irritability onset in adolescence (increasing trajectory class) was associated with female sex. This depression/mood type irritability trajectory class was also associated with depression genetic risk scores and depression diagnosis in adolescence. This class was also associated with ADHD genetic risk scores. Although twin studies have indicated genetic overlap between irritability and depression, previous analyses of this same sample failed to observe an association between irritability and depression genetic risk scores (8, 29). This has raised questions about the primary classification of severe irritability as a mood problem. Indeed, it appears that ICD-11 is going to take a different approach than DSM-5 and conceptualize irritability as a specifier of oppositional defiant disorder and not include severe irritability/disruptive mood dysregulation disorder as a mood disorder (the approach taken in DSM-5). However, ICD-11 now includes irritability as an alternative symptom to depressed mood in dysthymic disorder in children and adolescents (similar to DSM-5 for depression). Our findings suggest that the association between irritability and depression genetic risk scores may be specific to a type of irritability that has an onset during adolescence.
Regardless of whether the DSM-5 or ICD-11 stance on categorizing severe irritability is most valid, neither has taken a developmental approach. Our findings suggest that development matters. This view is not new (e.g., see reference 30), and it has been applied to other phenotypes, including antisocial behavior (31), but perhaps has been forgotten, since it poses substantial challenges to clinicians and researchers. A transdiagnostic conceptualization of irritability, such as that used in the National Institute of Mental Health’s Research Domain Criteria or the p-factor framework (32), could provide a helpful research framework for conceptualizing irritability dimensionally across multiple levels. Our study suggests that if such a framework were to be implemented, it should be developmentally informed, taking into account age and age at onset.
In terms of how the trajectory classes relate to psychiatric diagnoses, the diagnostic rates reported here are relatively low, because our sample is a population-based cohort. Nevertheless, there are some notable observations. Interestingly, while the high-persistent neurodevelopmental/ADHD-like irritability trajectory class was not associated with depression PRS, individuals in this class showed a risk of adolescent depression similar to that for the increasing depression/mood irritability trajectory. Thus, both trajectory classes were associated with risk of depression in adolescence, although the mechanisms of this association are likely different. For example, increased risk for depression in the early-onset irritability type may have been driven by environmental factors, such as increased life events associated with irritability, rather than genetic risk for depression, although further research is needed. It is established that child neurodevelopmental disorders such as ADHD, as well as oppositional defiant disorder and conduct problems, are risk factors for later depression, so it is perhaps not surprising that the neurodevelopmental irritability trajectory was associated with depression, although emerging research suggests that the presence of irritability in individuals with ADHD confers additional risk of depression (33).
Consistent with previous work (2), we found elevated rates of other psychiatric disorders among individuals with elevated irritability. Rates of oppositional defiant disorder were particularly high in the elevated irritability trajectory classes and followed a developmental pattern similar to that of irritability levels. This is not surprising given that irritability is a core component of oppositional defiant disorder; indeed, irritability was defined by terms from the oppositional defiant disorder section of the Development and Well-Being Assessment. However, a large proportion (39%−99%) of individuals in the elevated irritability trajectory classes did not have oppositional defiant disorder, which suggests that irritability among individuals without oppositional defiant disorder is important and adds to the idea that irritability is transdiagnostic. Interestingly, despite previous research that found associations between irritability and anxiety as well as depression (2), we did not find strong evidence that the rates of generalized anxiety disorder in adolescence differed between those in each of the irritability trajectory classes. Previous research on links between irritability and depression has often included anxiety symptoms in the same measure (29) or found the same pattern of results for depression and anxiety (6). Our findings suggest specificity to depression in adolescence.
One explanation for the apparent existence of different irritability types is that irritability is simply a feature of different underlying diagnoses (e.g., depression and ADHD). However, the low rates of these diagnoses in the population-based trajectory classes (Tables 2 and 3) suggest that this is not the case (e.g., only a small minority of those in the increasing irritability trajectory class had a diagnosis of depression), and associations with PRSs were similar when we excluded individuals with diagnoses (see Table S7 in the online supplement). In addition, sex differences for the high-persistent and increasing irritability trajectory classes were not as pronounced as typically reported for depression and ADHD (34). These observations suggest that the different irritability types are not simply a feature of different underlying diagnoses (ADHD and depression).
Our findings should be considered in light of some limitations. First, ALSPAC is a longitudinal birth cohort study that suffers from nonrandom attrition, and individuals with increased genetic liability to a disorder and with higher levels of psychopathology are more likely to drop out of the study (35, 36). However, our trajectory analyses used full-information maximum likelihood estimation (23) so that complete data on irritability were not required. Moreover, inverse probability-weighted analyses suggested that missingness of genetic data did not have a large effect on our findings. It is possible that associations between depression PRS and (increasing) irritability were inflated by some of the cases in the depression GWAS involving (adolescent-onset) irritability as a symptom. In addition, while PRSs are useful indicators of genetic liability, ADHD and depression PRSs currently explain a minority of common variant liability to the disorder (20, 21). Our analyses were therefore underpowered to detect associations between PRS and irritability trajectories: our analyses had 80% power to detect odds ratios of 1.35 for the increasing trajectory and 1.60 for the high-persistent trajectory (37). Thus, while the effect sizes we observed are consistent with similar types of analyses reported elsewhere (38), PRSs should be regarded as indicators of genetic liability rather than as predictors of psychopathology. We deliberately elected to use depression PRS derived from adult samples that reflect “typical” depression. Childhood or prepubertal depression is rare and considered to be atypical not only in age at onset but also in terms of other features (e.g., see reference 39). The associations that we observed between adolescent-onset irritability and depression PRS are therefore consistent with previous work demonstrating associations between irritability and depression in adolescence and adulthood.
We did not include covariates in our analyses of ADHD and major depressive disorder diagnoses because we were interested in describing observed associations, which means that we cannot infer any causal associations between irritability and diagnosis. Different methods, such as Mendelian randomization, would be needed to investigate such research questions (40). Finally, our aim was to use a developmental approach to investigate nosology. However, diagnostic and subgroup overlap is the rule in psychiatry, and this study was no exception. Our neurodevelopmental/ADHD-like and depression/mood irritability classes were both associated with ADHD and depression diagnoses and ADHD genetic risk scores. We observed different patterns for these classes rather than identifying completely distinct groups. Finally, there were difficulties in determining the optimum number of classes using growth mixture modeling. Given that this is the first study, to our knowledge, to investigate irritability trajectories across childhood and adolescence, we emphasize that further research is needed in this area, including testing the replicability of these trajectories in different samples.
In conclusion, our study identified different developmental trajectories of irritability, including one with characteristics typical of neurodevelopmental/ADHD-like problems—early onset, male preponderance, and clinical and genetic links with ADHD—and one with characteristics typical of depression/mood problems—later onset, female preponderance, and clinical and genetic links with depression. Both groups were associated with risk of adolescent depression, and both were associated with ADHD genetic risk scores. Overall, these findings suggest that the developmental context of irritability may be important in its conceptualization, and this has implications for treatment as well as nosology (2).
1 : Irritability in children: what we know and what we need to learn. World Psychiatry 2017; 16:100–101Crossref, Medline, Google Scholar
2 : Practitioner review: definition, recognition, and treatment challenges of irritability in young people. J Child Psychol Psychiatry 2018; 59:721–739Crossref, Medline, Google Scholar
3 : Adult outcomes of youth irritability: a 20-year prospective community-based study. Am J Psychiatry 2009; 166:1048–1054Link, Google Scholar
4 : Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol Psychiatry 2006; 60:991–997Crossref, Medline, Google Scholar
5 : Longitudinal outcome of youth oppositionality: irritable, headstrong, and hurtful behaviors have distinctive predictions. J Am Acad Child Adolesc Psychiatry 2009; 48:404–412Crossref, Medline, Google Scholar
6 : The status of irritability in psychiatry: a conceptual and quantitative review. J Am Acad Child Adolesc Psychiatry 2016; 55:556–570Crossref, Medline, Google Scholar
7 : Three dimensions of oppositionality in youth. J Child Psychol Psychiatry 2009; 50:216–223Crossref, Medline, Google Scholar
8 : Adolescent irritability: phenotypic associations and genetic links with depressed mood. Am J Psychiatry 2012; 169:47–54Link, Google Scholar
9 : Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry 2011; 168:129–142Link, Google Scholar
10 : Irritability in ADHD: associations with depression liability. J Affect Disord 2017; 215:281–287Crossref, Medline, Google Scholar
11 : Genetic associations between the symptoms of attention-deficit/hyperactivity disorder and emotional lability in child and adolescent twins. J Am Acad Child Adolesc Psychiatry 2014; 53:209–220.e4Crossref, Medline, Google Scholar
12 : Investigating the genetic underpinnings of early-life irritability. Transl Psychiatry 2017; 7:
13 : Childhood antecedents and risk for adult mental disorders. Annu Rev Psychol 2015; 66:459–485Crossref, Medline, Google Scholar
14 : Cohort profile: the children of the 90s—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013; 42:111–127Crossref, Medline, Google Scholar
15 : Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 2013; 42:97–110Crossref, Medline, Google Scholar
16 : The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry 2000; 41:645–655Crossref, Medline, Google Scholar
17 : The DAWBA bands as an ordered-categorical measure of child mental health: description and validation in British and Norwegian samples. Soc Psychiatry Psychiatr Epidemiol 2011; 46:521–532Crossref, Medline, Google Scholar
18 : PRSice: polygenic risk score software. Bioinformatics 2015; 31:1466–1468Crossref, Medline, Google Scholar
19 : Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res 2013; 22:278–295Crossref, Medline, Google Scholar
20 : Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 2018; 50:668–681Crossref, Medline, Google Scholar
21 : Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet 2019; 51:63–75Crossref, Medline, Google Scholar
22 : Genome-wide association for major depression through age at onset stratification: Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. Biol Psychiatry 2017; 81:325–335Crossref, Medline, Google Scholar
23 : MPlus User’s Guide, 7th ed. Los Angeles, Muthén & Muthén, 1998–2012Google Scholar
24 : Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res 2000; 24:882–891Crossref, Medline, Google Scholar
25 : Assessing the evidence for shared genetic risks across psychiatric disorders and traits. Psychol Med 2018; 48:1759–1774Crossref, Medline, Google Scholar
26 : Auxiliary variables in mixture modeling: three-step approaches using Mplus. Struct Equ Modeling 2014; 21:329–341Crossref, Google Scholar
27 : Association of genetic risk variants with attention-deficit/hyperactivity disorder trajectories in the general population. JAMA Psychiatry 2016; 73:1285–1292Crossref, Medline, Google Scholar
28 : Sex-specific manifestation of genetic risk for attention deficit hyperactivity disorder in the general population. J Child Psychol Psychiatry 2018; 59:908–916Crossref, Medline, Google Scholar
29 : A genetically informed study of the longitudinal relation between irritability and anxious/depressed symptoms. J Am Acad Child Adolesc Psychiatry 2015; 54:377–384Crossref, Medline, Google Scholar
30 : Epidemiological approaches to developmental psychopathology. Arch Gen Psychiatry 1988; 45:486–495Crossref, Medline, Google Scholar
31 : Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychol Rev 1993; 100:674–701Crossref, Medline, Google Scholar
32 : All for one and one for all: mental disorders in one dimension. Am J Psychiatry 2018; 175:831–844Link, Google Scholar
33 : Irritability in ADHD: associations with depression liability. J Affect Disord 2017; 215:281–287Google Scholar
34 : Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J Child Psychol Psychiatry 2003; 44:1092–1115Crossref, Medline, Google Scholar
35 : Selective drop-out in longitudinal studies and non-biased prediction of behaviour disorders. Br J Psychiatry 2009; 195:249–256Crossref, Medline, Google Scholar
36 : Association of genetic risk for schizophrenia with nonparticipation over time in a population-based cohort study. Am J Epidemiol 2016; 183:1149–1158Crossref, Medline, Google Scholar
37 : Power and predictive accuracy of polygenic risk scores. PLoS Genet 2013; 9:
38 : Characterizing developmental trajectories and the role of neuropsychiatric genetic risk variants in early-onset depression. JAMA Psychiatry 2018; 76:306–313Crossref, Google Scholar
39 : Depression in adolescence. Lancet 2012; 379:1056–1067Crossref, Medline, Google Scholar
40 : Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol 2004; 33:30–42Crossref, Medline, Google Scholar



