Cortical and Subcortical Brain Morphometry Differences Between Patients With Autism Spectrum Disorder and Healthy Individuals Across the Lifespan: Results From the ENIGMA ASD Working Group
Abstract
Objective:
Neuroimaging studies show structural differences in both cortical and subcortical brain regions in children and adults with autism spectrum disorder (ASD) compared with healthy subjects. Findings are inconsistent, however, and it is unclear how differences develop across the lifespan. The authors investigated brain morphometry differences between individuals with ASD and healthy subjects, cross-sectionally across the lifespan, in a large multinational sample from the Enhancing Neuroimaging Genetics Through Meta-Analysis (ENIGMA) ASD working group.
Method:
The sample comprised 1,571 patients with ASD and 1,651 healthy control subjects (age range, 2–64 years) from 49 participating sites. MRI scans were preprocessed at individual sites with a harmonized protocol based on a validated automated-segmentation software program. Mega-analyses were used to test for case-control differences in subcortical volumes, cortical thickness, and surface area. Development of brain morphometry over the lifespan was modeled using a fractional polynomial approach.
Results:
The case-control mega-analysis demonstrated that ASD was associated with smaller subcortical volumes of the pallidum, putamen, amygdala, and nucleus accumbens (effect sizes [Cohen’s d], 0.13 to –0.13), as well as increased cortical thickness in the frontal cortex and decreased thickness in the temporal cortex (effect sizes, −0.21 to 0.20). Analyses of age effects indicate that the development of cortical thickness is altered in ASD, with the largest differences occurring around adolescence. No age-by-ASD interactions were observed in the subcortical partitions.
Conclusions:
The ENIGMA ASD working group provides the largest study of brain morphometry differences in ASD to date, using a well-established, validated, publicly available analysis pipeline. ASD patients showed altered morphometry in the cognitive and affective parts of the striatum, frontal cortex, and temporal cortex. Complex developmental trajectories were observed for the different regions, with a developmental peak around adolescence. These findings suggest an interplay in the abnormal development of the striatal, frontal, and temporal regions in ASD across the lifespan.
Autism spectrum disorder (ASD) is a childhood-onset neurodevelopmental disorder that affects about 1.4% of the population (1–3). ASD is usually diagnosed before age 6, and it often leads to lifelong impaired functioning and problems in social adaptation. Although ASD is highly heritable and considered to be a brain-based disorder, the biological underpinnings of the disorder and its development over the lifespan remain largely unclear.
Much research has focused on the role of anatomical brain abnormalities in ASD (4–7). Both larger (7) and smaller (8) volumes of striatal structures have been reported in ASD, as well as smaller hippocampal volumes (9) and, in childhood, larger amygdala volumes (10). Increased intracranial volume (11), total gray matter, and cortical thickness have also been reported in ASD (12), with more specific cortical effects observed mainly in the frontal (13) and temporal lobes (14). These structural abnormalities play a crucial role in current theories on the neurobiology of autism. Specifically, altered frontal and striatal volumes and disrupted fronto-striatal connectivity are key components in the executive function deficit theory of ASD (15–17). On the other hand, abnormal amygdala volume, specifically in childhood (18, 19), plays a central role in the social theories of ASD (10, 20, 21).
However, the neuroimaging literature reflects considerable heterogeneity in the direction and effect size of these morphometric brain differences (12, 22, 23), with a recent large-scale study by Haar et al. (12) even indicating overall increased gray/white matter measures but very small local effects of ASD on brain morphometry. This heterogeneity in the literature may be due to various factors. First, variation in case-control differences of brain structures may be due to age differences between study samples. Research has suggested the presence of altered patterns of cortical and subcortical development in ASD, generally with abnormally higher volumes in childhood followed by a more rapid volumetric decline during adolescence and adulthood (13, 24–26). Second, factors such as sex, medication use, symptom severity, and presence of comorbidities may also affect case-control differences in brain structures. Third, method-based factors, such as variation in data acquisition, processing, and analysis protocols, may influence the results reported across different studies.
Several recent studies have used the anatomical differences in ASD as the basis for multivariate analyses with the ultimate goal of using these differences as a tool for categorizing ASD (12, 27, 28). These efforts, however, are yet to be validated in clinical settings (29). The heterogeneity of anatomical differences in the various samples may also underlie the lack of consistent results in this line of research.
The present study addresses several of these issues. This collaboration was established as part of Enhancing Neuroimaging Genetics Through Meta-Analysis (ENIGMA), the worldwide imaging genetics consortium aimed at unifying analytic methods across a range of neuropsychiatric disorders. We use the ENIGMA processing and analysis pipelines to merge individual subject data from 49 existing ASD case-control cohorts (of which 16 cohorts were collected previously as part of the ABIDE consortium [6], and 17 as part of ABIDE-II [30]) to determine whether and which changes in subcortical volumes and cortical thickness and surface area underlie the ASD phenotype across the lifespan. By unifying processing and analysis, we were able to eliminate a large part of the methodological noise between individual studies. Additionally, we were able to investigate directly the effects of sex, IQ, and symptom severity across this extensive sample. Last but not least, studies employing small sample sizes are liable to overestimate effect sizes (31). With 43 of the 49 included cohorts employing samples of fewer than 100 subjects each, it is important to test whether results of small-scale studies remain robust within this large cohort.
Based on the literature, we expected the ASD group to show smaller subcortical volumes specifically in the putamen and caudate but larger volumes in the hippocampus and amygdala. We furthermore expected generally increased gray matter volumes and cortical thickness in the ASD group, specifically increased thickness in the frontal and temporal cortices (32). Given the broad age range of the sample, we also charted in detail the development of these morphological features over the lifespan in ASD, albeit based on cross-sectional data. Based on previous studies, we expected to see the largest effects of ASD during childhood, with normalization of features through adolescence and adulthood (13, 24–26).
Method
Contributing Sites
ENIGMA ASD is an open cohort, aimed at bringing together MRI data from a wide range of ASD studies. The working group was started in 2015 and remains open for any new groups working with ASD patients of any age. The working group implemented a data freeze to execute the present subcortical volume analyses in March 2017, at which point we included a total of 1,571 patients with ASD and 1,651 healthy control subjects from 49 participating research groups spanning 13 countries. Both the ABIDE and ABIDE-II consortia were included in the present cohort (6, 30). Basic demographic and clinical information for the cohort is summarized in Table 1 and Figure 1; details of the contributing samples can be found in Table S1 in the data supplement that accompanies the online edition of this article. All contributing sites had local ethical approval for the sharing of meta-analytic test statistics, and 48 of the 49 sites had approval for sharing anonymized individual data. Even though we included samples from the entire ASD spectrum, the vast majority of the included individuals did not have an intellectual disability, so the IQ scores between the patient and control groups are comparable.
| Characteristic | Control Group (N=1,651) | ASD Group (N=1,571) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Agea (years) | 15.83 | 8.41 | 15.41 | 8.64 |
| IQb,c | 111 | 19.04 | 103 | 20.02 |
| N | % | N | % | |
| Femaleb | 393 | 23.8 | 224 | 14.3 |
| Medication used | 0 | 0.0 | 233 | 14.8 |
| Comorbiditiese | 0 | 0.0 | 148 | 9.4 |
TABLE 1. Characteristics of Participants in a Mega-Analysis of Brain Morphometry in Autism Spectrum Disorder (ASD) and Healthy Control Subjects
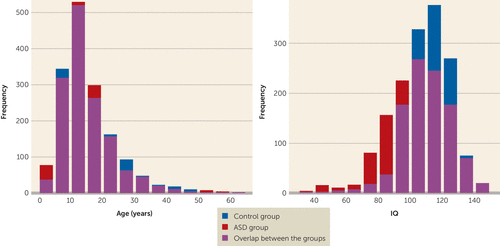
FIGURE 1. Distributions of Age and IQ Within the Full Sample of Patients With Autism Spectrum Disorder (ASD) and Healthy Control Subjects in a Mega-Analysis of Brain Morphometry
FreeSurfer Segmentation
Structural T1-weighted MRI scans acquired at the various contributing sites were segmented using standardized and publicly available ENIGMA imaging protocols (http://enigma.ini.usc.edu/protocols/imaging-protocols/). These automated protocols, based on FreeSurfer (version 5.3) segmentations, are fully validated and allow maximal uniformity and comparability across sites. For each participant, left and right subcortical volumes, cortical thickness, and cortical surface area measures were calculated. The mean of left and right volumes were used for most subsequent analyses. Standardized quality control depended on the automatic detection of segmentation outliers for each volume, followed by visual inspection of outlying volumes. Poorly segmented regions were removed from further analyses. Detailed information on the quality control procedure is provided in the online data supplement.
Case-Control Analyses
The main aim of this study was to investigate differences in cortical and subcortical morphometry related to ASD status. To accomplish this, individual segmentation subcortical volumes, cortical thickness, and cortical surface area were merged for participants over all sites into one mega-analysis. The effect of diagnosis (patient, control group) on each region of interest was calculated using a linear mixed-effects model (using the nlme package in R), including a polynomial fit for age, sex, and IQ as fixed factors and age-by-diagnosis and age-by-sex interactions. For subcortical volumes, total intracranial volume was added as a fixed factor. Scan site was added as a random factor. The false discovery rate adjustment was used to correct p values for multiple comparisons.
Several additional sensitivity analyses were performed to investigate how sex, IQ, medication use, comorbidities, ASD severity, hemisphere effects, or total intracranial volume differences might have influenced the main effect of ASD status on morphometric measures. Age and sex were available for all incorporated studies. For IQ, medication use, comorbidities, and ASD severity, data were available for some samples (see the online data supplement for the available data per site). Given the large variation in available detail on medication use and comorbidity assessment, these were included in the analyses as a dichotomous measure (current medication versus none, current comorbidity versus none). For ASD severity, the most widely available measure was the total score on the Autism Diagnostic Observation Schedule–Generic (ADOS) (33), which was used as an estimate of ASD severity. For a more detailed comparison with the Haar et al. study (12), a permutation-based post hoc analysis was performed.
To allow for comparisons across the different sites as well as to control for any unobserved effects that may influence the mega-analysis, we repeated the same analysis of the diagnosis effect in a more conservative meta-analysis, running a linear regression model for each site separately. The I2 statistic was calculated to estimate the heterogeneity of the diagnostic effects across sites, indicating the percentage of variation across studies that is due to heterogeneity rather than to chance.
Age Effects Modeling
Given the importance of developmental trajectories when estimating the effects of ASD, we used a fractional polynomial approach to estimate the optimal fit for development of the volume, thickness, or surface area with age (using the mfp package in R). For all regions of interest that showed a significant effect of age or age-by-diagnosis interaction in the mega-analysis, the optimal model was estimated for the ASD and healthy control groups using one- and two-term curvilinear models, choosing the best-fitting model out of 44 possible two-term models, with possible powers of −2, −1, −0.5, 0, 0.5, 1, 2 and 3 (these models also included sex, IQ, and scan site as covariates).
Power Calculation
Using G*Power, version 3.1.9.2, we calculated the minimal effect sizes observable given 1,571 participants with ASD and 1,651 healthy control subjects. At a minimum desired power level of 0.8 and a significance threshold of 0.05 (two-tailed), we have the statistical power to observe effect sizes (Cohen’s d) >0.108.
Results
Case-Control Differences in Subcortical and Cortical Partitions
Sex and IQ were not distributed equally between the ASD and healthy control groups. The control group had a larger proportion of females than the ASD group (23.8% compared with 14.25%; p<0.001), as well as a significantly higher mean IQ (111 compared with 103, p<0.001). Both sex and IQ were incorporated as covariates in the main mega-analysis to correct for these differences. Additional sensitivity analyses without correction for IQ as well as analyses with subgroups balanced on sex and IQ are reported in the online data supplement. The effects of medication use and associations with symptom severity were also investigated.
The results of the subcortical mega-analysis are presented in Table 2 and Figure 2. Even though the hippocampus is technically a cortical area, we have chosen to add hippocampal volume to the subcortical results, since it is segmented as a subcortical volume in FreeSurfer. ASD was associated with significantly smaller mean volumes of the putamen (p<0.001, d=−0.10), the pallidum (p<0.05, d=−0.08), the amygdala (p<0.05; d=−0.08), and the nucleus accumbens (p<0.001, d=−0.13), as well as a larger mean volume for the lateral ventricles (p<0.001, d=0.11) and a larger mean intracranial volume (p<0.001, d=0.13). Additional sensitivity analyses correcting the subcortical volumes for intracranial gray matter volume instead of total intracranial volume are reported in the data supplement.
| Region and Structure | Control Group (N) | ASD Group (N) | Diagnosis | Age | Age by Diagnosis | Sex | IQ | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohen’s d | pb | Cohen’s d | pb | pb | Cohen’s d | pb | Cohen's d | pb | |||
| Subcortical | |||||||||||
| Lateral ventricles | 1,569 | 1,482 | 0.11 | 0.010 | 0.16 | 0.001 | 0.529 | –0.10 | 0.064 | 0.17 | 0.050 |
| Thalamus | 1,597 | 1,494 | 0.00 | 0.989 | 0.07 | 0.042 | 0.654 | –0.11 | 0.034 | 0.01 | 0.110 |
| Caudate | 1,604 | 1,517 | –0.05 | 0.206 | –0.19 | 0.001 | 0.230 | –0.15 | 0.003 | 0.00 | 0.150 |
| Putamen | 1,600 | 1,518 | –0.10 | 0.013 | –0.12 | 0.001 | 0.882 | –0.22 | <0.001 | 0.00 | 0.140 |
| Pallidum | 1,596 | 1,509 | –0.08 | 0.046 | –0.26 | 0.001 | 0.261 | 0.01 | 0.839 | 0.00 | 0.140 |
| Hippocampus | 1,595 | 1,507 | –0.05 | 0.222 | 0.14 | 0.001 | 0.521 | –0.05 | 0.310 | 0.02 | 0.100 |
| Amygdala | 1,601 | 1,508 | –0.08 | 0.046 | 0.13 | 0.001 | 0.230 | –0.16 | 0.002 | 0.01 | 0.110 |
| Nucleus accumbens | 1,596 | 1,518 | –0.13 | 0.002 | –0.19 | 0.001 | 0.230 | –0.22 | <0.001 | 0.13 | 0.060 |
| Intracranial volume | 1,606 | 1,522 | 0.13 | 0.009 | 0.14 | 0.001 | 0.261 | –0.24 | <0.001 | 0.02 | 0.100 |
| Frontal | |||||||||||
| Superior frontal | 1,574 | 1,657 | 0.17 | <0.001 | –0.30 | <0.001 | <0.001 | 0.08 | 0.039 | –0.03 | 0.731 |
| Rostral middle frontal | 1,572 | 1,658 | 0.20 | <0.001 | –0.39 | <0.001 | <0.001 | 0.03 | 0.379 | –0.02 | 0.818 |
| Caudal middle frontal | 1,574 | 1,656 | 0.08 | 0.057 | –0.24 | <0.001 | <0.001 | 0.08 | 0.025 | 0.05 | 0.397 |
| Pars triangularis | 1,572 | 1,653 | 0.11 | 0.006 | –0.31 | <0.001 | <0.001 | 0.03 | 0.342 | –0.01 | 0.818 |
| Pars orbitalis | 1,573 | 1,656 | 0.12 | 0.002 | –0.28 | <0.001 | <0.001 | 0.03 | 0.490 | –0.07 | 0.319 |
| Pars opercularis | 1,572 | 1,654 | 0.01 | 0.733 | –0.25 | <0.001 | 0.002 | 0.00 | 0.920 | 0.00 | 0.982 |
| Medial orbitofrontal | 1,572 | 1,656 | 0.15 | <0.001 | –0.37 | <0.001 | <0.001 | –0.03 | 0.406 | –0.09 | 0.143 |
| Lateral orbitofrontal | 1,574 | 1,655 | 0.05 | 0.294 | –0.32 | <0.001 | <0.001 | –0.03 | 0.367 | –0.02 | 0.805 |
| Precentral | 1,569 | 1,655 | –0.06 | 0.132 | –0.10 | 0.006 | 0.544 | 0.04 | 0.239 | 0.12 | 0.020 |
| Paracentral | 1,574 | 1,653 | –0.01 | 0.733 | –0.29 | <0.001 | <0.001 | 0.02 | 0.492 | 0.06 | 0.362 |
| Frontal pole | 1,568 | 1,655 | 0.10 | 0.011 | –0.25 | <0.001 | <0.001 | –0.02 | 0.567 | –0.02 | 0.805 |
| Insula | |||||||||||
| Insula | 1,567 | 1,651 | –0.07 | 0.113 | –0.13 | <0.001 | 0.454 | –0.05 | 0.195 | –0.02 | 0.818 |
| Cingulate | |||||||||||
| Rostral anterior (frontal) | 1,569 | 1,652 | 0.02 | 0.585 | –0.20 | <0.001 | 0.008 | 0.02 | 0.541 | –0.08 | 0.228 |
| Caudal anterior (frontal) | 1,566 | 1,651 | 0.03 | 0.523 | –0.16 | <0.001 | 0.012 | 0.05 | 0.197 | –0.08 | 0.228 |
| Posterior (parietal) | 1,568 | 1,657 | 0.13 | 0.002 | –0.22 | <0.001 | 0.002 | 0.02 | 0.634 | –0.06 | 0.375 |
| Isthmus (parietal) | 1,572 | 1,650 | 0.08 | 0.044 | –0.21 | <0.001 | 0.001 | –0.02 | 0.542 | –0.02 | 0.787 |
| Parietal | |||||||||||
| Superior parietal | 1,572 | 1,654 | –0.01 | 0.819 | –0.34 | <0.001 | <0.001 | 0.06 | 0.107 | –0.01 | 0.846 |
| Inferior parietal | 1,572 | 1,657 | 0.02 | 0.639 | –0.32 | <0.001 | <0.001 | 0.01 | 0.713 | –0.02 | 0.805 |
| Supramarginal | 1,572 | 1,652 | –0.07 | 0.079 | –0.27 | <0.001 | <0.001 | 0.00 | 0.992 | 0.03 | 0.726 |
| Postcentral | 1,569 | 1,655 | –0.03 | 0.523 | –0.25 | <0.001 | <0.001 | 0.03 | 0.467 | 0.02 | 0.787 |
| Precuneus | 1,573 | 1,658 | 0.03 | 0.526 | –0.34 | <0.001 | <0.001 | 0.04 | 0.287 | –0.06 | 0.362 |
| Temporal | |||||||||||
| Superior temporal | 1,569 | 1,652 | –0.08 | 0.062 | –0.04 | 0.313 | 0.387 | 0.00 | 0.942 | 0.05 | 0.397 |
| Middle temporal | 1,570 | 1,653 | –0.10 | 0.014 | –0.14 | <0.001 | 0.150 | 0.02 | 0.540 | 0.07 | 0.319 |
| Inferior temporal | 1,571 | 1,655 | –0.14 | <0.001 | –0.15 | <0.001 | 0.044 | –0.05 | 0.154 | –0.05 | 0.453 |
| Banks of the superior temporal sulcus | 1,562 | 1,647 | –0.03 | 0.523 | –0.15 | <0.001 | 0.098 | –0.02 | 0.555 | 0.03 | 0.787 |
| Fusiform | 1,570 | 1,655 | –0.19 | <0.001 | –0.17 | <0.001 | 0.024 | –0.06 | 0.097 | –0.01 | 0.914 |
| Transverse temporal | 1,574 | 1,655 | –0.12 | 0.003 | –0.16 | <0.001 | 0.039 | 0.09 | 0.010 | 0.05 | 0.397 |
| Entorhinal | 1,559 | 1,641 | –0.21 | <0.001 | 0.00 | 0.947 | 0.683 | –0.01 | 0.823 | 0.05 | 0.453 |
| Temporal pole | 1,565 | 1,648 | –0.13 | 0.001 | 0.03 | 0.424 | 0.481 | –0.03 | 0.345 | 0.03 | 0.731 |
| Parahippocampal | 1,569 | 1,653 | –0.10 | 0.014 | –0.06 | 0.123 | 0.556 | 0.05 | 0.157 | 0.05 | 0.453 |
| Occipital | |||||||||||
| Lateral occipital | 1,569 | 1,652 | –0.05 | 0.202 | –0.21 | <0.001 | <0.001 | –0.02 | 0.526 | –0.02 | 0.787 |
| Lingual | 1,572 | 1,655 | –0.04 | 0.417 | –0.24 | <0.001 | <0.001 | –0.03 | 0.478 | –0.01 | 0.883 |
| Cuneus | 1,571 | 1,651 | 0.07 | 0.086 | –0.27 | <0.001 | <0.001 | 0.01 | 0.786 | –0.07 | 0.278 |
| Pericalcarine | 1,571 | 1,648 | 0.00 | 0.934 | –0.11 | 0.003 | 0.150 | 0.05 | 0.160 | 0.00 | 0.987 |
TABLE 2. Model Outcomes in a Mega-Analysis of Brain Morphometry in Patients With Autism Spectrum Disorder (ASD) and Healthy Control Subjectsa
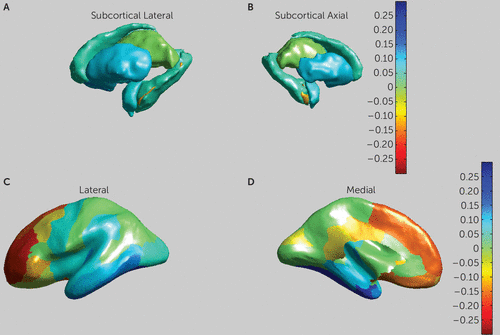
FIGURE 2. Effect Sizes for all Subcortical and Cortical Partitions in a Mega-Analysis of Brain Morphometry in Patients With Autism Spectrum Disorder (ASD) and Healthy Control Subjectsa
a Panels A and B are medial and lateral views of the striatum. Panels C and D are medial and lateral views of cortical thickness. Yellow to red hues indicate higher d values, corresponding to larger volumes in patients with ASD. Blue hues indicate lower volumes in subjects with ASD. Images are in Montreal Neurological Institute space (MNI152).
The effects of ASD diagnosis on cortical thickness are presented in Table 2. We observed a significantly increased overall cortical thickness (p<0.003, d=0.41) in the ASD group, as well as more specifically in nine of the 34 cortical partitions, located in the middle and superior frontal, orbitofrontal, inferior frontal, and posterior cingulate areas. We observed decreased cortical thickness in the ASD group in seven partitions, located in the temporal, entorhinal, and parahippocampal areas (see Figure 2). We also found increased overall gray matter volume (p<0.052, d=0.23) in the ASD group. No effects of ASD diagnosis on cortical surface area were found.
Post hoc analyses per hemisphere indicated that for the lateral ventricles (left, p<0.004; right, p<0.006), the putamen (p<0.03, p<0.05), and the nucleus accumbens (p<0.008; p<0.02), both hemispheres contribute to the overall effect. For the hippocampus and amygdala, only the right hemisphere showed a significant effect (p<0.05 and p<0.03, respectively). Increased cortical thickness in the ASD group was observed in the frontal cortex for both hemispheres, as well as decreased thickness in the temporal cortex. In the right hemisphere, significantly thicker cortex in the ASD group was furthermore observed in the cingulate cortex, and decreased thickness in the parietal cortex (see Table S8 in the data supplement).
To investigate differential effects between sites, a meta-analysis was performed by treating every site independently and aggregating the results. The results from the case-control meta-analysis confirmed smaller pallidum (p<0.04, d=−0.09), amygdala (p<0.03, d=−0.09), and nucleus accumbens (p<0.01, d=−0.1) volumes in the ASD group, as well as a larger volume for the lateral ventricles (p<0.003, d=0.13) and a larger intracranial volume (p<0.016, d=0.06). The meta-analysis also showed increased cortical thickness in the ASD group in three of 11 frontal partitions and decreased thickness in eight of nine temporal partitions as well as in the supramarginal gyrus (see Table S2 in the data supplement). Overall, the meta-analysis showed smaller effect sizes and higher standard error of effect sizes than the mega-analysis. The I2 test indicates moderate to high heterogeneity across sites for all effect sizes (I2=15.19–64.63). Individual test statistics for the case-control comparison per site are listed in the data supplement (see Table S12).
Age Effects
Main linear effects of age were observed for all subcortical volumes (see Table 2). However, no interaction effects between diagnosis and age were found. We calculated fractional polynomial fits for the age effect for all the above-mentioned regions of interest, estimating the polynomial fit for these volumes (Figure 3; see also Table S11 in the data supplement). The fractional polynomial approach indicates that the optimal model for the age effect in all subcortical volumes contains the powers of 0.5 and 2 (see Figure 2). These results indicate that the developmental curves of the ASD and healthy control groups follow similar trajectories over time, which confirms the observed lack of detectable age-by-diagnosis interactions.
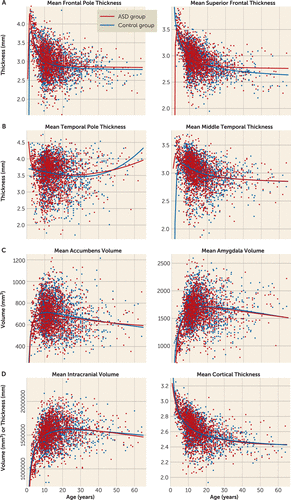
FIGURE 3. Fractional Polynomial Best Model Fits for Age in a Mega-Analysis of Brain Morphometry in Patients With Autism Spectrum Disorder (ASD) and Healthy Control Subjectsa
a Models are shown (with separate fits for the ASD and control groups) for frontal cortical thickness (panel A), temporal cortical thickness (panel B), and subcortical volumes with significant diagnosis and age or age-by-diagnosis effects (panel C), as well as total intracranial volume and total cortical thickness (panel D). (Additional plots are provided in Figure S2 in the online data supplement.)
Main linear effects of age on cortical thickness were observed in 30 of 34 partitions, all of them showing a negative relationship between age and thickness (see Table 1). The four partitions that did not show a significant effect all overlapped with the partitions that showed a negative relationship between thickness and ASD diagnosis (temporal, entorhinal, and parahippocampal areas). A quadratic effect of age was observed in the insula. Age-by-diagnosis interactions were observed in 24 of the partitions that also showed a linear age effect. Fractional polynomial plots were calculated for these partitions as well, showing complex developmental curves, including both quadratic and cubic effects across the partitions (see Figure 3). These visualizations indicate that individuals with ASD show a peak in cortical thickness differences around adolescence, with both the greater thickness in the frontal cortex and the lesser thickness in the temporal cortex peaking around this age.
Sex and IQ Effects
The mega-analysis shows significant effects of sex on intracranial volume and volumes of the lateral ventricles, thalamus, caudate, putamen, amygdala, and nucleus accumbens, indicating larger volumes in males than females. No interactions between diagnosis and sex were observed. Effects of IQ on brain volumes were observed for volumes of the putamen, hippocampus, amygdala, and nucleus accumbens, with larger volumes associated with higher IQ (see Table 2). The three-way interaction of diagnosis, age, and sex rendered no significant results.
Effects of sex on cortical thickness were observed in transverse temporal, caudal-middle-frontal, and superior frontal partitions, all of them indicating a thicker cortex in males. Participants with a higher IQ showed greater cortical thickness in precentral and rostral anterior cingulate partitions and lower thickness in medial orbitofrontal and caudal anterior cingulate partitions.
Medication, Comorbidity, and Symptom Severity Effects
Within the ASD group, further mega-analyses were performed to test for any effects of medication use, comorbidities, and ASD symptom severity on subcortical volumes (see the online data supplement). Neither medication use nor the presence of comorbidities significantly influenced subcortical volumes within this sample. Cortical thickness was associated with medication use only in the inferior-temporal partition (d=−0.47, p<0.002), but not with comorbidity. ASD symptom severity analyses showed that higher ADOS scores were associated with larger intracranial and lateral ventricle volumes and lower volumes in the putamen, nucleus accumbens, thalamus, amygdala, and hippocampus (see Table S9 in the data supplement). Greater thickness in the frontal areas and lower thickness in the temporal areas were associated with higher ADOS scores. Interestingly, higher ADOS scores were additionally associated with increased thickness in cingulate, parietal, and occipital regions, while lower thickness in the insula was associated with higher ADOS scores.
Discussion
We investigated subcortical brain volumes, cortical thickness, and surface area in the largest sample to date of patients with ASD and typically developing healthy control subjects across a wide age range. ASD was found to be associated with significantly smaller volumes for the putamen, pallidum, and nucleus accumbens and larger volumes of the lateral ventricles. The ASD group also showed generally larger intracranial volume, total gray matter, and total cortical thickness, but no differences in surface area. Our analyses indicate a split in the direction of cortical thickness effects between the frontal and temporal cortices, where the ASD group showed increased cortical thickness in the frontal cortex but decreased thickness in the temporal cortex. The effect sizes of these cortical and subcortical group differences ranged from −0.21 to 0.20, indicating small to moderate effects and significant overlap in the distribution of brain morphometry measures between the ASD and control groups, in line with effect sizes reported by the ENIGMA attention deficit hyperactivity disorder (ADHD), schizophrenia, and bipolar disorder working groups (34–36).
Increased cortical thickness in general, as well as increased lateral ventricle volumes, are in line with results found in the meta-analysis by Haar et al. (12), although those authors did not find any alterations in basal ganglia volumes or frontal cortical thickness. Decreased temporal cortical thickness and increased frontal thickness, however, were observed in another recent large-scale meta-analysis on brain morphometry in ASD (37). Our sensitivity analyses, based on the permutation testing used in the Haar et al. (12) study, indicate that the discrepancies with the present results can be attributed mainly to differences in sample size, as we replicate previous results using the permutation method on our larger cohort sample. This comparison further indicates the necessity of large-scale cohort studies and stresses the caution needed when interpreting effects with these limited effect sizes.
The involvement of the pallidum, putamen, and nucleus accumbens indicates an important role for the socio-motivational and cognitive and motor systems of the striatum in the neurobiology of ASD (38). Taken together with the increased cortical thickness in the frontal cortical regions, which are involved mainly in cognitive control, these findings are in line with previous studies relating aberrant frontal-striatal connectivity to the repetitive behavior and executive functioning deficits observed in ASD (6, 14, 17, 39). Nucleus accumbens deficits have additionally been suggested to support the theory of social reward–based differences underlying part of the behavioral phenotype in ASD (15). In contrast to some earlier findings (19), but in line with others (10), we also found a slightly smaller amygdala volume in ASD. Further research is needed to investigate whether changes in nucleus accumbens and amygdala volumes are related to social and reward processes in ASD. Decreased thickness in the temporal cortex in ASD may be further related to both social (40) and language deficits in the disorder (41). Our post hoc analyses on ASD severity further indicate that cortical and subcortical morphometry is related to ASD severity, following the same direction as the group effect, indicating that these alterations indeed serve a functional role in the ASD etiology.
The age distribution in the present sample ranged from 2 years to 64 years, providing an unprecedented cross-sectional view of the development of brain morphometry in ASD and healthy control subjects over the lifespan. Our subcortical results indicate complex—linear and quadratic—age effects in the thalamus, hippocampus, amygdala, nucleus accumbens, and lateral ventricles. The fractional polynomial fits for these volumes indicate that across both diagnostic groups, the subcortical volumes follow a quadratic growth model with a distinct peak around puberty, which is in line with previous analyses of the development of these regions (42). As opposed to some previous studies and reviews (e.g., 26), we found no evidence for age-by-diagnosis interaction effects in any of the investigated subcortical volumes (43). Cortical thickness, on the other hand, showed large-scale effects of both age and age-by-diagnosis interaction. We observed a general decline in cortical thickness over age, in line with previous studies (13, 32). However, as compared with the control subjects, the ASD patients in general showed the strongest group differences during childhood and adolescence, with normalized or even reversed thickness results in adulthood. Interestingly, this was observed both for the increased thickness in the frontal areas and the decreased thickness in the temporal lobe in ASD. These results indicate a complex maturation pattern for the subcortical, frontal, and temporal structures in ASD, peaking around adolescence. Specifically, the balance of frontal, temporal, and striatal maturation may prove a valuable marker for the development of ASD symptoms and treatment response, although further longitudinal studies are needed to verify the predictive validity of these morphometric measures.
Our findings also replicate previously reported main effects of sex (44) and IQ (45) on brain volumes, with generally larger subcortical volumes and increased thickness found in males and in participants with a higher IQ. The mega-analytic approach can statistically correct for differences in sex and IQ between participating sites, removing some of the outcome variance associated with different distributions of these factors between sites. We observed that males have, on average, larger basal ganglia volumes and increased cortical thickness, but the patient groups had a larger proportion of males, indicating that sex could not have confounded the ASD effect. Although we found no evidence of a sex-by-diagnosis interaction, the increased volumes and thickness in both males and females with ASD could be taken as evidence for the “extreme male brain” hypothesis (46). Although our study was not optimally designed to validate this hypothesis, other recent large-scale studies have found considerable evidence for gender effects in brain morphometry in ASD (47). We aim to replicate the analysis by Ecker et al. (47) in a future report on the ENIGMA ASD cohort. Neither medication use nor comorbidity had any large-scale influence on brain morphometry in ASD. Within the ASD sample, overall effects of symptom severity were largely consistent with the direction of the between-group effects. This supports the inference that the observed differences in morphometry are indeed related to the phenotypic expression of ASD in this cohort.
Since individual participant data were available for almost the entire sample, we could compare meta- and mega-analytic approaches in this data set. The main difference between these approaches is that in a meta-analysis, within- and between-group variance is estimated for each separate site, whereas for the mega-analysis, variance is estimated within group. Our results indicate that the meta-analysis is generally less sensitive to group differences, with smaller effect sizes and higher standard errors. The meta-analysis also allowed us to investigate the effects of ASD on brain morphometry per site (as presented in the online data supplement). This analysis indicates significant heterogeneity in the direction and size of the effects between participating sites. The heterogeneity of effect sizes as expressed with the I2 measure was moderate to high for all volume differences found.
This heterogeneity is very important to take into account when interpreting single-sample studies. Even though all the data in the present study were processed using the same analysis and quality-control pipeline and were corrected for effects of age, sex, and IQ identically across sites, we still found significant differences in the estimated main effects between sites. Some of these differences may be due to random sampling differences of the ASD population, in particular within the smaller samples. However, this is less likely to be the case in larger samples employing hundreds of subjects. Alternatively, the within-group heterogeneity may be indicative of different biological mechanisms or subtypes underlying ASD and may therefore be informative for further classification studies. In any case, the existence of this heterogeneity underlines the importance of large-scale studies such as ENIGMA in developing reliable benchmarks for the different major psychiatric disorders. The establishment of these benchmarks allows us to more accurately tackle the heterogeneity due to measurement differences and biological differences in neurodevelopmental studies. Planned multivariate factor analyses and subtyping analyses may additionally provide further insight into different biological mechanisms in ASD.
One of the main goals of the ENIGMA consortium is to unify analytical methods not only across samples, but also across different disorders. The recent report of the ENIGMA ADHD working group (36) is based on the same analysis pipeline and mega-analysis as the present study, using a similarly sized sample of individuals with ADHD and control subjects. The ENIGMA ASD and ADHD results suggest similar decreases in volumes in the putamen, amygdala, and nucleus accumbens, whereas differences are found in pallidum volume in ASD but not ADHD. Additionally, age analyses of subcortical volumes using fractional polynomials suggest different patterns of neural development in ASD and ADHD. Whereas patients with ASD show volume growth curves similar to those of control subjects, patients with ADHD show a significant diagnosis-by-age interaction, with different developmental models most clearly seen in volumes of the nucleus accumbens and putamen for ADHD patients (36). These partly overlapping striatal volume differences offer a fascinating starting point for further investigation of the shared and unique neurobiological underpinnings of ADHD and ASD, as direct comparisons between these two cohorts have not yet been completed at the time of writing.
Some limitations should be considered when interpreting these findings. Our various participating sites used different scanners and acquisition protocols, and although we controlled for the effects of scan site in our models, we cannot fully exclude potential influence from these measurement protocols on the data. We were also unable to obtain longitudinal data for the out samples and therefore could not conduct a within-subject analysis of brain development in ASD. Future large-scale efforts should, in our opinion, be aimed at also standardizing acquisition protocols and long-term follow-ups.
In summary, this study showed the abnormal development of cortical thickness and subcortical volumes in ASD in the largest sample to date, as obtained by the ENIGMA ASD working group. In ASD patients compared with healthy control subjects, we observed smaller volumes for the putamen, amygdala, nucleus accumbens, and pallidum, increased frontal cortical thickness, and decreased temporal cortical thickness. Our age analyses show that subcortical differences in ASD remain relatively stable over the lifespan, while cortical alterations in ASD show a peak in childhood and early adolescence and taper off over adulthood. Future functional activation and resting-state connectivity studies will want to take into account these differences in maturation and focus on unraveling how the balance between frontal, temporal, and subcortical alterations influences the expression of the ASD phenotype across the lifespan. No differences in the development of brain morphometry were observed between males and females with ASD.
1 : Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). Lancet 2006; 368:210–215Crossref, Medline, Google Scholar
2 : Prevalence and characteristics of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 2016; 65:1–23Crossref, Medline, Google Scholar
3 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC, American Psychiatric Association 2013Google Scholar
4 : Neuroanatomy of autism. Trends Neurosci 2008; 31:137–145Crossref, Medline, Google Scholar
5 : Mapping early brain development in autism. Neuron 2007; 56:399–413Crossref, Medline, Google Scholar
6 : The Autism Brain Imaging Data Exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry 2014; 19:659–667Crossref, Medline, Google Scholar
7 : Pallidum and lateral ventricle volume enlargement in autism spectrum disorder. Psychiatry Res 2016; 252:40–45Crossref, Medline, Google Scholar
8 : The autism puzzle: diffuse but not pervasive neuroanatomical abnormalities in children with ASD. Neuroimage Clin 2015; 8:170–179Crossref, Medline, Google Scholar
9 : Amygdala and hippocampus enlargement during adolescence in autism. J Am Acad Child Adolesc Psychiatry 2010; 49:552–560Crossref, Medline, Google Scholar
10 : Brain anatomy of autism spectrum disorders, II: focus on amygdala. Epidemiol Psychiatr Sci 2013; 22:309–312Crossref, Medline, Google Scholar
11 : Microcephaly and macrocephaly in autism. J Autism Dev Disord 1999; 29:113–119Crossref, Medline, Google Scholar
12 : Anatomical abnormalities in autism? Cereb Cortex 2016; 26:1440–1452Crossref, Medline, Google Scholar
13 : Longitudinal changes in cortical thickness in autism and typical development. Brain 2014; 137:1799–1812Crossref, Medline, Google Scholar
14 : Structural gray matter differences during childhood development in autism spectrum disorder: a multimetric approach. Pediatr Neurol 2015; 53:350–359Crossref, Medline, Google Scholar
15 : Social and monetary reward processing in autism spectrum disorders. Mol Autism 2012; 3:7Crossref, Medline, Google Scholar
16 : Aberrant striatal functional connectivity in children with autism. Biol Psychiatry 2011; 69:847–856Crossref, Medline, Google Scholar
17 : Fronto-striatal circuitry and inhibitory control in autism: findings from diffusion tensor imaging tractography. Cortex 2012; 48:183–193Crossref, Medline, Google Scholar
18 : Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders. Arch Gen Psychiatry 2015; 69:53–61Crossref, Google Scholar
19 : The amygdala is enlarged in children but not adolescents with autism, the hippocampus is enlarged at all ages. J Neurosci 2004; 24:6392–6401Crossref, Medline, Google Scholar
20 : The amygdala theory of autism. Neurosci Biobehav Rev 2000; 24:355–364Crossref, Medline, Google Scholar
21 : The social motivation theory of autism. Trends Cogn Sci 2012; 16:231–239Crossref, Medline, Google Scholar
22 : Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur Psychiatry 2008; 23:289–299Crossref, Medline, Google Scholar
23 : Brain structure anomalies in autism spectrum disorder: a meta-analysis of VBM studies using anatomic likelihood estimation. Hum Brain Mapp 2012; 33:1470–1489Crossref, Medline, Google Scholar
24 : Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 2001; 57:245–254Crossref, Medline, Google Scholar
25 : Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res 2011; 1380:138–145Crossref, Medline, Google Scholar
26 : Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. Lancet Neurol 2015; 14:1121–1134Crossref, Medline, Google Scholar
27 : Describing the brain in autism in five dimensions: magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. J Neurosci 2010; 30:10612–10623Crossref, Medline, Google Scholar
28 : Multivariate searchlight classification of structural magnetic resonance imaging in children and adolescents with autism. Biol Psychiatry 2011; 70:833–841Crossref, Medline, Google Scholar
29 : From estimating activation locality to predicting disorder: a review of pattern recognition for neuroimaging-based psychiatric diagnostics. Neurosci Biobehav Rev 2015; 57:328–349Crossref, Medline, Google Scholar
30 : Enhancing studies of the connectome in autism using the Autism Brain Imaging Data Exchange II. Sci Data 2017; 4:170010Crossref, Medline, Google Scholar
31 : Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013; 14:365–376Crossref, Medline, Google Scholar
32 : Cortical thickness abnormalities in autism spectrum disorders through late childhood, adolescence, and adulthood: a large-scale MRI study. Cereb Cortex 2017; 27:1721–1731Crossref, Medline, Google Scholar
33 : The Autism Diagnostic Observation Schedule–Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000; 30:205–223Crossref, Medline, Google Scholar
34 : Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 2015; 4:547–53Google Scholar
35 : Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry 2016; 21:1710–1716Crossref, Medline, Google Scholar
36 : Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry 2017; 4:310–319Crossref, Medline, Google Scholar
37 : Comparative multimodal meta-analysis of structural and functional brain abnormalities in autism spectrum disorder and obsessive-compulsive disorder. Biol Psychiatry 2017; 82:83–102Crossref, Medline, Google Scholar
38 : Neural systems mediating decision-making and response inhibition for social and nonsocial stimuli in autism. Prog Neuropsychopharmacol Biol Psychiatry 2015; 60:112–120Crossref, Medline, Google Scholar
39 : Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain 2002; 125:1594–1606Crossref, Medline, Google Scholar
40 : Social perception in autism spectrum disorders: impaired category selectivity for dynamic but not static images in ventral temporal cortex. Cereb Cortex 2014; 24:37–48Crossref, Medline, Google Scholar
41 : Different functional neural substrates for good and poor language outcome in autism. Neuron 2015; 86:567–577Crossref, Medline, Google Scholar
42 : Typical development of basal ganglia, hippocampus, amygdala, and cerebellum from age 7 to 24. Neuroimage 2014; 96:67–72Crossref, Medline, Google Scholar
43 : Changes in the developmental trajectories of striatum in autism. Biol Psychiatry 2009; 66:327–333Crossref, Medline, Google Scholar
44 : Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol 1996; 366:223–230Crossref, Medline, Google Scholar
45 : Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci 2009; 29:11772–11782Crossref, Medline, Google Scholar
46 : Sex differences in the brain: implications for explaining autism. Science 2005; 310:819–823Crossref, Medline, Google Scholar
47 : Association between the probability of autism spectrum disorder and normative sex-related phenotypic diversity in brain structure. JAMA Psychiatry 2017; 74:329–338Crossref, Medline, Google Scholar



