Improving Depression Outcome by Patient-Centered Medical Management
Abstract
Specific challenges that profoundly affect the outcome of treatment for depression include 1) patient engagement and retention in care and optimization of treatment adherence, 2) optimization of symptom and side effect control by medication adjustments using measurement-based care procedures, 3) restoration of daily functioning and quality of life, and 4) prevention or at least mitigation of symptomatic relapse or recurrence. According to data from the Sequenced Treatment Alternatives to Relieve Depression study, some 10%–15% of patients will not return for treatment after an initial thorough evaluation visit; an additional 20%–35% will not complete the first acute-phase treatment step, and another 20%–50% will not complete 6 months of continuation treatment. Among patients who stay in treatment, over 50% exhibit poor adherence. Thus, most patients do not overcome the first two challenges. There are no systematic, widely agreed-upon psychosocial approaches to any of these four major challenges. The authors propose “patient-centered medical management” to address each of the four challenges, using psychoeducational, behavioral, cognitive, interpersonal, and dynamic models and methods. A renewed emphasis on the development and testing of systematic approaches to overcoming these common clinical challenges could enhance the chances of patient recovery and care system cost efficiencies.
[AJP AT 175: Remembering Our Past As We Envision Our Future
July 1933: Psychotherapeutics at Stockbridge
Horace K. Richardson: “Frequently, in the simpler situations, very few interviews are required in order that he [the patient] discover for himself what part of the adaptive machinery is at fault, and for him to develop a technique of handling the maladjustment on a more satisfactory level in the future.” (Am J Psychiatry 1933; 90:45–56)]
Sixty years of treatment research for depression has produced advances in pharmacotherapy, refinements in the delivery of electroconvulsive therapy (ECT), newer neuromodulation treatments, and the development of time-limited, symptom-targeted, manualized psychotherapies. Clinicians now have a broad range of treatment options to control or eliminate symptoms, restore function, and enhance longer-term outcomes with continuation and maintenance treatments (1–3) for patients with mood disorders.
Consequently, clinical practice guidelines for depression (1, 4–7) now recognize four possible initial management strategies: medication, psychotherapy, their combination, and active surveillance (watchful waiting). The choice among these strategies logically depends on the clinical context (e.g., symptom severity, clinical acuity, concomitant conditions, and social support) as well as the patient’s treatment goals.
Whatever the treatment chosen, the goals of treatment are total and sustained symptom relief (or at least optimal symptom control, if sustained remission is elusive); restoration of function and quality of life (ideally to premorbid levels); and prevention or at least mitigation of the risks and impacts of relapse (5, 8–10). In addition, where applicable, ideal outcomes should include changes in lifestyle and habits to promote medical and mental health, reduce the risk of depressive relapse, and enhance resilience to life stresses. The achievement of each outcome may contribute to the achievement of the others. For example, symptom reduction and functional restoration each contributes to relapse mitigation and prevention. Problem resolution reduces symptoms and may reduce the risk of relapse. Symptom reduction may enhance problem solving and vice versa.
Challenges in the Delivery of Pharmacotherapy and Neuromodulation Therapies
Figure 1 broadly summarizes the six main domains that can profoundly affect clinical outcomes and provides some examples for each domain. The elements in the sociocultural and care system domains are not under clinician or patient control, whereas those in the other domains are. In this article, we focus on what clinicians and patients can do to improve outcomes when medication or neuromodulation therapies are called for. Once the decision is made to initiate pharmacological or somatic treatments for a mood disorder, many challenges remain in the actual delivery of treatment to achieve optimal symptom control, functional recovery, and relapse mitigation in the real world (5, 8, 11, 12).
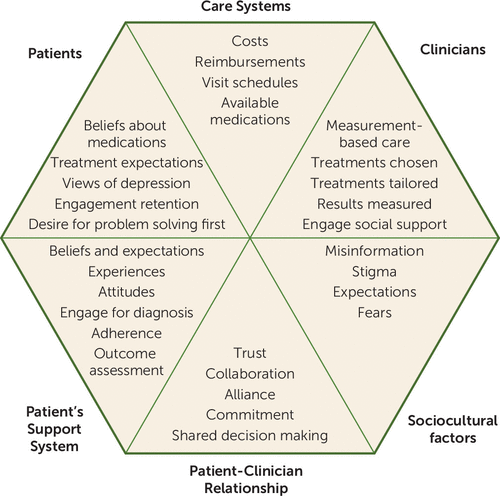
FIGURE 1. Factors Affecting Depression Outcomes With Pharmacotherapy or Somatic Therapies
Figure 2 highlights where collaboration between patient and clinician plays an essential role in the management of depressed patients—especially those who wish to avoid treatment with medications or somatic therapies. Indeed, the importance of this relationship and collaboration was emphasized by McKay et al. (13), who used data from the 1985 National Institute of Mental Health (NIMH) study that compared interpersonal therapy, cognitive-behavioral therapy (CBT), imipramine plus clinical management, and placebo plus clinical management to find that when the placebo plus clinical management was compared with imipramine plus clinical management, more of the variance in depressive symptom outcome was due to the psychiatrist than to the medication, although both medication and psychiatrist contributed meaningfully. That is, the relationship context was at least as important—if not more so—than whether the patient received placebo or medication.

FIGURE 2. Essential Patient-Clinician Collaborations in Patient-Centered Medical Management of Depression
The effective implementation of patient-centered medical management depends critically on the patient-clinician relationship and collaboration. Patient-centered medical management defines and provides alternative means to address the four essential clinical tasks defined in Table 1 in order to optimize outcomes when medication or neurostimulation is the chosen treatment. While there may be many ways to accomplish each task, there are few systematic, agreed-upon approaches regarding how to accomplish each task. Furthermore, the degree to which accomplishing each task affects symptom control, functioning, and prognosis varies widely, depending on the patient, clinician behaviors, care system factors, and incentives as well as the nature of the illness, its treatments, and other parameters.
| Tasks | Examples of Methods to Address the Task |
|---|---|
| Engagement, retention, and adherence | Establish collaboration and alliance |
| Elicit and address treatment misconceptions and beliefs | |
| Align personal expectations with treatment aims | |
| Align expectations about timing of outcomes | |
| Agree on how to measure results | |
| Motivational interviewing | |
| Track medication taking | |
| Identify “misses” and their causes | |
| Elicit side effects | |
| Discuss options to treat or manage side effects | |
| Symptom control | |
| Select treatment | Make shared decisions by discussing pros and cons of treatments |
| Tailor and optimize treatment | Measurement-based care: |
| Monitor progress with rating scales (e.g., symptoms, function levels, side effect burden) | |
| Tailor treatment dosages and sequences | |
| Manage side effects | |
| Adjust and optimize dosages | |
| Address comorbidities | |
| Revise treatment(s) | Second opinion |
| Monitor progress with brief rating scales (symptoms, functioning, side effects, health behaviors) | |
| Switch treatments | |
| Augment treatments | |
| Restore function | Identify and prioritize relationship deficiencies and goals |
| Consider marital therapy, occupational therapy, interpersonal therapy, or cognitive-behavioral therapy | |
| Promote healthier lifestyle | |
| Treat comorbid conditions (e.g., substance use disorders) | |
| Treat comorbid general medical conditions | |
| Relapse mitigation | Teach prodromal symptom recognition |
| Resilience training and stress management | |
| Relapse planning | |
| Increase protective factors and reduce risk factors |
TABLE 1. Clinical Tasks and Examples of Methods to Address Each Task in Patient-Centered Medication Management of Depression
The magnitude of these challenges is daunting. According to data from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, some 10%–15% of patients will not return for treatment after an initial thorough evaluation visit; an additional 20%–35% will not complete the first acute-phase treatment step, and another 20%–50% will not complete 6 months of continuation treatment. Among patients who stay in treatment, over 50% exhibit poor adherence (14). Because STAR*D used diligently implemented measurement-based care and provided clinical research coordinators at each clinical site (15), these figures are likely overly optimistic for community clinical practice.
Another way to estimate the magnitude of some of these challenges is illustrated by Pence et al. (16), who specified a depression treatment cascade analogous to the HIV treatment cascade (17, 18). Pence et al. (16) used available evidence to put numerical estimates on five key parameters that affect the public health impact of these steps in care. Their estimates included population prevalence of depression in primary care settings (12.5%), likelihood of depression being recognized clinically (47%), likelihood of treatment initiation (50%), likelihood of receiving adequate treatment (40%), and likelihood of achieving remission (65%). These estimates were aimed at the outcome of symptom remission (without considering functional restoration or relapse prevention). Taken together, the estimates suggest that only 6% of depressed patients in primary care settings would achieve remission in acute-phase treatment. Estimates for remission in psychiatric settings have not been made with this model.
Treatment Tasks
Each treatment task (Table 1) is essential to optimizing the chances of recovery, and each is typically addressed in a stepwise manner, informed by the patient’s specific needs. The tasks include 1) engaging and retaining the patient in treatment (and whenever possible, the patient’s support system or persons) and optimizing adherence to both medications and behavioral changes needed; 2) selecting, implementing, and tailoring the acute treatments to optimize depressive symptom control; 3) restoring day-to-day function and quality of life; and 4) selecting and tailoring longer-term medication use, preparing for early detection of and responses to symptom exacerbations, and making lifestyle and behavioral changes that minimize the risk and personal impact of relapse or recurrence. The successful completion of each task depends on efforts by both the clinician and the patient.
Table 1 also provides a few examples of how each task could be addressed by specific clinical activities designed to accomplish it. A depressive episode within the bipolar spectrum, of course, entails the additional tasks of selecting, initiating, and optimizing a mood stabilizer.
Although these tasks are typically sequenced, they may also overlap. For example, during symptom reduction, ongoing attention is often paid to retention and adherence. And when symptoms are reduced, function usually improves as a consequence (19). In addition, the goal of one task—for example, functional restoration—is a critical goal itself and a means to further reduce symptoms. Furthermore, improving adherence to pharmacotherapy will minimize relapse risk, which in turn will play a key role in functional restoration and enhancing quality of life.
An iterative process is often needed to accomplish each task because we do not know which of several possible methods will be most effective for any given patient. Usually the obstacles to completing the task are defined, one of many potential methods to address a given problem is selected, and the intervention is tried. If it fails, a second step is taken. For example, a reminder system might be used to address erratic adherence. If that fails, a cognitive approach to understanding and redressing a patient’s misconceptions about medication taking may be indicated.
Obstacles to achieving these four important tasks occur at different times during the treatment process (20), and they often require different approaches (21). The choice of which tasks to address and when depends on patient contexts. Which methods are chosen currently rests on “clinician judgment,” informed by shared decision making. For example, some patients are already convinced of the importance of their medication treatment and have expectations that are well aligned with their likely experience, and others may have unrealistic expectations that, when not met, can lead to poor adherence or quitting treatment altogether. Engagement may be rapid for patients in the former group but require greater effort with those in the latter group. Expectations and attitudes are best addressed initially—prior to treatment initiation—to create the essential patient-clinician collaboration.
Engagement, Retention, and Adherence
The first task is to engage and retain (22) patients in treatment and to engender their reliable participation in taking treatments or making behavioral changes to increase the chances of attaining symptomatic or functional outcomes that reduce the risk of relapse (12, 23).
Engagement
About 10% of depressed outpatients do not fill the initial prescription, and up to 33% do not return for even one follow-up visit after the initial evaluation and prescription of medication (24, 25). Even in the well-resourced care environments provided in STAR*D, which included additional nursing staff support and medications at minimal or no cost, 15%−30% of patients dropped out of treatment during each 12-week treatment step (15, 26, 27). Of those who benefited from treatment and entered continuation-phase therapy, another 40% left continuation-phase treatment (15).
A host of factors contribute to these engagement and retention problems, including lack of belief in the benefit of treatment, lack of understanding about the importance of the clinician-patient relationship, and the complexity, cost, and inconvenience of treatment, to name just a few. This is true not only in depression but in all general medical conditions (28). In depression, the less educated and socially disadvantaged patients have higher rates of nonengagement and premature discontinuation of acute treatment even when efforts are made to reduce barriers and improve access to care (26, 27).
Difficulties in engaging patients in treatment are well recognized in several mental health fields, including substance abuse and eating disorders (29, 30), as well as in general medicine and surgery (28). A range of possible approaches to address these issues could include motivational interviewing (31), which was initially developed to engage and retain patients with substance use disorders. This procedurally specified intervention focuses on patients’ motivations for change, reservations about the treatment process, and readiness to engage in therapy. Would this approach help engage and retain depressed patients in treatment? Could it be targeted to selected patients who could be identified by a brief self-report measure?
Retention
Even among patients who are responding to antidepressant therapy, up to 50% do not persist in the recommended 6–9 months of continuation treatment (15). Extrapolation from controlled studies of continuation-phase therapies suggests that a large proportion of those who exit treatment prematurely would not have been able to sustain a response or reach remission (32, 33). In fact, in controlled studies of antidepressant discontinuation, the risk of relapse begins to increase within the first few weeks after stopping medication, and study participants who are switched to placebo experience about twice the risk of relapse across 6 months as those who remain on active medication (34). In contrast, naturalistic studies document that the “gateway” to recovery begins with a period of at least 8 weeks essentially free of symptoms (35). Thus, when an effective treatment is found, every effort should be made to ensure that it is continued throughout this critical transition from acute-phase to continuation-phase pharmacotherapy.
Given the significant costs and health burdens associated with persistent depressive episodes, the failure to retain these patients can be both disabling for patients and costly to society (36). Moreover, early attrition from treatment has been found to be associated with a number of correlates of poorer prognosis, including higher rates of comorbidities and greater global illness severity (37). Better engagement of patients—and their support systems—would set the stage for shared decision making during treatment selection, which in turn could lead to better adherence once treatment is initiated.
Adherence
Poor adherence is perhaps the largest contributor to pharmacotherapy failures. As noted earlier, as many as 10% of initial prescriptions for antidepressants are never filled, and another 20%−30% of depressed outpatients who begin a course of antidepressant therapy discontinue treatment prematurely (24, 25). While interventions to enhance adherence have been developed for persons with schizophrenia and bipolar disorder, little attention has been focused on adherence in nonpsychotic major depression.
The importance of anticipating and managing adherence in pharmacotherapy is exemplified by the manual for pharmacotherapists created by Fawcett and colleagues for the landmark NIMH Treatment of Depression Collaborative Research Program (11). The manual concisely outlined the essentials of supportive clinical management within the context of pharmacotherapy and anticipated many of the suggestions presented here. Despite the several advantages of the newer antidepressants that may favor better adherence (i.e., fewer side effects, fewer steps for dose titration), adherence remains an issue, with estimates of poor adherence often over 50% (less than 75%−80% adherence to prescribed doses) (14). Thus, psychoeducation about when and what sorts of wanted and unwanted medication effects are possible can help patients deal with their ambivalence about taking medications.
For patients with more highly recurrent depressive episodes, sustained adherence to pharmacotherapy is necessary across years (1–3, 6). Nevertheless, it appears that such ongoing care is more the exception than the rule. For example, one study using pharmacy utilization data found that only about 30% of patients taking antidepressants have sustained prescription fill rates of 90%−100% of prescribed doses (38).
We have found that simply asking patients about their willingness to fill the initial prescription is helpful, as are questions at each subsequent visit and questions about the frequency of medication taking (or the number of unused pills or capsules at the end of the prescription interval). An example question would be: “Based on what you’ve learned about your condition and this form of treatment, how likely are you to fill this prescription and take the medication?”
Now, with the availability of cloud computing–based support and mobile apps, patients and families can more easily monitor symptoms and track psychological experiences or daily activities to detect impending relapses earlier, allowing more intensive interventions to be delivered in a more timely fashion. While prescribers may want to familiarize themselves with various web sites, our patients are usually the best persons for choosing the most helpful apps for themselves. Yet, the cost-effectiveness of such strategies has not yet been demonstrated, and neither clinical practice guidelines nor consumer-focused materials recommend specific self-help strategies, other tools, or brief psychotherapies that target medication adherence (6, 39). Moreover, it is unknown when such strategies should be recommended, or for which patients (14).
It is sobering that the thoughts, feelings, beliefs, unspoken assumptions, and conflicts that are known to underpin variable and inconsistent adherence by depressed patients have rarely been the subject of innovative psychological approaches. Aside from a few reports of cognitive-behavioral strategies (40, 41), there is a dearth of well-controlled studies that address adherence in depressed patients. It would be worthwhile to develop and then prospectively evaluate a range of targeted interventions focusing on thoughts, feelings, behaviors, and attitudes that are associated with nonadherence, which could improve clinical outcomes for depressed patients who have a history of significant difficulties in taking prescribed medications.
For some patients, however, nonadherence does not seem to be related to distorted thoughts or feelings about ingesting medication or suffering from a mental disorder, but rather is due to unacceptable side effects. Indeed, problematic side effects are the leading reason patients give for stopping taking antidepressants during the first 4 weeks of treatment. Side effects are most salient when patients believe that their doctor is not listening to their concerns. Even after months of therapy, disagreeable side effects continue to “push” nonadherent behavior and unilateral decision making (42).
For others, poor adherence may be driven by nagging doubts about the illness model or whether the medication is even needed (43). Either way, a promising strategy could be psychoeducation tailored to inform the patient and help adjust his or her internal cost-benefit analysis, specifically by addressing doubts in an evidence-based fashion and helping align patients’ expectations with the evidence on what to expect. If doubts are identified, they should be accepted, explored, and addressed.
Engage and Optimize Social Support
It is well known that social support has a protective effect that minimizes the risk of becoming depressed during stressful times (44). Conversely, low levels of social support and high levels of interpersonal discord have been found to be associated with higher treatment dropout rates and lower remission and recovery rates (45, 46). These data suggest that interventions that focus on strengthening the depressed person’s level of social support could be expected to improve recovery rates and decrease the risk of relapse or recurrence.
Consistent with this notion, several small-scale studies of depressed patients have found that focused couples interventions can have symptom-reducing effects that are comparable to individual therapies such as CBT (47). Moreover, the work of Miklowitz and colleagues has shown that more broadly focused family-focused interventions significantly improve longer-term outcomes in patients with bipolar disorder (48, 49). What has yet to be shown, however, is whether the outcomes of pharmacotherapy-treated individuals with low levels of social support or high levels of interpersonal difficulties can be reliably enhanced by improving social supports with couples-focused or a family-focused therapy from the outset of a new course of treatment.
Aligning Treatment Expectations
Many people suffering from major depression seek treatment because of distressing difficulties in their current interpersonal, marital, or occupational roles (50). Yet, once we diagnose a depressive disorder, we sometimes focus more on the resolution of the signs and symptoms of the depressive episode and fail to focus adequately on the patient’s primary goals. Explicitly ensuring that the patient and care provider are collaborating to achieve the shared goals may go a long way toward promoting a stronger, more productive treatment alliance.
How, when, and for whom we should shift the primary target of treatment from symptom relief and remission to functional restoration is neither clear nor evidence based. Nevertheless, common sense suggests that the process of establishing a strong collaboration would be facilitated by an open and early discussion of the goals and desired outcomes of treatment. But how and to which patients this discussion is to be tailored deserve greater focus and evaluation.
Case example: Aligning expectations to the patient’s own context
“Mr. A,” a 57-year-old high school teacher, had a history of mild, persistent depression that began in his youth and more severe major depressive episodes beginning in his mid-30s. Mr. A’s most recent episode, which lasted 14 months, led him to accept early retirement from his tenured position. Now doing much better on the combination of an antidepressant and a second-generation antipsychotic, Mr. A’s residual symptoms (a score of 9 on the Quick Inventory of Depressive Symptomatology) included residual low energy, reduced libido, and social isolation. Despite his gaining 15 pounds over the past 6 months, Mr. A and his psychiatrist were reluctant to stop the antipsychotic this early in the process of recovery.
Mr. A, had divorced 7 years earlier, and he had sporadic contact with his two adult children. He hardly ever saw his several adult friends, and he had “given up” on dating. He was not participating in any of several previously enjoyable hobbies and instead spent most of his waking hours watching television and browsing the Internet. He had had several previous courses of psychotherapy and concluded that they hadn’t helped that much. His psychiatrist suggested that in his state of partial remission, he might be more likely than before to benefit from a focused psychotherapy program, and cited several losses and life transitions that adversely affected his current quality of life. When Mr. A politely declined the nudge back to therapy, his psychiatrist encouraged him to begin using a daily planner to keep track of his activities and moods. They agreed to use secure e-mail so that Mr. A could regularly report on his progress on a weekly basis. Using this approach, it was clear that Mr. A felt worse when he was alone and underactive. Mr. A and his psychiatrist worked together to schedule daily self-help activities, including regular light aerobic exercise, morning sun exposure, and reaching out to his friends and former colleagues. As Mr. A’s activity level and social interactions increased, his mood and subjective quality of life improved.
Symptom Control
After engagement, retention, and adherence, the usual next major clinical task is to optimally control symptoms while minimizing treatment side effects and, ideally, to reach sustained remission from depressive symptoms. Preferentially, this aim is most easily accomplished by using measurement-based care procedures (51, 52). These procedures were developed and tested in the Texas Medication Algorithm Project (53–55) and the German Algorithm Project (56–61), although the term “measurement-based care” was coined when the process of measurement-based care used in STAR*D was first described (62).
Measurement-based care enables clinicians and patients to make treatment decisions tailored to each patient through a step-by-step evaluation of treatment response and medication tolerance. After treatment initiation, the dosage is adjusted or the medication is changed to minimize side effects, maximize safety, and optimize the therapeutic benefit for each patient according to a predefined dosage adjustment plan that is informed by symptom and side effect scales administered at each visit (62–65).
In fact, measurement-based care procedures are analogous to the medication and placebo management procedures used in the Treatment of Depression Collaborative Research Program (11), which recommended scheduled dosage adjustments informed and tailored individually by regular, systematic symptom and side effect measurements using a 47-item checklist. In 1993, the Agency for Healthcare Policy and Research Clinical Practice Guidelines for Depression (8) also recommended a stepwise dosing process based on regular assessment of symptoms and side effects.
Restoration of Function
Wellness and Lifestyle Interventions
Ample evidence indicates that many depressed people could benefit from lifestyle changes, such as increasing activity and exercise, stopping smoking, adopting a more regular sleep-wake cycle, reducing alcohol intake, and improving nutrition and diet. Lifestyle modifications can reduce depressive symptoms, improve function, and, over time, promote recovery and reduce the risk of relapse (66–69). However, neither supportive medication management nor the various kinds of widely practiced supportive psychotherapy routinely incorporate strategies to promote lifestyle changes. And, in the absence of evidence from controlled trials, it seems unlikely that changes will be incorporated in clinical practice guidance in any specific actionable way. We believe it is time to change the “don’t ask, don’t tell” approach to lifestyle management and consider the availability of the plethora of self-help materials on the web as a potentially cost-effective means to effect change as well as to develop therapeutic approaches to achieve these lifestyle modifications.
Targeting Functional Restoration
When symptoms are improved, so too are longer-term functional outcomes (70–72). And, since many patients value improvements in quality of life and function above symptomatic improvement (73), a patient-centered approach to treatment planning should ensure that patients’ vocational, interpersonal, and leisure time activities that are associated with well-being have normalized. In this respect, there is strong evidence that the additive benefits of combining psychotherapy and pharmacotherapy often extend well beyond symptom reduction. Psychotherapies that aim to repair marital or occupational relationships can be the difference between “better” and “recovered” (74) or between more and fewer relapses or recurrences (75). Yet information on which therapies to use, for how long, and with which patients is not included in clinical practice guidelines and is often not delivered in practice (76).
Relapse Mitigation
There are many potential approaches to the prevention or mitigation of symptomatic relapses or recurrences (4, 35, 77, 78). They are best chosen for and tailored to each patient, although a systematic approach to considering and selecting among potential options has not been agreed upon, and it is not a systematic part of residency training. To address this important issue, we illustrate by way of a few examples.
Clinical experience and some data suggest that perceived resilience plays a significant role in moderating the risk of depressive onset or recurrence in high-risk individuals (79, 80). Such findings raise the question: Can specific exercises be developed that improve resilience or help “inoculate” vulnerable individuals against the impact of stress and be used prospectively to improve the lives of those at high risk? One such group of interventions, which incorporate mindfulness-based meditation strategies, has shown some promise on both clinical (81) and physiologic (82) outcomes.
Furthermore, it is logical to assume that effectively treating concurrent psychiatric conditions should improve the longer-term outcomes of patients with major depression (for example, addressing panic attacks and phobic avoidance behavioral patterns may reduce relapse risk in patients with co-occurring major depression). Similarly, given the association of heavy drinking or drug use with nonrecovery from depression (83–86), targeted interventions that reduce or eliminate substance use disorders should enhance the outcomes of the mood disorder. Naturalistic data collected in the United States (85) and Ireland (87) suggest that this is indeed possible, although the uncontrolled nature of the observations leaves open the possibility that being able to reduce problem drinking or drug use is simply a good prognostic sign. As residual sleep disturbances or reemerging insomnia are associated with an increased relapse risk in major depression (88, 89), they likewise represent a risk factor that, when treated, should be expected to improve outcomes and enhance quality of life, whether the intervention is an adjunctive pharmacotherapy (90) or targeted CBT (91).
The management of concurrent general medical conditions can also affect the longer-term outcomes of mood disorders. Flare-ups of autoimmune diseases, such as systemic lupus erythematosus (92) and multiple sclerosis (93), or infectious diseases that provoke neuroinflammatory responses, such as HIV/AIDS (94) and hepatitis C (95), increase the risk of depressive symptoms, as do some medications used to treat these conditions, including glucocorticoids (96) and interferon-alpha (97). Traumatic brain injuries or other debilitating conditions that profoundly reduce physical mobility and quality of life may also be treatable risk factors for depressive symptom exacerbation (98–100). The medications required for the management of many general medical conditions may also affect the pharmacodynamics or pharmacokinetics of antidepressant medications, and this needs to be proactively managed to reduce the risk of relapse in the longer term.
Pregnancy may increase the risk for depression. Many women planning to become pregnant may consider discontinuing previously successful antidepressant medications to avoid exposing the fetus to drugs. For them, consideration of sequential cognitive, behavioral, and interpersonal psychotherapies with established efficacy in pregnancy-related depression may be useful (101–103).
Prodromal detection and intervention is another approach (10) that seems useful clinically. For example, some patients will tend toward social withdrawal, and some will develop insomnia first at the initiation of relapse, which can be countered when recognized. Others may be able to identify, for example, a particular sequence of symptoms or particular behaviors that develop when a relapse is beginning to unfold. Early recognition of these prodromes allows for early treatment adjustments or behavioral changes to mitigate the relapse.
Case example: Recognizing the signature of the relapse prodrome through the patient’s social system
“Ms. B,” a 43-year-old saleswoman with bipolar disorder who had two past hospitalizations and whose mother had bipolar disorder, was familiar with the waxing and waning of symptoms, both manic and depressive, in this condition. While treated largely with lithium (1200–1500 mg/day) and an antidepressant, Ms. B had learned to track her symptoms using a daily monitoring tool. Nevertheless, her psychiatrist encouraged her to regularly bring her husband, as a corroborating informant. On this occasion, when asked, “How are things going?” she responded that all was well and that her life and work were routine and satisfactory. When the same question was posed to her husband in her presence, he responded, “What about your visits to the all-night convenience store at 4 a.m. nearly every night in the last 2 weeks? You were talking world events and politics with the cashier. That’s new.” Indeed, without this information, we would not have recognized this prodrome. As a result, medication adjustments were made and a potential full relapse was avoided.
Corroborating informants can also play essential roles in providing the history of illness and information on treatment response and medication adherence as well as assisting in relapse mitigation (10, 104).
Sequential or Combined Therapies With Psychotherapy
Combined treatment strategies are now widely practiced for the management of depressive disorders. A strong case can also be made for a greater use of sequential psychotherapies for higher-risk patients who have benefited from pharmacotherapy, to enhance relapse prevention, improve long-term outcomes, and optimize psychosocial functioning (1). Although less well established, there is a literature documenting the value of focused psychotherapies to help minimize the risk of relapse or recurrence when antidepressant medications are being discontinued (105, 106).
In addition, a recent series of studies that evaluated the utility of adjunctive psychotherapy in patients who have not responded well to antidepressant medications found meaningful levels of symptom reduction, of a magnitude comparable to those found with commonly used adjunctive medications (107–110).
The use of psychotherapy in combination with or in sequence with neuromodulation strategies has only recently received attention (111, 112). And the use of therapy combined with ECT has received little attention until recently, likely in part because of the amnestic effects of acute-phase ECT, which could interfere with learning and could be disruptive to the development of a psychotherapeutic relationship, or because of a sense of therapeutic nihilism on the clinician’s part, who may judge that such patients are just “too ill” to benefit from psychotherapy.
The potential for psychotherapy to improve outcomes after ECT is no longer theoretical. One recent trial found that CBT after a course of ECT significantly reduced the risk of relapse or recurrence (compared with continuation ECT and pharmacotherapy) (111). Moreover, the investigators found no evidence to suggest that patients’ perceptions of residual cognitive difficulties adversely affected therapeutic outcomes. Furthermore, early studies suggest that CBT may enhance or broaden the more modest antidepressant effects of transcranial magnetic stimulation therapy (112).
In sum, there are various ways to prevent or reduce relapses, such as learning to detect early signs of relapse, avoiding or managing factors that increase the risk of relapse, enhancing social support and social relationships, and making occupational or other environmental revisions. There is a substantial need for the development and testing of a range of relapse prevention therapies in the context of pharmacotherapy or neuromodulation therapy.
Implications of Patient-Centered Medical Management
Patient-centered medical management is premised on the notion that treatment outcomes entail symptoms plus function plus longer-term course and that both behavioral and attitudinal changes are often required. This perspective raises several questions: 1) whether, when, and how to incorporate composite outcomes in clinical and health service research, as well as care system management; 2) whether the new methods needed to accomplish these essential clinical tasks should be based on just one or a variety of psychotherapeutic models (e.g., cognitive, behavioral, interpersonal, dynamic); 3) how to accomplish these tasks in practice; and 4) where to find research funding to develop, test, and target these new methods to accomplish these essential tasks. It is very likely that methods developed for depression will help improve the delivery of many medical and surgical treatments (28).
The notion of composite outcomes in mental health is not new. The Agency for Healthcare Policy and Research’s Depression Guidelines Panel (8) and others (1, 5, 9, 11, 113) recognized that symptoms, function, quality of life, and the mitigation or prevention of relapse and recurrence were the aims of treatment, but symptoms and function are usually measured separately because they occur on different time scales. On the other hand, researchers and clinicians want to know whether some interventions with similar effects on symptoms may differ in their effects on function or prognosis. A composite measure may be quite informative at critical decision points in the longer-term management of these conditions. Alternatively, a composite measure of symptoms and function could be used repeatedly to gauge longer-term outcomes, especially in patients with waxing and waning courses of illness.
In terms of the therapeutic innovations, we currently conceptualize psychotherapies (e.g., cognitive, behavioral, interpersonal, psychodynamic) according to how we believe they work or how they explain the disorders or the problems associated with them. By contrast, medical and surgical practitioners conceptualize what they do by the objectives to be achieved. Medications, for example, are grouped according to their objectives (e.g., antibiotics, antihypertensives, analgesics), as medications in each group have different mechanisms of action. Surgeons choose among different surgical (e.g., wires, plates, joint replacement) and nonsurgical approaches (e.g., casting) to address the same clinical problem (e.g., a fractured clavicle or hip). Treatment selection is based on each patient’s situation, risk factors, personal priorities, personal burden, complication risks, occupation, and so on.
Perhaps a similar approach, at least in the case of patient-centered medical management—namely, specifying the objective to be achieved (e.g., engagement, adherence) and then selecting among possible approaches based on our understanding of the patient’s psychological and psychosocial strengths—would help us develop innovative approaches that could be tailored to each particular patient. This reframing does not exclude the development and use of the therapeutic relationship to effect change or to address difficulties caused by prior developmental difficulties or personality disorders.
Once the objective is defined, an agreed-upon metric can be selected so that diverse approaches to the same objective (e.g., adherence, relapse prevention) could be compared. For example, is motivational interviewing and shared decision making more effective in improving medication adherence than a reminder-based intervention, and if so, for which patients groups? Or we could ask which of several alternative approaches to repairing the damage done to the patient’s marriage by a recent manic episode is most effective, and for which patients. Training methods and videos to deliver these interventions could be developed and disseminated via cloud computing. This type of approach might also help improve the quality and outcomes of medical and surgical conditions, where these same four tasks pertain. Such approaches are currently being used in diabetes treatment (114, 115).
In terms of accomplishing these tasks in practice, psychiatrists and primary care practitioners acting alone cannot deliver all aspects of the care of depression to a panel of patients. Unfortunately, the current implementation of team care in mental health clinics can marginalize psychiatrists to writing prescriptions. The system in medical and surgical clinics whereby the physician orders each major treatment task in response to an assessment of the patient’s individual level of need leaves much of the individual doctor-patient relationship intact while effectively allocating and utilizing the panoply of skills of the diverse treatment team. Requiring that each major treatment task be chosen and separately ordered by the physician encourages him or her to assess carefully each patient’s context and tailor the choices to each patient. Greater treatment effectiveness and economy would be expected by increasing the relevance of treatment to the patient.
In summary, we have identified four essential clinical tasks in the optimal delivery of pharmacotherapy or neuromodulation therapies to patients with mood disorders. Systematically addressing each task with methods specifically chosen for or tailored to each patient (patient-centered medical management) should make recovery more likely and resource utilization more cost-effective. Psychiatrists should be trained and prepared to deliver these methods themselves as well as to oversee their delivery by the relevant treatment team members. Research support to develop, test, and target innovative ways to accomplish these clinical tasks would seem to be a wise and much-needed investment.
1 : A review of the current guidelines for depression treatment. J Clin Psychiatry 2010; 71:e15Crossref, Medline, Google Scholar
2 : Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder, section 3: pharmacological treatments. Can J Psychiatry 2016; 61:540–560Crossref, Medline, Google Scholar
3 : World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry 2013; 14:334–385Crossref, Medline, Google Scholar
4 : Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder, section 2: psychological treatments. Can J Psychiatry 2016; 61:524–539Crossref, Medline, Google Scholar
5 American Psychiatric Association: Practice Guideline for the Treatment of Patients With Major Depressive Disorder, 3rd edition. Washington, DC, American Psychiatric Association, 2010 (https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf)Google Scholar
6 : Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol 2015; 29:459–525Crossref, Medline, Google Scholar
7 : Pharmacological treatment of unipolar depressive disorders: summary of WFSBP guidelines. Int J Psychiatry Clin Pract 2017; 21:166–176Crossref, Medline, Google Scholar
8 Agency for Healthcare Policy and Research, Depression Guidelines Panel: Clinical Practice Guideline No 5: Depression in Primary Care, 2: Treatment of Major Depression (AHCPR publication 93-0551). Rockville, Md, US Dept of Health and Human Services, Agency for Healthcare Policy and Research, 1993Google Scholar
9 : Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology 2006; 31:1841–1853Crossref, Medline, Google Scholar
10 : Cognitive-Behavioral Therapy for Bipolar Disorder, 2nd ed. New York, Guilford, 2005Google Scholar
11 : Clinical management: imipramine/placebo administration manual. Psychopharmacol Bull 1987; 23:309–324Medline, Google Scholar
12 : Consensus recommendations for improving adherence, self-management, and outcomes in patients with depression. CNS Spectr 2007; 12(suppl 13):1–27Medline, Google Scholar
13 : Psychiatrist effects in the psychopharmacological treatment of depression. J Affect Disord 2006; 92:287–290Crossref, Medline, Google Scholar
14 : Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci 2012; 9:41–46Medline, Google Scholar
15 : Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163:1905–1917Link, Google Scholar
16 : The depression treatment cascade in primary care: a public health perspective. Curr Psychiatry Rep 2012; 14:328–335Crossref, Medline, Google Scholar
17 : Rethinking prevention of HIV type 1 infection. Clin Infect Dis 2010; 51:725–731Crossref, Medline, Google Scholar
18 : The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800Crossref, Medline, Google Scholar
19 : Efficacy of antidepressants on measures of workplace functioning in major depressive disorder: a systematic review. J Affect Disord 2018; 227:406–415Crossref, Medline, Google Scholar
20 : Distinguishing functional from syndromal recovery: implications for clinical care and research. J Clin Psychiatry 2015; 76:e832–e834Crossref, Medline, Google Scholar
21 : Sequential treatment of mood and anxiety disorders. J Clin Psychiatry 2005; 66:1392–1400Crossref, Medline, Google Scholar
22 : Engaging depressed patients: an essential step in optimizing care. Am J Psychiatry 2017; 174:506–507Link, Google Scholar
23 : Physical activity and exercise in the treatment of depression. Front Psychiatry 2012; 3:106Crossref, Medline, Google Scholar
24 : Adherence and persistence across antidepressant therapeutic classes: a retrospective claims analysis among insured US patients with major depressive disorder (MDD). CNS Drugs 2017; 31:421–432Crossref, Medline, Google Scholar
25 : Adherence to depression treatment in primary care: a randomized clinical trial. JAMA Psychiatry 2017; 74:1129–1135Crossref, Medline, Google Scholar
26 : Predictors of attrition during initial (citalopram) treatment for depression: a STAR*D report. Am J Psychiatry 2007; 164:1189–1197Link, Google Scholar
27 : What predicts attrition in second step medication treatments for depression? A STAR*D Report. Int J Neuropsychopharmacol 2009; 12:459–473Crossref, Medline, Google Scholar
28 : Adherence to medication. N Engl J Med 2005; 353:487–497Crossref, Medline, Google Scholar
29 : Dropping out of substance abuse treatment: a clinically oriented review. Clin Psychol Rev 1992; 12:93–116Crossref, Google Scholar
30 : Client-related predictors of early treatment drop-out in a substance abuse clinic exclusively employing individual therapy. J Subst Abuse Treat 2004; 26:189–195Crossref, Medline, Google Scholar
31 : Toward a theory of motivational interviewing. Am Psychol 2009; 64:527–537Crossref, Medline, Google Scholar
32 : Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 2003; 361:653–661Crossref, Medline, Google Scholar
33 : Preventing relapse and recurrence of depression: a brief review of therapeutic options. CNS Spectr 2006; 11(suppl 15):12–21Crossref, Medline, Google Scholar
34 : Trajectories of relapse in randomised, placebo-controlled trials of treatment discontinuation in major depressive disorder: an individual patient-level data meta-analysis. Lancet Psychiatry 2017; 4:230–237Crossref, Medline, Google Scholar
35 : A new empirical definition of major depressive episode recovery and its positive impact on future course of illness. J Clin Psychiatry 2016; 77:1065–1073Crossref, Medline, Google Scholar
36 : The costs of depression. Psychiatr Clin North Am 2012; 35:1–14Crossref, Medline, Google Scholar
37 : Adherence to antidepressant combinations and monotherapy for major depressive disorder: a CO-MED report of measurement-based care. J Psychiatr Pract 2014; 20:118–132Crossref, Medline, Google Scholar
38 : Low-intensity treatment of depression in primary care: is it problematic? Gen Hosp Psychiatry 2000; 22:78–83Crossref, Medline, Google Scholar
39 : Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2016; 164:350–359Crossref, Medline, Google Scholar
40 : Systematic review of multifaceted interventions to improve depression care. Gen Hosp Psychiatry 2007; 29:91–116Crossref, Medline, Google Scholar
41 : Cognitive behavioural therapy for adherence and depression in patients with HIV: a three-arm randomised controlled trial. Lancet HIV 2016; 3:e529–e538Crossref, Medline, Google Scholar
42 : Risk factors for non-adherence to antidepressant treatment in patients with mood disorders. Eur J Clin Pharmacol 2014; 70:89–98Crossref, Medline, Google Scholar
43 : Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 1999; 47:555–567Crossref, Medline, Google Scholar
44 : Joint trajectories of spousal social support and depressive symptoms in older age. J Aging Health (Epub ahead of print, Mar 1, 2018)Google Scholar
45 : Predictors of relapse in unipolar depressives: expressed emotion, marital distress, and perceived criticism. J Abnorm Psychol 1989; 98:229–235Crossref, Medline, Google Scholar
46 : Expressed emotion and relapse of psychopathology. Annu Rev Clin Psychol 2007; 3:329–352Crossref, Medline, Google Scholar
47 : Efficacy of couple therapy as a treatment for depression: a meta-analysis. Psychiatr Q 2008; 79:121–132Crossref, Medline, Google Scholar
48 : Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry 2007; 64:419–426Crossref, Medline, Google Scholar
49 : “Family matters”: a systematic review of the evidence for family psychoeducation for major depressive disorder. J Marital Fam Ther 2017; 43:245–263Crossref, Medline, Google Scholar
50 : Broader conceptualization of remission assessed by the remission from depression questionnaire and its association with symptomatic remission: a prospective, multicenter, observational study. BMC Psychiatry 2016; 16:352Crossref, Medline, Google Scholar
51 : Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry 2011; 72:1136–1143Crossref, Medline, Google Scholar
52 : A tipping point for measurement-based care. Psychiatr Serv 2017; 68:179–188Link, Google Scholar
53 : Clinical results for patients with major depressive disorder in the Texas Medication Algorithm Project. Arch Gen Psychiatry 2004; 61:669–680Crossref, Medline, Google Scholar
54 : The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on Medication Treatment of Major Depressive Disorder. J Clin Psychiatry 1999; 60:142–156Crossref, Medline, Google Scholar
55 : Mental health care from the public perspective: the Texas Medication Algorithm Project. J Clin Psychiatry 1999; 60(suppl 3):16–20Medline, Google Scholar
56 : Effectiveness and feasibility of a standardized stepwise drug treatment regimen algorithm for inpatients with depressive disorders: results of a 2-year observational algorithm study. J Clin Psychiatry 2002; 63:782–790Crossref, Medline, Google Scholar
57 : Algorithms for optimizing the treatment of depression: making the right decision at the right time. Pharmacopsychiatry 2003; 36(suppl 3):S222–S229Crossref, Medline, Google Scholar
58 : Algorithms and collaborative-care systems for depression: are they effective and why? A systematic review. Biol Psychiatry 2006; 59:1029–1038Crossref, Medline, Google Scholar
59 : Algorithm-guided treatment of depression reduces treatment costs: results from the randomized controlled German Algorithm Project (GAPII). J Affect Disord 2011; 134:249–256Crossref, Medline, Google Scholar
60 : How effective is algorithm-guided treatment for depressed inpatients? Results from the randomized controlled multicenter German Algorithm Project 3 trial. Int J Neuropsychopharmacol 2017; 20:721–730Crossref, Medline, Google Scholar
61 : A standardized stepwise drug treatment algorithm for depression reduces direct treatment costs in depressed inpatients: results from the German Algorithm Project (GAP3). J Affect Disord 2018; 228:173–177Crossref, Medline, Google Scholar
62 : Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006; 163:28–40Link, Google Scholar
63 : Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry 2015; 172:1004–1013Link, Google Scholar
64 : Isn’t it about time to employ measurement-based care in practice? Am J Psychiatry 2015; 172:934–936Link, Google Scholar
65 : Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009; 70(suppl 6):26–31Crossref, Medline, Google Scholar
66 : Exercise as an augmentation treatment for nonremitted major depressive disorder: a randomized, parallel dose comparison. J Clin Psychiatry 2011; 72:677–684Crossref, Medline, Google Scholar
67 : Effects of exercise on depressive symptoms in older adults with poorly responsive depressive disorder: randomised controlled trial. Br J Psychiatry 2002; 180:411–415Crossref, Medline, Google Scholar
68 : Treatment of the depressed alcoholic patient. Curr Psychiatry Rep 2012; 14:610–618Crossref, Medline, Google Scholar
69 : Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ 2014; 348:g1151Crossref, Medline, Google Scholar
70 : Restoring function in major depressive disorder: a systematic review. J Affect Disord 2017; 215:299–313Crossref, Medline, Google Scholar
71 : Early improvement in work productivity predicts future clinical course in depressed outpatients: findings from the CO-MED trial. Am J Psychiatry 2016; 173:1196–1204Link, Google Scholar
72 : Daily activity level improvement with antidepressant medications predicts long-term clinical outcomes in outpatients with major depressive disorder. Neuropsychiatr Dis Treat 2017; 13:803–813Crossref, Medline, Google Scholar
73 : Remission in depressed outpatients: more than just symptom resolution? J Psychiatr Res 2008; 42:797–801Crossref, Medline, Google Scholar
74 : Couple-based intervention for depression: an effectiveness study in the National Health Service in England. Fam Process 2017; 57:275–292Crossref, Medline, Google Scholar
75 : Couple therapy as a treatment for depression, II: the effects of relationship quality and therapy on depressive relapse. J Consult Clin Psychol 1993; 61:516–519Crossref, Medline, Google Scholar
76 : Functional recovery in major depressive disorder: providing early optimal treatment for the individual patient. Int J Neuropsychopharmacol 2018; 21:128–144Crossref, Medline, Google Scholar
77 : World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 2: maintenance treatment of major depressive disorder: update 2015. World J Biol Psychiatry 2015; 16:76–95Crossref, Medline, Google Scholar
78 : Effectiveness of preventive cognitive therapy while tapering antidepressants versus maintenance antidepressant treatment versus their combination in prevention of depressive relapse or recurrence (DRD study): a three-group, multicentre, randomised controlled trial. Lancet Psychiatry 2018; 5:401–410Crossref, Medline, Google Scholar
79 : Neural markers of resilience in adolescent females at familial risk for major depressive disorder. JAMA Psychiatry 2018 75:493–502Crossref, Medline, Google Scholar
80 : Resilience in high-risk adolescents of mothers with recurrent depressive disorder: the contribution of fathers. J Adolesc 2018; 65:207–218Crossref, Medline, Google Scholar
81 : Mindfulness-based interventions for psychiatric disorders: a systematic review and meta-analysis. Clin Psychol Rev 2018; 59:52–60Crossref, Medline, Google Scholar
82 : Mindfulness mediates the physiological markers of stress: systematic review and meta-analysis. J Psychiatr Res 2017; 95:156–178Crossref, Medline, Google Scholar
83 : The role of hazardous drinking reductions in predicting depression and anxiety symptom improvement among psychiatry patients: a longitudinal study. J Affect Disord 2016; 206:169–173Crossref, Medline, Google Scholar
84 : Patterns of marijuana use among psychiatry patients with depression and its impact on recovery. J Affect Disord 2017; 213:168–171Crossref, Medline, Google Scholar
85 : Prognostic effect of the variable course of alcoholism on the 10-year course of depression. Am J Psychiatry 1994; 151:701–706Link, Google Scholar
86 : Five-year outcome of major depressive disorder in primary health care. Psychol Med 2014; 44:1369–1379Crossref, Medline, Google Scholar
87 : A 5-year follow-up of depressed and bipolar patients with alcohol use disorder in an Irish population. Alcohol Clin Exp Res 2014; 38:1049–1058Crossref, Medline, Google Scholar
88 : Ongoing or re-emerging subjective insomnia symptoms after full/partial remission or recovery of major depressive disorder mainly with the selective serotonin reuptake inhibitors and risk of relapse or recurrence: a 52-week follow-up study. J Affect Disord 2011; 134:257–265Crossref, Medline, Google Scholar
89 : Predicting relapse with individual residual symptoms in major depressive disorder: a reanalysis of the STAR*D data. Psychopharmacology (Berl) 2017; 234:2453–2461Crossref, Medline, Google Scholar
90 : Evaluation of adjunct extended-release quetiapine fumarate on sleep disturbance and quality in patients with major depressive disorder and an inadequate response to on-going antidepressant therapy. Int J Neuropsychopharmacol 2013; 16:1755–1765Crossref, Medline, Google Scholar
91 : Efficacy of cognitive-behavioral therapy for insomnia combined with antidepressant pharmacotherapy in patients with comorbid depression and insomnia: a randomized controlled trial. J Clin Psychiatry 2016; 77:e1316–e1323Crossref, Medline, Google Scholar
92 : Depressive symptoms are associated with tumor necrosis factor alpha in systemic lupus erythematosus. J Neuroinflammation 2016; 13:5Crossref, Medline, Google Scholar
93 : Multiple immune-inflammatory and oxidative and nitrosative stress pathways explain the frequent presence of depression in multiple sclerosis. Mol Neurobiol 2018; 55:6282–6306Crossref, Medline, Google Scholar
94 : Neuropathogenesis of human immunodeficiency virus infection. Handb Clin Neurol 2018; 152:21–40Crossref, Medline, Google Scholar
95 : Hepatitis C virus infection, and neurological and psychiatric disorders: a review. J Adv Res 2017; 8:139–148Crossref, Medline, Google Scholar
96 : Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry 2012; 169:491–497Link, Google Scholar
97 : Interferon-related depression: a primer on mechanisms, treatment, and prevention of a common clinical problem. Curr Neuropharmacol 2016; 14:743–748Crossref, Medline, Google Scholar
98 : A systematic review of the benefits of mindfulness-based interventions following transient ischemic attack and stroke. Int J Stroke 2013; 8:465–474Crossref, Medline, Google Scholar
99 : Neuropsychological rehabilitation and psychotherapy of adult traumatic brain injury patients with depression: a systematic review and meta-analysis. J Neurosurg Sci 2018; 62:24–35Medline, Google Scholar
100 : Psychotherapeutic and psychosocial interventions for managing stress in multiple sclerosis: the contribution of mindfulness-based interventions. Neurologia 2016; 31:113–120Medline, Google Scholar
101 : A pragmatic randomized clinical trial of behavioral activation for depressed pregnant women. J Consult Clin Psychol 2017; 85:26–36Crossref, Medline, Google Scholar
102 : Internet delivered cognitive behavior therapy for antenatal depression: a randomised controlled trial. J Affect Disord 2017; 221:56–64Crossref, Medline, Google Scholar
103 : A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affect Disord 2018; 232:316–328Crossref, Medline, Google Scholar
104 : Mental Health and Psychopathology: A MacArthur Foundation Research Network Series: Cognitive Therapy for Depressed Adolescents. New York, Guilford, 1994Google Scholar
105 : Treatment of recurrent depression: a sequential psychotherapeutic and psychopharmacological approach. CNS Drugs 2003; 17:1109–1117Crossref, Medline, Google Scholar
106 : A lifetime approach to major depressive disorder: the contributions of psychological interventions in preventing relapse and recurrence. Clin Psychol Rev 2015; 41:16–26Crossref, Medline, Google Scholar
107 : Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry 2007; 164:739–752Link, Google Scholar
108 : Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet 2013; 381:375–384Crossref, Medline, Google Scholar
109 : Effectiveness of supplementary cognitive-behavioral therapy for pharmacotherapy-resistant depression: a randomized controlled trial. J Clin Psychiatry 2017; 78:1126–1135Crossref, Medline, Google Scholar
110 : A randomised controlled trial of intensive short-term dynamic psychotherapy for treatment resistant depression: the Halifax Depression Study. J Affect Disord 2017; 214:15–25Crossref, Medline, Google Scholar
111 : Cognitive-behavioral therapy as continuation treatment to sustain response after electroconvulsive therapy in depression: a randomized controlled trial. Biol Psychiatry 2014; 76:194–202Crossref, Medline, Google Scholar
112 : Simultaneous rTMS and psychotherapy in major depressive disorder: clinical outcomes and predictors from a large naturalistic study. Brain Stimul 2018; 11:337–345Crossref, Medline, Google Scholar
113 : Outcomes on the pharmacopsychometric triangle in bupropion-SR vs buspirone augmentation of citalopram in the STAR*D trial. Acta Psychiatr Scand 2012; 125:342–348Crossref, Medline, Google Scholar
114 : Comparative effectiveness of telemedicine strategies on type 2 diabetes management: a systematic review and network meta-analysis. Sci Rep 2017; 7:12680Crossref, Medline, Google Scholar
115 : Effect of a 36-month pharmaceutical care program on pharmacotherapy adherence in elderly diabetic and hypertensive patients. Int J Clin Pharm 2011; 33:642–649Crossref, Medline, Google Scholar



