Effects of Patient Preferences on Outcomes in the Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) Study
Abstract
Objective:
The Predictors of Remission in Depression to Individual and Combined Treatments [PReDICT] study aimed to identify clinical and biological factors predictive of treatment outcomes in major depressive disorder among treatment-naive adults. The authors evaluated the efficacy of cognitive-behavioral therapy (CBT) and two antidepressant medications (escitalopram and duloxetine) in patients with major depression and examined the moderating effect of patients’ treatment preferences on outcomes.
Method:
Adults aged 18–65 with treatment-naive major depression were randomly assigned with equal likelihood to 12 weeks of treatment with escitalopram (10–20 mg/day), duloxetine (30–60 mg/day), or CBT (16 50-minute sessions). Prior to randomization, patients indicated whether they preferred medication or CBT or had no preference. The primary outcome was change in the 17-item Hamilton Depression Rating Scale (HAM-D), administered by raters blinded to treatment.
Results:
A total of 344 patients were randomly assigned, with a mean baseline HAM-D score of 19.8 (SD=3.8). The mean estimated overall decreases in HAM-D score did not significantly differ between treatments (CBT: 10.2, escitalopram: 11.1, duloxetine: 11.2). Last observation carried forward remission rates did not significantly differ between treatments (CBT: 41.9%, escitalopram: 46.7%, duloxetine: 54.7%). Patients matched to their preferred treatment were more likely to complete the trial but not more likely to achieve remission.
Conclusions:
Treatment guidelines that recommend either an evidence-based psychotherapy or antidepressant medication for nonpsychotic major depression can be extended to treatment-naive patients. Treatment preferences among patients without prior treatment exposure do not significantly moderate symptomatic outcomes.
Choosing the initial form of treatment is the most fundamental decision clinicians face in caring for patients with major depressive disorder. Current guidelines recommend that most patients should initially be treated with either an antidepressant medication or an evidence-based psychotherapy (1), based on the roughly equivalent efficacy, on average, of psychotherapy and medication treatments for major depression (2). This average equivalency, however, masks the substantial variability in outcomes that exists across patients. There is great need to develop methods for identifying the best treatment for a particular individual depressed patient. Variously referred to as personalized medicine, precision medicine, or personalized intervention, this approach aims to match each individual patient to a treatment most likely to prove beneficial through identifying moderators of likely outcomes to specific treatment options (3, 4).
Few clinical or socio-demographic variables have emerged as prescriptive factors to allow specific differential recommendations of psychotherapy versus pharmacotherapy, and the identified factors are not consistent across studies. Previously identified moderators from large trials include lower cognitive dysfunction and greater work dysfunction predicting better outcomes with cognitive therapy and imipramine, respectively (5); a history of early childhood trauma and better response to a cognitive-behavioral analysis system of psychotherapy compared with nefazodone (6); being married, being unemployed, experiencing a greater number of recent life events, or prior treatment with antidepressants associated with superior improvement with cognitive therapy compared with pharmacotherapy (7, 8); and psychomotor activation predicting better overall outcome with a selective serotonin reuptake inhibitor (SSRI) compared with interpersonal therapy (9).
Studies of biological measures predicting outcomes in depression have primarily been conducted in studies of a single treatment modality (10). Though valuable, these studies do not inform clinicians about which treatment is likely to be better or worse for a given patient, necessary information for clinical decision making. An exception to this uncertainty was a recent positron emission tomography (PET) study, which found that metabolic activity in the right anterior insula differentially predicted treatment outcomes in patients with major depression treated with the SSRI escitalopram or cognitive-behavioral therapy (CBT) (11, 12). However, this finding awaits replication and formal experimental testing.
In the absence of useful treatment-prediction moderators for major depression, treatment guidelines recommend that patient preferences be considered for making treatment recommendations (1). Surveys of treatment- and non-treatment-seeking depressed patients have found that roughly 70% express a preference for psychotherapy over medication (13). The relative preference for psychotherapy may be even greater among ethnic minority groups, including Latinos (14) and African Americans (15), although these conclusions remain uncertain. In contrast, preference for medication has been associated with greater severity of illness (16).
Most randomized trials evaluating the effects of patient preference for medication or psychotherapy treatment have found no effect of preference on treatment efficacy (17), although two meta-analyses that examined the effects of preference across all studies (not limited to randomized trials) found a small effect of better outcomes among patients receiving their preferred treatment (18, 19). Treatment dropout or attrition is another important outcome related to preferences, with meta-analyses demonstrating that patients who do not receive their preferred treatment are more likely to terminate care prematurely (19).
An important potential confound for prediction medicine and the impact of preference on outcomes is patients’ prior lifetime treatment exposures (20). Prior treatment may introduce a selection bias in that patients’ willingness to participate in a randomized trial comparing active treatments might be affected by their prior experience with a particular treatment. A second, and highly salient, concern for personalized interventions is the possibility of persisting psychological (21, 22) or biological (23, 24) effects from previous treatments that affect response to treatments in a randomized trial. These considerations emphasize the value of treatment-naive patient samples for examining the effects of patient preferences and biological variables in predicting treatment outcomes.
To address the need for personalized medicine approaches for major depression, we conducted the Emory Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) study that aimed to identify patient-level biological and psychological factors that predict short- and long-term outcomes to three differing treatments: CBT, escitalopram, and duloxetine, a serotonin norepinephrine reuptake inhibitor (SNRI). To minimize confounding of associations between treatments and outcomes that could arise from patients’ prior treatment exposures, participation in this trial was limited to treatment-naive patients with major depressive disorder. Herein, we report the primary clinical results from the initial 12-week randomized monotherapy treatment phase, along with the effects of treatment preferences on outcomes. Based on our prior work (25), we hypothesized that there would be no effect of matching to preference on depression symptom outcomes but that mismatched patients would be more likely to terminate the trial prematurely.
Method
Study Overview
We previously published a detailed description of the PReDICT study rationale, methods, and design (26). The study was conducted through the Emory University Mood and Anxiety Disorders Program and involved two clinics: 1) the primary Mood and Anxiety Disorders Program Clinic at Emory University, including a satellite location in Stockbridge, Georgia, and 2) a purely Spanish-speaking clinic at Grady Hospital in Atlanta (27). The Emory Institutional Review Board and the Grady Hospital Research Oversight Committee granted study approval.
Patients
Patients were recruited primarily through advertising, although a minority of patients were referred from primary care clinics. All patients provided written, informed consent prior to beginning study procedures. The study enrolled men and women, aged 18–65 years, who met DSM-IV criteria for current major depressive disorder and who had never previously received treatment for a mood disorder. We defined “previous treatment” as patient-reported treatment for major depression, dysthymia, or depressive disorder not otherwise specified with either 1) a marketed antidepressant at a minimum effective dose for 4 or more consecutive weeks or 2) 4 or more sessions of an evidence-based and structured psychotherapy for depression (i.e., CBT, behavior therapy, interpersonal therapy, or behavioral marital therapy). Patients who had received prior supportive therapy were eligible, but they were not permitted to participate in such psychotherapy during the study.
Eligible patients had a primary psychiatric diagnosis of nonpsychotic major depressive disorder and a 17-item Hamilton Depression Rating Scale (HAM-D) (28) total score ≥18 at screening and ≥15 at the baseline visit. Exclusion criteria included a lifetime history of bipolar disorder, primary psychotic disorder, or dementia, or a current (past 12 months) diagnosis of obsessive-compulsive disorder, eating disorder, or dissociative disorder. Additionally, patients were excluded if they met DSM-IV criteria for substance abuse within 3 months, substance dependence within 12 months of the randomization visit, or if their urine tested positive for drugs of abuse. Additional exclusionary criteria included any lifetime treatment with citalopram, escitalopram, or duloxetine. Women who were, or planned on becoming, pregnant or breast-feeding during the trial were excluded, as were any patients with significant uncontrolled medical conditions, or any condition that could interfere with the study or the interpretation of the study results.
Diagnostic and Outcome Measures and Procedures
The diagnosis of major depressive disorder was made using the Structured Clinical Interview for DSM-IV Axis I Disorders (29) and confirmed through an evaluation by a study psychiatrist. In addition to the HAM-D, depressive symptoms were evaluated using the Montgomery-Åsberg Depression Rating Scale (MADRS) (30), and two patient self-report measures, the Beck Depression Inventory-I (BDI) (31) and the Quick Inventory of Depressive Symptomatology, Self-Report (32). Anxiety was assessed with the Hamilton Anxiety Rating Scale (HAM-A) (33). Demographic variables and family history of psychiatric illness were collected via self-report.
Assessment of Preferences
As part of the informed consent process, patients read paragraphs describing the medication and CBT treatments used in the study. The study physician also explained that, on average, people with major depression are equally likely to benefit from CBT or medication, and that the study’s goal was to identify characteristics of individuals that could be used to choose specific treatments for patients in the future. Patients were informed that their preference would not influence the treatment they received because treatment was assigned randomly and that in order to take part in the study they needed to be willing to start the type of treatment assigned to them. Preference was assessed by a single question asking patients if their preferred treatment was: “no preference,” “cognitive behavior therapy,” or “medication.” Those who expressed a preference then indicated whether the strength of their preference was “mild,” “moderate,” or “very strong” (25).
Randomization
Patients were randomly assigned 1:1:1 to one of three possible treatments: 1) escitalopram 10–20 mg/day, 2) duloxetine 30–60 mg/day, or 3) CBT, 16 individual sessions. In order to ensure equal allocation across treatment groups, prior to opening the study for enrollment, the treatment assignment was generated using randomized permuted blocks. Separate randomization blocks were stratified for the English- and Spanish-language clinics. The treatment assignments were placed in sealed opaque envelopes by Emory employees uninvolved in the study. At a patient’s baseline visit, after the study psychiatrist confirmed that all eligibility criteria for randomization had been met, within the patient’s clinic the study coordinator opened the next-in-sequence envelope to determine the treatment assignment.
Study Visits and Treatments
After randomization, patients returned at weeks 1–6, 8, 10, and 12 to complete the symptom rating scales conducted by trained raters blind to treatment assignment, self-reports, and a brief assessment by a study physician. Spanish-speaking raters and physicians also performed assessments at the English-speaking site in order to enhance treatment and assessment consistency. Patients received gift cards worth approximately $5.00 per visit to offset travel-related costs. Patients who did not achieve remission (defined below) at the end of week 12 were eligible to enter a 12-week combined treatment phase, in which combined psychotherapy and medication was provided; results of this phase of the study will be reported in a subsequent article.
Pharmacotherapy.
Study medications were compounded in the Emory Investigational Drug Service pharmacy and contained the equivalent of either 10 mg of escitalopram or 30 mg of duloxetine. Patients randomly assigned to medication were started on one capsule per day, and if the patient had not meaningfully improved by week 4, the dose was increased to two capsules once daily, though the treating psychiatrist could raise the dose at week 3 if deemed clinically necessary. If the response to treatment plateaued during the trial, or if remission was not achieved by week 6, an increase to two capsules per day was required. If adverse events were sufficiently distressing to the patient, the dose was reverted to one capsule per day. To assess adherence, serum concentrations of escitalopram and duloxetine were measured at week 12. The week-12 sample was also tested for the presence of 10 other antidepressants to ensure that patients were not surreptitiously using other antidepressants.
CBT.
Doctoral-level and experienced master's-level providers trained in the specific CBT protocol for the study provided the therapy utilizing a standardized treatment protocol (34). To complete the 16 visits during the 12 weeks of intervention, patients randomly assigned to CBT met with their therapist twice per week for the first 4 weeks and then weekly for the remaining 8 weeks, though some flexibility in the timing of visits was permitted when necessary. Therapist supervision occurred weekly, sessions were videotaped, and competency was rated, using the Cognitive Therapy Scale (35), by the Beck Institute for Cognitive Therapy and the Academy of Cognitive Therapy. Anytime a session score dropped below 40, the session was reviewed in weekly supervision with the therapist and additional training was undertaken to assure improvement and continued scores above 40.
Concomitant Medications
Medications used to manage acute chronic medical conditions were permitted. Patients were not permitted to use other psychoactive medications, with the exception of hypnotics, which were permitted up to three times per week when requested by patients.
Statistical Analysis
Baseline characteristics were compared across treatment groups using one-way analyses of variance for continuous variables and chi-square for categorical variables. For treatment outcomes, three analysis sets were defined. The primary analysis for this article was the change in HAM-D score, performed on the intent-to-treat population, defined as all randomly assigned patients. Change in HAM-D score over the course of treatment was modeled using a linear-mixed model, with time as a continuous predictor (growth curve model) and individual-level random effects for both intercept (initial depression severity) and slope (change in depression severity over time). The effects of interest were the treatment group-by-time interactions. Main and interaction effects of clinic (Spanish versus English language) were tested, as well as time, treatment group, and time-by-treatment group interactions. Similar models were developed to assess change in scores on the MADRS, BDI, Quick Inventory of Depressive Symptomatology, Self-Report, and HAM-A.
Secondarily, for the purpose of assessing categorical outcomes, we used a last observation carried forward data set, defined as all randomly assigned patients who initiated treatment and had at least one follow-up rating assessment. Four mutually exclusive categorical outcomes were defined based on the last valid HAM-D rating: 1) nonresponse: <30% reduction from baseline; 2) partial response: 30%−49% reduction from baseline; 3) response without remission: ≥50% reduction from baseline, but HAM-D score >7; and 4) remission: HAM-D score ≤7. For these outcomes, we tested for clinic-by-treatment group interactions.
Because the PReDICT study was designed to identify biomarkers predictive of treatment outcome, we also analyzed a per-protocol completer data set. This sample comprised randomly assigned patients who met all inclusion/exclusion criteria, had no major protocol violations, completed the 12 weeks of treatment, and whose week-12 pharmacokinetic measures did not contradict the randomized treatment assignment (i.e., patients assigned to medication who had no detectable drug, or those assigned to CBT who had a detectable antidepressant at week 12). Categorical outcomes in the per-protocol sample employed the same definitions as the last observation carried forward sample, with the exception that remission was defined as a HAM-D score ≤7 at both weeks 10 and 12 (the a priori definition specified in the protocol).
For testing predictors of remission and completion, we performed two sets of analyses for each outcome: a set with each measure as a predictor of remission (main effect) and another set testing each measure as a possible moderator of the remission effect (simultaneous main and interaction effects). All analyses were performed using logistic regression with a similar structure. However, the effects of preference and preference matching on remission were assessed using logistic regression with targeted coding of the baseline groups, reflecting the fact that both preference and preference matching were also treatment specific. To assess odds of remission in various preference groups, appropriate frequency weights were used to account for the fact that the probability of a match was not the same in the medication and CBT groups.
Results
The disposition of patients through the study is presented in Figure S1 in the data supplement accompanying the online version of this article. Five hundred fifteen individuals consented to participate in the trial, and 344 were randomly assigned: 114 to escitalopram, and 115 each to the CBT and duloxetine arms. Twenty-eight patients did not return for a postrandomization assessment, and thus the last observation carried forward data set comprised 316 patients; 234 met all criteria for inclusion in the per-protocol completer sample.
Demographic and Clinical Characteristics
Baseline clinical and demographic characteristics of the randomized sample are summarized in Table 1. Demographic characteristics did not differ between the treatment groups. The only clinical characteristic that significantly differed between the groups was age at onset, which was slightly younger in the duloxetine group. The mean HAM-D score indicated moderate severity (mean=19.8 [SD=3.8]), and more than half (52%) of the participants were in their first major depressive episode. Hispanic participants were more socio-economically disadvantaged than black or white participants, with lower levels of full-time employment and education, and were less likely to have private insurance.
| Characteristic | All Patients (N=344) | Cognitive-Behavioral Therapy Group (N=115) | Escitalopram Group (N=114) | Duloxetine Group (N=115) | Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | df | p | |
| Age (years) | 40.0 | 11.7 | 40.0 | 11.3 | 41.6 | 12.1 | 38.3 | 11.4 | 2.27 | 2, 341 | 0.105 |
| Age at first episode (years) | 30.7 | 14.2 | 31.8 | 13.5 | 32.8 | 15.2 | 27.5 | 13.4 | 4.59 | 2, 336 | 0.011 |
| Current episode duration (weeks) | 119.0 | 232.7 | 117.7 | 232.3 | 101.2 | 153.9 | 137.6 | 290.3 | a | 0.601 | |
| Measure | |||||||||||
| Hamilton Depression Rating Scale (17-item) | 19.8 | 3.8 | 19.7 | 3.7 | 20.1 | 3.7 | 19.6 | 3.9 | 0.560 | 2, 341 | 0.572 |
| Quick Inventory of Depressive Symptomatology, Self-Report | 14.0 | 4.0 | 13.9 | 4.0 | 14.1 | 3.9 | 14.0 | 4.2 | 0.117 | 2, 338 | 0.890 |
| Beck Depression Inventory | 22.9 | 7.3 | 22.2 | 7.5 | 23.1 | 7.2 | 23.4 | 7.3 | 0.895 | 2, 338 | 0.410 |
| Hamilton Anxiety Rating Scale | 16.1 | 5.2 | 16.2 | 4.9 | 16.7 | 5.4 | 15.4 | 5.2 | 1.855 | 2, 341 | 0.158 |
| N | % | N | % | N | % | N | % | χ2 | df | p | |
| Sex | 0.332 | 2 | 0.847 | ||||||||
| Male | 148 | 43.0 | 51 | 44.3 | 50 | 43.9 | 47 | 40.9 | |||
| Female | 196 | 57.0 | 64 | 55.7 | 64 | 56.1 | 68 | 59.1 | |||
| Race | 7.64 | 4 | 0.106 | ||||||||
| White | 164 | 47.7 | 61 | 53.0 | 47 | 41.2 | 56 | 48.7 | |||
| Black | 64 | 18.6 | 13 | 11.3 | 28 | 24.6 | 23 | 20.0 | |||
| Other | 116 | 33.7 | 41 | 35.7 | 39 | 34.2 | 36 | 31.3 | |||
| Ethnicity | 0.387 | 2 | 0.824 | ||||||||
| Hispanic | 102 | 29.7 | 32 | 27.8 | 36 | 31.6 | 34 | 29.6 | |||
| Non-Hispanic | 242 | 70.3 | 83 | 72.2 | 78 | 68.4 | 81 | 70.4 | |||
| Married/cohabitating | 0.121 | 2 | 0.941 | ||||||||
| Yes | 169 | 49.1 | 57 | 49.6 | 57 | 50.0 | 55 | 47.8 | |||
| No | 175 | 50.9 | 58 | 50.4 | 57 | 50.0 | 60 | 52.2 | |||
| Education level | 2.096 | 4 | 0.718 | ||||||||
| ≤12 years or trade school | 105 | 30.5 | 37 | 32.2 | 32 | 28.1 | 36 | 31.3 | |||
| Some college | 96 | 27.9 | 27 | 23.5 | 34 | 29.8 | 35 | 30.4 | |||
| ≥4-year college degree | 143 | 41.6 | 51 | 44.3 | 48 | 42.1 | 44 | 38.3 | |||
| Employed full-time | 0.659 | 2 | 0.719 | ||||||||
| Yes | 185 | 54.1 | 61 | 53.5 | 59 | 51.8 | 65 | 57.0 | |||
| No | 157 | 45.9 | 53 | 46.5 | 55 | 48.2 | 49 | 43.0 | |||
| Current anxiety disorder | 0.337 | 2 | 0.845 | ||||||||
| Yes | 205 | 59.6 | 71 | 61.7 | 67 | 58.8 | 67 | 58.3 | |||
| No | 139 | 40.4 | 44 | 38.3 | 47 | 41.2 | 48 | 41.7 | |||
| Previous episodes | 5.603 | 4 | 0.231 | ||||||||
| 1 | 177 | 52.2 | 67 | 59.8 | 59 | 52.7 | 51 | 44.3 | |||
| 2 | 63 | 18.6 | 17 | 15.2 | 20 | 17.9 | 26 | 22.6 | |||
| ≥3 | 99 | 29.2 | 28 | 25.0 | 33 | 29.5 | 38 | 33.0 | |||
| Chronic episode (≥2 years) | 106 | 31.5 | 38 | 33.9 | 31 | 27.9 | 37 | 32.5 | 1.01 | 2 | 0.603 |
| History of suicide attempt | 25 | 7.4 | 8 | 7.0 | 4 | 3.6 | 13 | 11.4 | 5.12 | 2 | 0.077 |
| Insurance status | 3.12 | 4 | 0.538 | ||||||||
| Private | 137 | 41.6 | 42 | 38.2 | 50 | 45.5 | 45 | 41.3 | |||
| Public | 3 | 0.9 | 2 | 1.8 | 1 | 0.9 | 0 | 0 | |||
| None | 189 | 57.4 | 66 | 60.0 | 59 | 53.6 | 64 | 58.7 | |||
TABLE 1. Clinical and Demographic Characteristics at Baseline
Of the 344 randomly assigned patients, 21 (6.1%) had previously received an antidepressant medication of inadequate dose or duration to meet our definition of previous treatment. Seventy-eight (22.3%) reported receiving a non-evidence-based psychotherapeutic intervention or <4 sessions of an evidence-based psychotherapy for depression. Among patients having had prior psychotherapy, 43.8% (32/73) remitted compared with 48.9% (119/243) of those who had not (χ2=0.593, df=1, p=0.441). Remission rates also did not significantly differ between patients with (5/20, 25%) and without (146/296, 49.3%) prior antidepressant exposure (χ2=3.52, df=1, p=0.061). Similarly, treatment completion was unrelated to prior psychotherapy (p=0.98) or antidepressant exposure (p=0.107).
Treatment Features
The mean number of CBT sessions attended was 11.4 (SD=4.7) for the intent-to-treat sample and 14.3 (SD=1.4) for the completer sample. Sessions 2, 8, and 12 were rated for slightly over 35% of the CBT patients. The average Cognitive Therapy Scale scores were above 40 for all therapists except two whose average was just slightly below 40. Of the nine scheduled pharmacotherapy visits for medication-treated patients, the mean number of visits attended was 8.2 (SD=2.0) for the medication-treated intent-to-treat sample and 8.9 (SD=0.4) for the completer sample. The mean escitalopram dose at endpoint was 16.2 mg/day (SD=5.1), and 16.9 mg/day (SD=4.5) at week 12 for completers. The mean duloxetine dose at endpoint was 48.0 mg/day (SD=15.0), and 49.6 mg/day (SD=14.1) for completers. Among the drug-treated completers, 80% receiving escitalopram were titrated to 20 mg/day, and 71% of duloxetine-treated patients were titrated to 60 mg/day at some point during the 12 weeks. The mean serum drug concentrations at endpoint were 30.9 ng/ml (SD=19.7) for escitalopram and 44.6 ng/ml (SD=59.1) for duloxetine. Sedative medications were used by 22.4% of the CBT and duloxetine groups and by 25.4% of the escitalopram group; these counts did not significantly differ (χ2=0.48, p=0.79).
Per-Protocol Completion Rates
Patients randomly assigned to CBT were less likely to be per-protocol completers than patients in either of the medication groups (CBT: 60.0%; escitalopram: 75.4%; duloxetine: 68.7%, χ2=6.31, df=2, p=0.043). Per-protocol completers, compared with noncompleters, had lower depression severity (HAM-D: mean=19.2 [SD=3.5] compared with mean=21.1 [SD=4.0]) and lower anxiety (HAM-A: mean=15.4 [SD=4.7] compared with mean=17.4 [SD=5.9]) at baseline.
Outcomes
There were no significant clinic-by-treatment group interactions on HAM-D, and thus clinic effects were removed from the presented models. The mean estimated overall improvement on the primary outcome of the HAM-D was 10.9 points for the intent-to-treat sample, which did not differ significantly across the three groups (CBT: 10.2; escitalopram: 11.1; duloxetine: 11.2; F=0.53, df=2, 257, p=0.589). Among completers, the mean reduction in HAM-D score was 10.5, which also did not significantly differ between the CBT (mean=9.8), escitalopram (mean=11.1), and duloxetine (mean=10.7) groups (F=0.88, df=2, 231, p=0.415). The raw data means over time for the three treatment arms are shown in Figure 1; there was no significant time-by-treatment interaction, indicating the change over time did not differ across treatments. The rates of remission and response by treatment group are summarized in Table 2 and Table 3. Among the last observation carried forward sample, 47.8% remitted and an additional 12.3% achieved response without remission. Among completers, 46.6% remitted and another 21.4% achieved response without remission. Remission rates did not significantly differ between treatment arms in either the last observation carried forward (CBT: 41.9%; escitalopram: 46.7%; duloxetine: 54.7%; χ2=3.55, df=2, p=0.170) or per-protocol (CBT: 43.5%; escitalopram: 44.2%; duloxetine: 51.9%; χ2=1.36, df=2, p=0.507) samples.
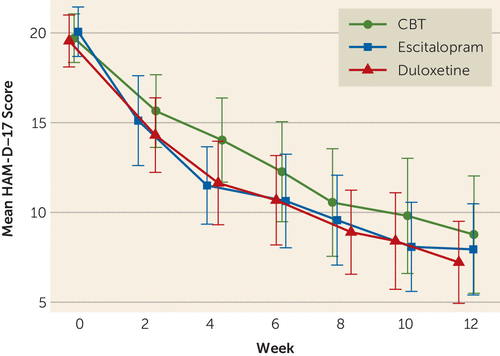
FIGURE 1. Modeled Change in the Mean Hamilton Depression Rating Scale (HAM-D) Score by Weeka
a CBT=cognitive-behavioral therapy; Error bars represent 95% confidence intervals.
| Cognitive-Behavioral Therapy (N=105) | Escitalopram (N=105) | Duloxetine (N=106) | Total Sample (N=316) | |||||
|---|---|---|---|---|---|---|---|---|
| Outcome | N | % | N | % | N | % | N | % |
| Non-responsea | 32 | 30.5 | 26 | 24.8 | 21 | 19.8 | 79 | 25.0 |
| Partial responseb | 19 | 18.1 | 12 | 11.4 | 16 | 15.1 | 47 | 14.9 |
| Response without remissionc | 10 | 9.5 | 18 | 17.1 | 11 | 10.4 | 39 | 12.3 |
| Remissiond | 44 | 41.9 | 49 | 46.7 | 58 | 54.7 | 151 | 47.8 |
TABLE 2. Response and Remission Rates in the Intent-to-Treat Last Observation Carried Forward Sample
| Cognitive-Behavioral Therapy (N=69) | Escitalopram (N=86) | Duloxetine (N=79) | All Completers (N=234) | |||||
|---|---|---|---|---|---|---|---|---|
| Outcome | N | % | N | % | N | % | N | % |
| Non-responsea | 15 | 21.7 | 15 | 17.4 | 10 | 12.7 | 40 | 17.2 |
| Partial responseb | 13 | 18.8 | 10 | 11.6 | 12 | 15.2 | 35 | 15.0 |
| Response without remissionc | 11 | 15.9 | 23 | 26.7 | 16 | 20.3 | 50 | 21.4 |
| Remissiond | 30 | 43.5 | 38 | 44.2 | 41 | 51.9 | 109 | 46.6 |
TABLE 3. Response and Remission Rates in the Per-Protocol Completer Sample
The modeled outcomes for each of the four depression scales and for the HAM-A are presented in Table S1 in the online data supplement. The primary result of no significant difference between the treatment groups in mean change on the HAM-D was replicated for the MADRS, Quick Inventory of Depressive Symptomatology, Self-Report, and BDI. Mean change on the HAM-A also did not significantly differ between the treatment arms.
Effect of Preferences on Outcomes
Preference data were obtained for 341 (99.1%) of the patients, of whom 225 (66%) expressed a treatment preference (CBT: N=121 [35.5%]; medication: N=104 [30.5%]). The 28 patients who did not return after randomization did not significantly differ from the rest of the sample in their expressed treatment preferences and were excluded from the outcome analyses.
Treatment preferences did not differ between patients with and without private insurance (Pearson χ2=0.254, p=0.88), but white non-Hispanic, black non-Hispanic, and Hispanic patients did significantly differ in their treatment preferences. In contrast to white non-Hispanic patients, who showed roughly equal distribution across the no-, CBT-, and medication-preference options, nearly one-half (47.5%) of black non-Hispanic patients preferred CBT, and few (19.8%) Hispanics preferred medication (Figure 2). The preference differences across the groups was statistically significant (χ2=17.16, df=4, p=0.002). The numbers of patients matched versus mismatched within each level of preference strength were as follows: mild: N=26 compared with N=24; moderate: N=54 compared with N=53; very strong: N=26 compared with N=40, respectively. Strength of preference did not differ between those preferring CBT compared with those preferring medication (Z=−1.74, p=0.08).
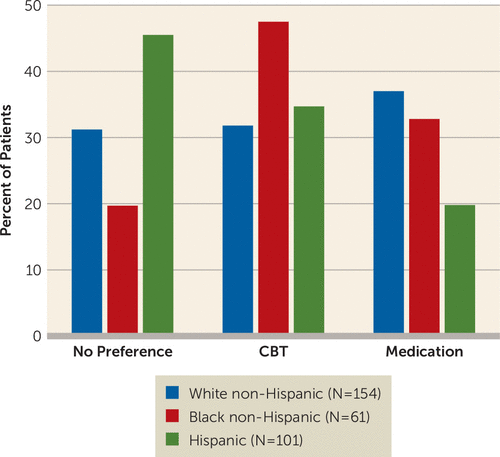
FIGURE 2. Treatment Preferences by Race and Ethnicitya
a CBT=cognitive-behavioral therapy.
Preferred treatment type did not significantly predict completion across the medication-, CBT-, and no-preference groups (completion rates: 77.9%, 71.9%, and 69.8%, respectively; χ2=1.93, df=2, p=0.38). However, among those expressing a preference, the completion rate was significantly higher among those matched compared with those mismatched to their preferred treatment (82.2% compared with 67.8%, respectively, χ2=6.19, df=1, p=0.013). This differential completion rate among matched and mismatched patients was driven by those with moderate or very strong preferences (mild: χ2=0.42, p=0.517; moderate: χ2=4.24, p=0.039; very strong: χ2=3.13, p=0.077) (Figure 3). There was also a main effect of baseline HAM-D severity on completion rates (greater severity among those less likely to complete: p=0.016), but this effect did not significantly differ between those matched compared with those mismatched to their preferred treatment (interaction effect: p=0.154).
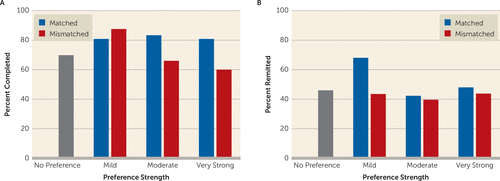
FIGURE 3. Completion and Remission Rates by Preference Strength Among Patients Matched or Mismatched to Their Preferred Treatmenta
a Completion rates are based on all randomly assigned patients; remission rates are based on the last observation carried forward sample. A) Completion rates were significantly lower overall among mismatched compared with matched patients. B) Matched and mismatched patients did not significantly differ in overall remission rates (see the article text). No preference: N=88; mild preference: N=50; moderate preference: N=107; very strong preference: N=66.
Of the 225 patients expressing a preference, 107 (47.6%) were matched to their preferred treatment, and 118 (52.4%) were mismatched. Last observation carried forward outcomes were available for 207 of the 225 patients who expressed a preference. Having any preference was associated with a non-significantly lower likelihood of remission on the HAM-D (odds ratio=0.74, p=0.31), which was similar among those preferring CBT (odds ratio=0.72) or medication (odds ratio=0.76) (Table 4). Being randomly assigned to the preferred treatment did not significantly increase the overall remission rate (odds ratio=1.42, p=0.31) or the remission rate specific to CBT or medication (Table 4 [also see Table S2 in the online data supplement]). Strength of preference also did not significantly affect remission rates (mild: 56.3%; moderate: 41%; very strong: 45.6%, χ2=3.04, df=2, p=0.22). As shown in Figure 3, remission rates among matched and mismatched patients did not significantly differ by strength of preference (mild: χ2=2.93, p=0.09; moderate: χ2=0.077, p=0.78; very strong: χ2=0.102, p=0.24).
| Treatment Preference (N=313)a | Preference Matching (N=207)b | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preference for Cognitive-Behavioral Therapy (N=112) | Preference for Medication (N=95) | Any Preference (N=207) | Matched to Cognitive-Behavioral Therapy (N=38) | Matched to Medication (N=65) | Matched to Preference (N=103) | |||||||||||||
| Outcome/Measure | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p |
| Hamilton Depression Rating Scale (17-item) | 0.72 | 0.36–1.42 | 0.34 | 0.76 | 0.40–1.46 | 0.42 | 0.74 | 0.42–1.32 | 0.31 | 1.24 | 0.55–2.77 | 0.60 | 1.68 | 0.72–3.90 | 0.23 | 1.42 | 0.72–2.80 | 0.31 |
| Beck Depression Inventory | 0.92 | 0.46–1.86 | 0.82 | 1.03 | 0.53–2.02 | 0.93 | 0.98 | 0.54–1.77 | 0.94 | 1.51 | 0.66–3.46 | 0.33 | 1.91 | 0.78–4.67 | 0.16 | 1.68 | 0.84–3.36 | 0.14 |
TABLE 4. Last Observation Carried Forward Remission Odds Ratios by Treatment Preference and Matching to Preference
Other Clinical Predictors of Outcomes
The logistic regression analyses of other clinical and sociodemographic variables found that baseline depression severity (HAM-D), baseline anxiety (HAM-A), and presence of a current anxiety disorder all predicted lower likelihood of remission in both the completer and last observation carried forward samples, but age, gender, race, chronicity, employment, marital status, and chronicity of episode did not (see Tables S3 and S4 in the online data supplement). Completion rates were also predicted by baseline depression and anxiety severity, with the additional finding that minority race/ethnicity patients were significantly less likely to complete the study than white patients (African American: 65.6%; Hispanic: 66.7%; Caucasian: 82.1%) (see Table S5 in the online data supplement). None of these analyses identified a statistically significant moderator of remission or completion between the three treatment groups.
Discussion
This study represents the largest randomized clinical trial, to our knowledge, conducted at a single institution in patients with major depressive disorder. Furthermore, none of the patients had ever previously received a minimally adequate course of an evidence-based treatment. Among the 344 randomly assigned patients, the average benefit from 12 weeks of treatment with CBT, escitalopram, or duloxetine did not meaningfully differ in terms of mean change in symptom severity or proportion of patients achieving remission. These results are consistent with other studies of depression that have reported roughly equivalent mean outcomes with evidence-based psychotherapy and medication treatments (36–40).
We found that patients matched to their preferred treatment did not achieve remission significantly more frequently than mismatched patients. This absence of difference in remission occurred despite a significantly higher rate of treatment completion among the matched patients. These results replicate a similarly designed randomized trial that compared escitalopram and CBT among previously treated patients (22). The results of these two trials, considered along with similar results from other large trials (16, 37, 38, 41–44), suggest that overall remission rates are roughly equivalent regardless of preference matching, even if dropout is greater among mismatched patients. However, retention itself is an important outcome of relevance, particularly for patients with chronic forms of depression. We found a clinically meaningful number needed to treat for increasing completion (6.94 for matched compared with mismatched patients). Thus, until better predictors of remission probability are identified (as reported in our companion study to the present article [see reference 45]), there may be value in using patient preferences to select initial treatments, particularly for those with moderate or strong preferences.
Consistent with other reports, we found that Hispanic and black non-Hispanic patients had treatment preferences that differed from white non-Hispanic patients (14, 15). Specifically, black patients were significantly more likely to prefer psychotherapy, and few Hispanic patients preferred medication.
The impact of matching to preference on the outcome of attrition is less clear. Consistent with prior work (44), we found that patients were more likely to complete the trial if they were matched to their treatment preference, though others have not found this effect (42, 46–48). Previous findings (25, 49) that preference for medication treatment (regardless of treatment received) predicted attrition were not replicated in the current analysis. The inconsistent results across studies may derive from differences in socioeconomic status or prior treatment experiences of the samples enrolled.
Other investigators have reported that preferences may affect indirect measures of outcome, such as treatment engagement (50), adherence (43), and working alliance. Of these, only the association between preference and strength of therapeutic alliance has been replicated across studies (44, 50, 51). These results suggest that patients who receive their preferred treatment may invest more fully in their treatment, which makes the finding that preference does not affect improvement all the more striking. This discrepancy implies that biological or psychosocial factors are stronger determinants of treatment efficacy than patient preference.
Strengths of the study include the large number of randomly assigned patients within a single institution, thereby minimizing confounding arising from potentially differing applications of treatments across study sites (52). The generalizability to the broader population of major depression patients would appear to be strong, given the diverse racial and ethnic composition of our sample. Because the PReDICT study enrolled treatment-naive patients, preferences were not influenced by prior treatment experiences, though this may limit generalization to patients with prior treatment experiences. The CBT treatment was delivered with high fidelity; however, dissemination may be hampered by the limited availability and quality of systematic CBT in community settings (53). The completion rate in the CBT arm (60%) was on the lower end of the range of psychotherapy arm completion rates from other large medication versus psychotherapy trials (55%−87%) (36–40). The completion rate across the two medication arms was 72%, similar to the rates observed in other trials (56%−89%) (36–40).
A potential limitation is that the maximum dose of duloxetine used was 60 mg/day; higher doses are often used in clinical practice, which suggests that the full efficacy of duloxetine may not have been detected. Another limitation, which is unavoidable in psychotherapy versus medication trials, is that patients were not blind to their treatment assignment and that CBT patients had substantially greater contact with the treatment providers. The majority of the randomly assigned patients were recruited through advertising, which may limit generalizability to spontaneously treatment-seeking samples. Other limitations to generalizability include the exclusion of patients with mild depressive symptoms or concomitant substance use disorders and those with very strong treatment preferences who were unwilling to be randomly assigned. Because many patients expressed no treatment preference, our analyses of the impact of preference on outcomes were underpowered, and larger samples might have detected statistically significant effects. Without a “patient’s choice” treatment arm, we could not address whether receiving one’s preferred treatment by choice, rather than by chance via randomization, would have led to improved outcomes. Assessing patients prior to randomization about their ambivalence toward continuing in treatment, particularly if they experienced side effects or minimal early improvement, may have informed the treatment discontinuation analyses (54).
There are two important implications of our results that can inform treatment guidelines. First, guideline recommendations that psychotherapy or antidepressant medications are equally appropriate for the initial treatment of major depression can be extended to treatment-naive patients. Second, because ethnic minorities comprised more than one-half of our study’s population, these treatment recommendations can be extended with confidence beyond the white non-Hispanic population, who comprised the majority of all prior randomized treatment studies comparing pharmacotherapy with psychotherapy.
In contrast to the finding that no clinical or socio-demographic variables significantly moderated differential remission rates in the PReDICT study, our neuroimaging data have proven to have predictive value. In the companion study to this article, we report that resting-state functional connectivity predicted remission and nonresponse rates with roughly 80% accuracy (45). This finding is consistent with our prior PET study that also found anterior insula metabolism to predict symptomatic outcomes among CBT- and SSRI-treated patients (11, 12). Other biological assessments examined to date in the PReDICT data set have not proven to serve as moderators of outcomes. Pharmacokinetic analyses and an ex-vivo assay measuring the degree of inhibition of the serotonin and norepinephrine transporters were not predictive of outcomes among the medication-treated patients (55). We also found that hypothalamic-pituitary-adrenal axis sensitivity, as measured by the dexamethasone-corticotropin-releasing hormone test, did not moderate remission outcomes (56). Given the poor replication of demographic and clinical moderators of outcomes to medication compared with psychotherapy treatments, the advancement of precision medicine for major depressive disorder hinges on the identification and refinement of biological markers.
1 : Practice Guideline for the Treatment of Patients with Major Depressive Disorder, 3rd ed. Washington, DC, American Psychiatric Publishing, 2010, pp 17Google Scholar
2 : Does baseline depression severity moderate outcomes between CBT and pharmacotherapy? An individual participant data meta-analysis. JAMA Psychiatry 2015; 72:1102–1109Crossref, Medline, Google Scholar
3 : A new initiative on precision medicine. N Engl J Med 2015; 372:793–795Crossref, Medline, Google Scholar
4 : Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry 2012; 17:1174–1179Crossref, Medline, Google Scholar
5 : Patient predictors of response to psychotherapy and pharmacotherapy: findings in the NIMH Treatment of Depression Collaborative Research Program. Am J Psychiatry 1991; 148:997–1008Link, Google Scholar
6 : Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci USA 2003; 100:14293–14296Crossref, Medline, Google Scholar
7 : Progressive resistance to a selective serotonin reuptake inhibitor but not to cognitive therapy in the treatment of major depression. J Consult Clin Psychol 2007; 75:267–276Crossref, Medline, Google Scholar
8 : Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. J Consult Clin Psychol 2009; 77:775–787Crossref, Medline, Google Scholar
9 : A novel approach for developing and interpreting treatment moderator profiles in randomized clinical trials. JAMA Psychiatry 2013; 70:1241–1247Crossref, Medline, Google Scholar
10 : Improving the prediction of treatment response in depression: integration of clinical, cognitive, psychophysiological, neuroimaging, and genetic measures. CNS Spectr 2008; 13:1066–1086Crossref, Medline, Google Scholar
11 : Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry 2013; 70:821–829Crossref, Medline, Google Scholar
12 : Preliminary findings supporting insula metabolic activity as a predictor of outcome to psychotherapy and medication treatments for depression. J Neuropsychiatry Clin Neurosci 2015; 27:237–239Crossref, Medline, Google Scholar
13 : Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry 2013; 74:595–602Crossref, Medline, Google Scholar
14 : Effectiveness of collaborative care in addressing depression treatment preferences among low-income Latinos. Psychiatr Serv 2010; 61:1112–1118Link, Google Scholar
15 : The acceptability of treatment for depression among African-American, Hispanic, and white primary care patients. Med Care 2003; 41:479–489Crossref, Medline, Google Scholar
16 : Assessing effectiveness of treatment of depression in primary care. Partially randomised preference trial. Br J Psychiatry 2000; 177:312–318Crossref, Medline, Google Scholar
17 : Should treatment for depression be based more on patient preference? Patient Prefer Adherence 2013; 7:1047–1057Crossref, Medline, Google Scholar
18 : The impact of client treatment preferences on outcome: a meta-analysis. J Clin Psychol 2009; 65:368–381Crossref, Medline, Google Scholar
19 : Client preferences affect treatment satisfaction, completion, and clinical outcome: a meta-analysis. Clin Psychol Rev 2014; 34:506–517Crossref, Medline, Google Scholar
20 : Antidepressant treatment history and drug-placebo separation in a placebo-controlled trial in major depressive disorder. Psychopharmacology (Berl) 2015; 232:3833–3840Crossref, Medline, Google Scholar
21 Weiner IB, Stricker G, Widiger TA, Behavior therapy and cognitive-behavioral therapy, Handbook of Psychology, vol. 8. 2nd ed: Clinical Psychology. Hoboken, NJ, Wiley, 2013, pp 291–319Google Scholar
22 : Therapist effectiveness: implications for accountability and patient care. Psychother Res 2011; 21:267–276Crossref, Medline, Google Scholar
23 : ‘It’s not over when it’s over’: persistent neurobiological abnormalities in recovered depressed patients. Psychol Med 2008; 38:307–313Crossref, Medline, Google Scholar
24 : Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry 2006; 59:106–113Crossref, Medline, Google Scholar
25 : Depression beliefs, treatment preference, and outcomes in a randomized trial for major depressive disorder. J Psychiatr Res 2012; 46:375–381Crossref, Medline, Google Scholar
26 : Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT): study protocol for a randomized controlled trial. Trials 2012; 13:106Crossref, Medline, Google Scholar
27 : Enhancing Hispanic participation in mental health clinical research: development of a Spanish-speaking depression research site. Depress Anxiety 2014; 31:258–267Crossref, Medline, Google Scholar
28 : Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
29 : Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0). New York, Biometrics Research Department, New York State Psychiatric Institute, 1995Google Scholar
30 : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
31 : An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
32 : The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003; 54:573–583Crossref, Medline, Google Scholar
33 : The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
34 : Cognitive Therapy of Depression. New York, Guilford, 1979Google Scholar
35 Young J, Beck AT: Cognitive Therapy Scale: Rating Manual. Philadelphia, University of Pennsylvania, 1980Google Scholar
36 : Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry 2005; 62:409–416Crossref, Medline, Google Scholar
37 : National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry 1989; 46:971–982Crossref, Medline, Google Scholar
38 : A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000; 342:1462–1470Crossref, Medline, Google Scholar
39 : Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol 2006; 74:658–670Crossref, Medline, Google Scholar
40 : Predictors and moderators of time to remission of major depression with interpersonal psychotherapy and SSRI pharmacotherapy. Psychol Med 2011; 41:151–162Crossref, Medline, Google Scholar
41 : Depression treatment preferences of VA primary care patients. Psychosomatics 2007; 48:482–488Crossref, Medline, Google Scholar
42 : The relation of patients’ treatment preferences to outcome in a randomized clinical trial. Behav Ther 2007; 38:209–217Crossref, Medline, Google Scholar
43 : Patients’ depression treatment preferences and initiation, adherence, and outcome: a randomized primary care study. Psychiatr Serv 2009; 60:337–343Link, Google Scholar
44 : Treatment preference, engagement, and clinical improvement in pharmacotherapy versus psychotherapy for depression. Behav Res Ther 2010; 48:799–804Crossref, Medline, Google Scholar
45 : Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. Am J Psychiatry 2017; 174:533–545Link, Google Scholar
46 : Patient preference compared with random allocation in short-term psychodynamic supportive psychotherapy with indicated addition of pharmacotherapy for depression. Psychother Res 2009; 19:205–212Crossref, Medline, Google Scholar
47 : Patient preference as a moderator of outcome for chronic forms of major depressive disorder treated with nefazodone, cognitive behavioral analysis system of psychotherapy, or their combination. J Clin Psychiatry 2009; 70:354–361Crossref, Medline, Google Scholar
48 : Are treatment preferences relevant in response to serotonergic antidepressants and cognitive-behavioral therapy in depressed primary care patients? Results from a randomized controlled trial including a patients’ choice arm. Psychother Psychosom 2011; 80:39–47Crossref, Medline, Google Scholar
49 : Patient treatment preference as a predictor of response and attrition in treatment for chronic depression. Depress Anxiety 2012; 29:896–905Crossref, Medline, Google Scholar
50 : Initial severity and differential treatment outcome in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Consult Clin Psychol 1995; 63:841–847Crossref, Medline, Google Scholar
51 : Treatment preferences affect the therapeutic alliance: implications for randomized controlled trials. J Consult Clin Psychol 2007; 75:194–198Crossref, Medline, Google Scholar
52 : Cognitive-behavior therapy versus pharmacotherapy: Now that the jury’s returned its verdict, it’s time to present the rest of the evidence. J Consult Clin Psychol 1996; 64:74–80Crossref, Medline, Google Scholar
53 : Beyond the label: Relationship between community therapists’ self-report of a cognitive behavioral therapy orientation and observed skills. Adm Policy Ment Health Ment Health Serv Res 2016; 43:36–43Crossref, Medline, Google Scholar
54 : Identifying risk for attrition during treatment for depression. Psychother Psychosom 2009; 78:372–379Crossref, Medline, Google Scholar
55 Owens MJ, Dunlop BW, Plott S, et al: Poster presentation: Estimates of serotonin or norepinephrine transporter occupancy do not predict efficacy in a 12 week trial. Presented at the proceedings of the 54th Annual Meeting of the American College of Neuropsychopharmacology, Hollywood, Fla, 2015Google Scholar
56 : Time-dependent effects of dexamethasone plasma concentrations on glucocorticoid receptor challenge tests. Psychoneuroendocrinology 2016; 69:161–171Crossref, Medline, Google Scholar



