Neural Predictors of Initiating Alcohol Use During Adolescence
Abstract
Objective:
Underage drinking is widely recognized as a leading public health and social problem for adolescents in the United States. Being able to identify at-risk adolescents before they initiate heavy alcohol use could have important clinical and public health implications; however, few investigations have explored individual-level precursors of adolescent substance use. This prospective investigation used machine learning with demographic, neurocognitive, and neuroimaging data in substance-naive adolescents to identify predictors of alcohol use initiation by age 18.
Method:
Participants (N=137) were healthy substance-naive adolescents (ages 12–14) who underwent neuropsychological testing and structural and functional magnetic resonance imaging (sMRI and fMRI), and then were followed annually. By age 18, 70 youths (51%) initiated moderate to heavy alcohol use, and 67 remained nonusers. Random forest classification models identified the most important predictors of alcohol use from a large set of demographic, neuropsychological, sMRI, and fMRI variables.
Results:
Random forest models identified 34 predictors contributing to alcohol use by age 18, including several demographic and behavioral factors (being male, higher socioeconomic status, early dating, more externalizing behaviors, positive alcohol expectancies), worse executive functioning, and thinner cortices and less brain activation in diffusely distributed regions of the brain.
Conclusions:
Incorporating a mix of demographic, behavioral, neuropsychological, and neuroimaging data may be the best strategy for identifying youths at risk for initiating alcohol use during adolescence. The identified risk factors will be useful for alcohol prevention efforts and in research to address brain mechanisms that may contribute to early drinking.
Underage drinking is widely recognized as one of the leading public health and social problems for adolescents in the United States. Teenage drinking is common in the United States, with approximately 66% of 18-year-olds reporting alcohol use (1). Adverse consequences of adolescent drinking include higher rates of violence, missing school, drunk driving, riding with a drunk driver, suicide, and risky sexual behavior, and it accounts for more than 5,000 deaths per year (2). Thus, being able to identify at-risk children before they initiate heavy alcohol use could have immense clinical and public health implications. However, few investigations have been conducted to gain a greater understanding of individual differences that could lead to adolescent substance use.
Previous findings have suggested that a mix of social, psychological, and biological mechanisms contribute to alcohol use during adolescence (3–5). Demographic risk factors for alcohol initiation include being male, having higher levels of psychological problems and externalizing behaviors, and having positive expectations about the effects of alcohol (5). Neuropsychological and neuroimaging data may provide quantification of underlying behavioral mechanisms of risk for substance use. Several studies suggest that poorer performance on tests of executive functioning (6), as well as having less brain activation relative to comparison participants during tasks of working memory, inhibition, and reward processing, can be used to predict which youths will initiate alcohol use during adolescence (7–11). In addition, having less volume in brain regions involved in impulsivity, reward sensitivity, and decision making appear to influence initiation of alcohol and other substance use during adolescence (7, 11). Understanding factors involved in the initiation and escalation of alcohol use during adolescence could provide crucial information for prevention and intervention efforts.
Machine learning approaches (12–17) are increasingly being used to generate predictions based on complex data like brain imaging (18, 19). Random forests (20) is a machine learning tool that can be used for feature selection and/or robust predictive modeling and that has been suggested to be superior to other machine learning techniques (21). The random forest technique consists of a complex partitioning of the predictor variable space and can be used when the number of predictor variables is much larger than the number of study participants, as is typically the case with neuroimaging data. Moreover, the random forest model has a low tendency to overfit, and the stepwise partitioning of the predictor space can yield high-order interactions among many predictor variables that cannot be identified using other classification procedures (22). Random forest models have been successfully used to detect a number of clinical outcomes and to predict behaviors (23), but they have not been used to identify predictors of substance use initiation in adolescents.
The present study uses random forest models in combination with multimodal imaging and neuropsychological test data to identify the best predictors of initiation to moderate to heavy alcohol use by age 18 in substance-naive adolescents. Based on previous research, initiation of substance use was expected to be associated with key demographic factors (e.g., being male, endorsing more externalizing behaviors and psychopathology, having positive expectations about alcohol use), neuropsychological performance (e.g., poorer performance on executive function tasks), and thinner cortices and less brain activation in key brain regions involved in executive functioning and decision making. Unlike other studies that focused on initiation of any alcohol use (11), we were interested in factors that predicted a pattern of more frequent and intense alcohol use, as these have been most consistently associated with poorer cognitive (7, 24) and social (5) outcomes.
Method
Participants
Participants were 137 healthy children and adolescents ages 12–14 (44% female) from a larger ongoing neuroimaging study recruited through flyers sent to households of students attending local middle schools (Table 1). Extensive screening and background information was obtained from the youths, one biological parent, and one other parent or close relative. All primary informants lived with the youths. The study protocol was executed in accordance with the standards approved by the University of California, San Diego, Human Research Protections Program.
| Variable | Continuous Nonusers (N=67) | Moderate to Heavy Alcohol Initiators (N=70) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | % | Mean | SD | % | t or χ2 | df | p | Cohen’s d | |
| Demographic and family variables | ||||||||||
| Sex (female) | 60 | 29 | 13.48 | 1, N=137 | <0.001 | 0.661 | ||||
| Follow-up age (range=16–19)b | 18.20 | 0.66 | 18.20 | 0.57 | 0.07 | 135 | 0.943 | 0.000 | ||
| Race (Caucasian) | 58 | 77 | 5.63 | 1, N=137 | 0.018 | 0.414 | ||||
| Baseline Hollingshead Index of Social Position score (socioeconomic status) | 24.03 | 16.75 | 21.11 | 11.50 | 1.19 | 135 | 0.235 | 0.203 | ||
| Family history density of alcohol or drug use disorder | 0.17 | 0.28 | 0.18 | 0.31 | 0.14 | 135 | 0.887 | 0.034 | ||
| Baseline Pubertal Development Scale score (girls only; N=60) | 15.05 | 3.15 | 14.95 | 3.15 | 0.12 | 58 | 0.908 | 0.032 | ||
| Baseline Pubertal Development Scale score (boys only; N=77) | 10.81 | 3.33 | 11.20 | 3.37 | 0.48 | 75 | 0.632 | 0.116 | ||
| Baseline grade in school | 6.76 | 0.74 | 7.00 | 0.87 | 1.73 | 135 | 0.086 | 0.297 | ||
| Birth order | 1.52 | 0.79 | 1.64 | 0.76 | 0.91 | 135 | 0.364 | 0.155 | ||
| Baseline % living with both parents | 79 | 81 | 0.12 | 1, N=137 | 0.732 | 0.059 | ||||
| Baseline % youth with biological parents married to each other | 78 | 77 | 0.00 | 1, N=137 | 0.948 | 0.000 | ||||
| Baseline youth behavior, mood, and cognition | ||||||||||
| Initiated dating by age 14 (%) | 22 | 61 | 21.37 | 1, N=137 | <0.001 | 0.860 | ||||
| Involved in extracurricular activities (%) | 82 | 89 | 1.15 | 1, N=137 | 0.283 | 0.184 | ||||
| Hours of video games played per week | 3.10 | 4.95c | 2.47 | 4.04c | 0.72 | 106 | 0.472 | 0.139 | ||
| Grade point average (4.0 scale) | 3.65 | 0.45 | 3.47 | 0.55 | 2.14 | 135 | 0.034 | 0.358 | ||
| CBCL externalizing disorder t score | 41.09 | 7.12d | 40.42 | 7.64d | 0.52 | 128 | 0.606 | 0.091 | ||
| CBCL internalizing t score | 44.48 | 8.80d | 42.11 | 7.65d | 1.65 | 128 | 0.102 | 0.287 | ||
| CBCL withdrawn t score | 52.00 | 3.79d | 51.42 | 2.69d | 1.00 | 128 | 0.319 | 0.176 | ||
| CBCL somatic complaints t score | 52.45 | 4.13d | 51.79 | 3.15d | 1.04 | 128 | 0.302 | 0.180 | ||
| CBCL anxious/depressed t score | 51.61 | 3.60d | 51.11 | 2.60d | 0.92 | 128 | 0.361 | 0.159 | ||
| CBCL social problems t score | 51.33 | 3.62d | 50.55 | 1.64d | 1.60 | 128 | 0.113 | 0.278 | ||
| CBCL thought problems t score | 51.98 | 3.90d | 51.03 | 2.31d | 1.70 | 128 | 0.091 | 0.296 | ||
| CBCL attention problems t score | 51.05 | 2.16d | 51.20 | 2.81d | 0.34 | 128 | 0.734 | 0.060 | ||
| CBCL delinquent behavior t score | 50.59 | 1.84d | 50.98 | 2.53d | 1.01 | 128 | 0.317 | 0.176 | ||
| CBCL aggressive behavior t score | 50.75 | 2.06d | 50.68 | 2.11d | 0.19 | 128 | 0.852 | 0.034 | ||
| CBCL total problem t score | 39.83 | 9.75d | 37.83 | 8.83d | 1.22 | 128 | 0.223 | 0.215 | ||
| Conduct Disorder Questionnaire total score | 0.45 | 1.08 | 1.17 | 1.88 | 2.75 | 135 | 0.007 | 0.470 | ||
| Beck Depression Inventory–II total score | 1.39 | 2.75e | 1.25 | 2.81e | 0.30 | 132 | 0.765 | 0.050 | ||
| State-Trait Anxiety Inventory total score | 26.22 | 6.66f | 26.68 | 6.48f | 0.40 | 130 | 0.690 | 0.070 | ||
| AEQ total score | 79.14 | 32.23 | 92.45 | 29.75 | 2.51 | 135 | 0.013 | 0.429 | ||
| AEQ global positive score | 37.81 | 8.37 | 40.83 | 9.53 | 1.97 | 135 | 0.051 | 0.336 | ||
| AEQ social behavior change score | 36.92 | 7.41 | 41.23 | 7.94 | 3.28 | 135 | 0.001 | 0.561 | ||
| AEQ improved performance score | 16.55 | 4.57 | 16.96 | 4.98 | 0.50 | 135 | 0.621 | 0.086 | ||
| AEQ sexual enhancement score | 20.15 | 5.06 | 21.00 | 4.07 | 1.09 | 135 | 0.279 | 0.185 | ||
| AEQ impaired performance score | 98.41 | 10.08 | 96.39 | 10.28 | 1.16 | 135 | 0.247 | 0.198 | ||
| AEQ increased arousal score | 24.24 | 5.44 | 24.96 | 4.78 | 0.82 | 135 | 0.414 | 0.141 | ||
| AEQ relaxation score | 41.88 | 9.83 | 43.87 | 7.90 | 1.31 | 135 | 0.193 | 0.293 | ||
| Baseline substance use | ||||||||||
| CDDR baseline lifetime smoking days | 0.03 | 0.17 | 0.11 | 0.65 | 1.03 | 135 | 0.305 | 0.168 | ||
| CDDR baseline lifetime drinking days | 0.00 | 0.00 | 0.31 | 1.50 | 1.72 | 135 | 0.089 | 0.292 | ||
| CDDR baseline lifetime marijuana use days | 0.00 | 0.00 | 0.09 | 0.44 | 1.59 | 135 | 0.115 | 0.289 | ||
| Total number of repetitions excluded during functional magnetic resonance imaging | 4.46 | 6.77 | 7.03 | 8.82 | 1.90 | 134 | 0.060 | 0.327 | ||
| Follow-up substance use | ||||||||||
| Age at first use of alcohol | 16.54 | 1.27g | 15.46 | 1.60 | 3.09 | 94 | 0.003 | 0.748 | ||
| Lifetime alcohol use occasions | 1.52 | 2.87g | 69.01 | 110.58 | 4.99 | 135 | 0.000 | 0.863 | ||
| Peak drinks on an occasion in past year | 0.46 | 0.96g | 9.30 | 4.60 | 15.41 | 135 | 0.000 | 2.66 | ||
| Lifetime marijuana use occasions | 0.06 | 0.38h | 108.61 | 256.94 | 3.46 | 135 | 0.001 | 0.597 | ||
| Reporting >30 lifetime marijuana use occasions (%) | 0 | 29 | ||||||||
| Lifetime other drug use | 0.00 | 0.00 | 7.04 | 38.05 | 1.52 | 135 | 0.132 | 0.261 | ||
TABLE 1. Demographic, Psychological, and Neuropsychological Variables for Adolescents Who Remained Nonusers or Transitioned to Moderate to Heavy Substance Use Patterns by Approximately Age 18 (N=137)a
Strict exclusionary criteria for project entry included experience with alcohol or drugs, defined as ≥10 total days in their life in which drinking had occurred, or >2 drinks in a week (i.e., two drinks on one occasion or one drink on two occasions in the same week); ≥3 lifetime experiences with marijuana and any use in the past 3 months; ≥5 lifetime cigarette uses; any history of other intoxicant use; any suggestion of prenatal exposure to alcohol (>2 drinks during a given week) or any illicit drug; premature birth (i.e., born before the 35th gestational week); a history of any neurological or DSM-IV axis I disorder (determined by the National Institute of Mental Health Diagnostic Interview Schedule for Children, version 4.0), head trauma, or loss of consciousness >2 minutes; chronic medical illness, learning disability or mental retardation, or use of medications potentially affecting the brain; contraindication to MRI (e.g., braces); inadequate comprehension of English; and noncorrectable sensory problems.
Measures
Substance use measures.
At baseline and follow-up assessments, the Customary Drinking and Drug Use Record (25) was administered to obtain quantity and frequency of lifetime and recent (past year) alcohol, marijuana, and other drug use, withdrawal/hangover symptoms, and DSM-IV abuse and dependence criteria. Breath alcohol testing and urine toxicology screens confirmed self-report data at baseline. Substance use information was updated every 6 months by telephone or in person after the participant’s baseline assessment. Parent and/or informant (sibling, friend, roommate) report of youth substance use was collected as collateral evidence.
Demographic information.
The Structured Clinical Interview (26) was administered to youths to ascertain information about the child’s sex, age, race, academic functioning (i.e., grade point average on a 4.0 scale), grade in school, family characteristics (i.e., birth order, living situation, parent’s marital status), dating status (e.g., never dated or history of dating), involvement in extracurricular activities, and hours of video games played per week.
Socioeconomic status.
Socioeconomic background information (i.e., educational attainment, occupation, and salary of each parent) was obtained from parents and converted to a Hollingshead Index of Social Position score (27).
Family background.
At baseline, the Family History Assessment Module (28) was administered to parents and youths to ascertain familial density of alcohol and drug use disorders in first- and second-degree relatives. Family history density scores were calculated by adding 0.5 for each biological parent and 0.25 per biological grandparent endorsed by either youth or parent as having alcohol use disorder or substance use disorder (possible scores range from 0 to 4).
Pubertal development.
The Pubertal Development Scale (29) determined the current level of pubertal development for girls and boys separately with five sex-specific items, with scores ranging from 1 (prepubertal) to 4 (postpubertal). Participants in this sample were, on average, early to midpubertal at baseline and were late to postpubertal at follow-up.
Psychopathology and mood.
The parent-administered Child Behavior Checklist (30) provided age- and gender-normed continuous measures of externalizing and internalizing psychopathology. T scores from the following subscales of the Child Behavior Checklist were used in analyses: withdrawn, somatic complaints, anxious/depressed, social problems, thought problems, attention problems, delinquent behavior, and aggressive behavior, as well as three summary scores representing externalizing, internalizing, and total problems. Youths completed the Conduct Disorder Questionnaire (31), which determined DSM-IV diagnostic criteria for conduct disorder; total symptom count was used in analyses. The Beck Depression Inventory–II (32) and the State-Trait Anxiety Inventory (33) assessed recent depressive and anxiety state symptoms in youths.
Alcohol Expectancy Questionnaire–Adolescent version.
Youths completed the Alcohol Expectancy Questionnaire–Adolescent version (AEQ) (34), which was developed to assess beliefs about the anticipated effects of alcohol. This version yields a total score (AEQ total score) and seven empirically derived factor scores indicating expectations for the effects of drinking alcohol: global positive changes (AEQ global positive), enhancement/impedance of social behavior (AEQ social behavior change), improvement in cognitive/behavioral functioning (AEQ improved performance), enhancement of sexuality (AEQ sexual enhancement), deterioration in cognitive/behavioral functioning (AEQ impaired performance), increased arousal (AEQ increased arousal), and promotion of relaxation/tension reduction (AEQ relaxation).
Neurocognition.
A comprehensive neuropsychological battery was completed by youths at baseline to assess cognitive functioning on several cognitive domains that could potentially affect initiation of alcohol and marijuana use during adolescence. (See Table 2 for neuropsychological tests and domains assessed, and see the supplementary references for neuropsychological testing materials found in the data supplement that accompanies the online edition of this article.)
| Continuous Nonusers (N=67) | Moderate to Heavy Alcohol Initiators (N=70) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Domain Assessed | Mean | SD | Mean | SD | t | df | p | Cohen’s d |
| Digit vigilance total time to complete (sec) | Sustained attention | 225.06 | 52.80a | 219.59 | 41.46a | 0.67 | 132 | 0.504 | 0.115 |
| Wechsler Abbreviated Scale of Intelligence (WASI) block design raw score | Spatial perception, visual abstract processing, problem solving | 47.27 | 13.81a | 42.86 | 13.13a | 1.89 | 132 | 0.060 | 0.327 |
| WASI matrix reasoning raw score | Nonverbal abstract problem solving, inductive reasoning | 27.80 | 3.62b | 26.41 | 3.34b | 2.31 | 131 | 0.023 | 0.399 |
| WASI vocabulary raw score | Word knowledge, verbal concept formation | 53.02 | 7.09a | 51.96 | 7.06a | 0.87 | 132 | 0.389 | 0.150 |
| WASI similarities raw score | Abstract verbal reasoning | 35.17 | 4.24a | 34.31 | 5.07a | 1.06 | 132 | 0.292 | 0.184 |
| Delis-Kaplan Executive Function System (D-KEFS) trails number-letter switching time to complete (sec) | Flexibility of thinking on a visual-motor task | 70.60 | 19.45 | 77.46 | 28.75 | 1.63 | 135 | 0.106 | 0.279 |
| D-KEFS towers total achievement score (raw) | Spatial planning, rule learning | 17.04 | 2.68 | 17.24 | 2.73 | 0.43 | 135 | 0.669 | 0.074 |
| D-KEFS color word interference inhibition time to complete (sec) | Inhibitory functioning | 57.39 | 14.25 | 59.60 | 13.12 | 0.95 | 135 | 0.346 | 0.161 |
| D-KEFS color word interference inhibition/switching time to complete (sec) | Inhibition/cognitive flexibility | 65.81 | 15.81 | 63.91 | 14.39 | 0.73 | 135 | 0.465 | 0.126 |
| Rey-Osterrieth Complex Figure (ROCF) copy accuracy score | Visuospatial functioning; organization | 29.35 | 3.02a | 28.63 | 3.01a | 1.39 | 132 | 0.168 | 0.239 |
| ROCF delay accuracy score | Spatial memory | 18.09 | 4.12c | 17.77 | 4.26c | 0.43 | 130 | 0.665 | 0.076 |
| Wechsler Intelligence Scale for Children, 3rd edition (WISC-III), digits forward raw score | Attention | 9.91 | 1.84a | 10.17 | 1.94a | 0.81 | 132 | 0.421 | 0.138 |
| WISC-III digits backward raw score | Short-term memory, attention | 6.44 | 1.74a | 6.19 | 2.05a | 0.76 | 132 | 0.447 | 0.131 |
| WISC-III arithmetic raw score | Computational skills, auditory memory, attention, problem solving | 22.23 | 3.36a | 22.46 | 3.34a | 0.39 | 132 | 0.701 | 0.069 |
| WISC-III coding raw score | Visual motor speed, visual memory, sustained effort and attention | 61.20 | 10.88a | 59.37 | 10.75a | 0.98 | 132 | 0.329 | 0.169 |
| WISC-III mazes raw score | Planning, perceptual organization, visual-motor coordination and speed, nonverbal reasoning | 23.48 | 3.71a | 22.77 | 3.20a | 1.19 | 132 | 0.235 | 0.205 |
| Wechsler Adult Intelligence Scale (WAIS-IV) letter-number sequence raw score | Attention, auditory short-term memory, sequencing ability | 10.69 | 1.94d | 10.09 | 1.88d | 1.65 | 108 | 0.103 | 0.314 |
| Hooper Visual Organization Test total raw score | Visuospatial functioning and spatial integration | 25.32 | 2.26d | 24.94 | 2.40d | 0.86 | 108 | 0.392 | 0.163 |
| California Verbal Learning Test (CVLT) list A total 1 to 5 raw score | Verbal learning and memory | 55.91 | 8.01a | 54.51 | 6.29a | 1.12 | 132 | 0.263 | 0.194 |
| CVLT list A trial 1 raw score | Verbal learning and memory | 7.72 | 1.93a | 7.37 | 1.64a | 1.13 | 132 | 0.262 | 0.195 |
| CVLT list A trial 5 raw score | Verbal learning and memory | 12.92 | 1.68a | 12.74 | 1.57a | 0.64 | 132 | 0.525 | 0.111 |
| CVLT short delay free raw score | Verbal learning and memory | 11.87 | 2.07a | 11.74 | 2.29a | 0.33 | 132 | 0.743 | 0.060 |
| CVLT short delay cued raw score | Verbal learning and memory | 12.15 | 1.94e | 12.19 | 1.70e | 0.12 | 129 | 0.907 | 0.022 |
| CVLT long delay free raw score | Verbal learning and memory | 12.11 | 1.91f | 12.30 | 1.79f | 0.60 | 130 | 0.550 | 0.103 |
| CVLT long delay cued raw score | Verbal learning and memory | 12.52 | 1.80f | 12.54 | 1.75f | 0.04 | 130 | 0.968 | 0.011 |
| Wide Range Achievement Test–3 Reading raw score | Premorbid functioning and intellectual capacity | 45.44 | 4.14a | 44.67 | 4.04a | 1.08 | 132 | 0.280 | 0.188 |
TABLE 2. Baseline Neuropsychological Test Variables in a Study of Neural Predictors of Alcohol Use Initiation During Adolescence
Follow-up procedures.
At baseline, 12- to 14-year-old youths underwent a baseline interview, neuropsychological testing, and a structural and functional neuroimaging session. Every 6 months, telephone or in-person interviews assessed current substance use and psychiatric functioning. At baseline, all participants were considered control participants and had never had more than 10 lifetime alcohol use occasions, with never more than one drink per occasion, and no more than three lifetime marijuana use episodes. Ninety-seven percent of the sample had never used alcohol, and 98% had never used marijuana. Rigorous follow-up procedures were used to ensure excellent follow-up rates (96%). At follow-up (approximately age 18), participants were classified as either continuous controls (baseline control who maintained abstinence during the follow-up, defined as 0–4 drinks on an occasion and <12 lifetime drinking occasions) or moderate to heavy drinking initiators (baseline controls who transitioned to moderate or heavy alcohol use, defined as 3–29 drinks on an occasion and 3–834 drinking occasions; see Figure 1 for classification). Participants who had fewer drinking days but drank significantly on those occasions (e.g., three lifetime occasions, 15 drinks per occasion) were classified as moderate to heavy drinkers to capture the fact that they had initiated significant levels of alcohol use. Sixty-seven participants (49%) were classified as continuous controls (of whom 61% continued to remain completely alcohol-naive at follow-up) and 70 (51%) as moderate to heavy drinking initiators (see Table 1; of the alcohol initiators, 27% met criteria for moderate drinking, and 73% met criteria for heavy drinking). Continuous nonusers were at least 17 years of age at follow-up to allow sufficient time to transition to alcohol use. Sixteen-year-old substance users were included because it was clear that onset of substance use had occurred by that age. For this sample, rates of alcohol initiation were consistent with those of the general U.S. adolescent population (1).
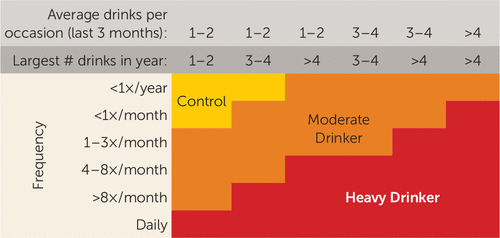
FIGURE 1. Substance Use Classification Chart for a Study of Neural Predictors of Alcohol Use Initiation During Adolescencea
a “Control” indicates continuously nondrinking participants. “Largest # drinks in year” refers to the largest number of alcoholic drinks consumed on one occasion in the past year. Reprinted with permission from American Psychiatric Association Publishing, Squeglia et al., Brain Development in Heavy-Drinking Adolescents. Am J Psychiatry 2015; 172:531–542.
Procedures
Image acquisition.
High-resolution anatomical and functional MRI (fMRI) images were collected at the University of California, San Diego, Center for Functional MRI on a 3-T CXK4 short-bore Excite-2 MR system (General Electric, Milwaukee) with an eight-channel phase-array head coil. Participants were placed on the scanner table, and the head was stabilized within the head coil using foam cushions (NoMoCo, La Jolla, Calif.). Scan sessions involved a 10-second scout scan to ensure good head placement and slice selection covering the whole brain, followed by a high-resolution T1-weighted sequence using a sagittally acquired spoiled gradient recalled sequence (field of view=24 cm, 256×256×192 matrix, 0.94×0.94×1 mm voxels, 176 slices, TR=20 ms, TE=4.8 ms, flip angle=12°, acquisition time=7 minutes and 26 seconds). Blood-oxygen-level-dependent (BOLD) response contrast was measured with T2*-weighted axially acquired echo-planar images (field of view=24 cm, 64×64 matrix, 3.75×3.75×3.8 mm voxels, 32 slices, TE=30 ms, TR=2,000 ms, flip angle=90°, ramped bandwidth=250 KHz). Field maps were acquired to minimize warping and signal dropout (∼4 minutes total) and employed two different echo times to assess field inhomogeneities and signal distortions using the same grid parameters under which echo-planar images were acquired.
Visual working memory task.
All participants were administered the same fast event-related visual working memory task (35) during fMRI acquisition, which has been shown to predict future initiation of alcohol use (8). Participants were instructed to indicate whether dot arrays presented with a 2,000 ms interstimulus interval were identical or different (i.e., one dot was of a different color). Each participant completed 30 trials of each level of complexity (two, four, or six dots) presented randomly, in addition to 69 null trials of 2,000 ms each interspersed to provide an optimized fast event-related sequence (256 repetitions in all; 8 minutes and 32 seconds). The six-dot condition is considered supra-span (i.e., higher than most people’s working memory span), and the two-dot condition is subspan (i.e., well within most people’s working memory load capacity) (36). None of the 137 total runs used during analysis had performance at or below chance level (50%) on the two-dot condition (i.e., low-capacity/easy condition). A greater BOLD response contrast (i.e., a larger fit coefficient) to the six-dot (supra-span) relative to the two-dot (subspan) condition was interpreted as having more cognitive energy expended to complete the challenging supra-span trials.
Data Analysis
Structural image processing.
FreeSurfer, version 5.0 (surfer.nmr.mgh.harvard.edu) was used for cortical surface reconstruction and cortical thickness estimation (37, 38) of the high-resolution T1-weighted MR data. The FreeSurfer program uses a series of automated imaging algorithms to produce measures of cortical thickness. One rater (L.M.S.), blind to participant characteristics, followed the reconstruction procedures (http://surfer.nmr.mgh.harvard.edu/fswiki/RecommendedReconstruction) to identify and correct any errors made during the cortical reconstruction. After inspection, an automated parcellation procedure divided each hemisphere into 34 independent cortical regions based on gyral and sulcal features (39; see Table 3 for a list of parcellated brain regions). Cortical thickness estimates of each region were extracted for subsequent statistical analyses.
| Variable | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Demographic and family variables | |||
| 1. Sex | X | X | X |
| 2. Baseline age | |||
| 3. Follow-up age | X | X | X |
| 4. Race | |||
| 5. Hollingshead Index of Social Position score (socioeconomic status) | X | X | X |
| 6. Family history density of alcohol or drug use disorder | |||
| 7. Pubertal Development Scale score | X | ||
| 8. Grade in school | |||
| 9. Birth order | |||
| 10. Living with both parents | |||
| 11. Parents’ marital status | |||
| Youth behavior, mood, and cognition | |||
| 12. Dating status | X | X | X |
| 13. Child involvement in extracurricular activities | |||
| 14. Hours of video games per week | |||
| 15. Grade point average | X | ||
| 16. CBCL externalizing t score | |||
| 17. CBCL internalizing t score | |||
| 18. CBCL withdrawn t score | |||
| 19. CBCL somatic complaints t score | |||
| 20. CBCL anxious/depressed t score | |||
| 21. CBCL social problems t score | |||
| 22. CBCL thought problems t score | |||
| 23. CBCL attention problems t score | |||
| 24. CBCL delinquent behavior t score | |||
| 25. CBCL aggressive behavior t score | |||
| 26. CBCL total problem t score | X | ||
| 27. Conduct Disorders Questionnaire total score | X | X | X |
| 28. Beck Depression Inventory-II total score | |||
| 29. State-Trait Anxiety Inventory total score | |||
| 30. AEQ total score | X | X | X |
| 31. AEQ global positive score | X | X | X |
| 32. AEQ social behavior change score | X | X | X |
| 33. AEQ improved performance score | |||
| 34. AEQ sexual enhancement score | |||
| 35. AEQ impaired performance score | |||
| 36. AEQ increased arousal score | |||
| 37. AEQ relaxation total score | |||
| 38. CDDR baseline lifetime smoking days (<5 for all participants) | |||
| 39. CDDR baseline lifetime drinking days (<10) | |||
| 40. CDDR baseline lifetime marijuana use days (<3) | |||
| 41. Repetitions excluded from functional magnetic resonance imaging (fMRI) series due to motion | X | ||
| Neuropsychological testing variables | |||
| 1. Digit vigilance total time to complete (sec) | X | X | |
| 2. WASI block design raw score | X | X | |
| 3. WASI matrix reasoning raw score | X | X | |
| 4. WASI vocabulary raw score | X | ||
| 5. WASI similarities raw score | |||
| 6. D-KEFS trails condition 4 (number-letter switching) time to complete (sec) | X | ||
| 7. D-KEFS towers total achievement raw score | |||
| 8. D-KEFS color word interference inhibition time to complete (sec) | |||
| 9. D-KEFS color word interference inhibition/switching time to complete (sec) | |||
| 10. ROCF copy accuracy score | |||
| 11. ROCF delay accuracy score | |||
| 12. WISC-III digits forward raw score | |||
| 13. WISC-III digits backward raw score | |||
| 14. WISC-III arithmetic raw score | |||
| 15. WISC-III coding raw score | |||
| 16. WISC-III mazes raw score | |||
| 17. WAIS-IV letter-number sequence raw score | |||
| 18. Hooper Visual Organization Test total raw score | |||
| 19. CVLT list A total 1 to 5 raw score | |||
| 20. CVLT list A trial 1 raw score | |||
| 21. CVLT list A trial 5 raw score | |||
| 22. CVLT short delay free raw score | |||
| 23. CVLT short delay cued raw score | |||
| 24. CVLT long delay free raw score | |||
| 25. CVLT long delay cued raw score | |||
| 26. WRAT–3 reading raw score | |||
| Cortical thickness and BOLD regionsb | |||
| 1. Banks of superior temporal sulcus | L-CT | ||
| 2. Caudal anterior cingulate | R-BOLD | ||
| 3. Caudal middle frontal | |||
| 4. Cuneus | |||
| 5. Entorhinal | |||
| 6. Fusiform | |||
| 7. Inferior parietal | |||
| 8. Inferior temporal | |||
| 9. Isthmus cingulate | |||
| 10. Lateral occipital | L-CT | ||
| 11. Lateral orbitofrontal | |||
| 12. Lingual | L-CT | ||
| 13. Medial orbitofrontal | |||
| 14. Middle temporal | R-CT, L-BOLD | ||
| 15. Parahippocampal | |||
| 16. Paracentral | |||
| 17. Pars opercularis | |||
| 18. Pars orbitalis | R-CT | ||
| 19. Pars triangularis | |||
| 20. Pericalcarine | |||
| 21. Postcentral | |||
| 22. Posterior cingulate | R-BOLD | ||
| 23. Precentral | |||
| 24. Precuneus | R-CT, L-BOLD, R-BOLD | ||
| 25. Rostral anterior cingulate | L-CT | ||
| 26. Rostral middle frontal | R-CT | ||
| 27. Superior frontal | R-CT | ||
| 28. Superior parietal | L-CT, R-CT | ||
| 29. Superior temporal | R-BOLD | ||
| 30. Supramarginal | L-CT | ||
| 31. Frontal pole | R-CT, R-BOLD | ||
| 32. Temporal pole | R-CT | ||
| 33. Transverse temporal | L-CT | ||
| 34. Insula |
TABLE 3. List of Variables Selected as Important in Each Model in a Study of Neural Predictors of Alcohol Use Initiation During Adolescencea
Functional image processing.
AFNI (40) was used to process functional images. Artifact and aberrant signal levels were examined in each repetition of each slice using an automated program developed by the University of California, San Diego, Laboratory of Cognitive Neuroimaging. Motion in time-series data was corrected by registering each acquisition to the maximally stable base volume with an iterated least squares algorithm (41) to estimate three rotational and three displacement parameters for each participant. An output file specifying adjustments made controlled for spin history effects in analyses if no significant task-correlated motion was found. To evaluate task-related motion, the reference vector was correlated with the six motion parameters for each data set. Data sets with significant task-correlated or bulk motion (>2 mm) were excluded from analyses. Two trained raters then scanned the time series to omit any remaining repetitions with visually discernible motion; if more than 15% of repetitions in a task were discarded, the run was not used (N=10, not described in this article).
Raw time-series data were standardized to percent signal change from baseline, and deconvolution was conducted with a reference function that convolved the behavioral stimuli with a hemodynamic response model while covarying for linear trends and motion correction, ignoring the first three repetitions (42). This resulted in a functional image in which every voxel contains a fit coefficient representing the change in signal across behavioral conditions, as well as the signal change percentage and threshold statistics. Standardized Talairach transformations were made for each high-resolution anatomical image, and functional data sets were warped in accordance to manage individual anatomical variability. Functional data were resampled into isotropic voxels (3 mm3), and a spatial smoothing Gaussian filter (full-width, half maximum, 5 mm) was applied to minimize the influence of individual anatomic variability. Coregistration of structural images to functional images was performed with a mutual information registration program (41) that robustly handles images with different signal characteristics and different spatial resolutions.
Volumetric and functional image alignment.
The AFNI Surface Mapper (SUMA) program (43) was used to align segmented volumetric and functional data sets to the same template space. SUMA programs allow for fine control over the mapping between volume and surface domains produced by the FreeSurfer segmentation process while maintaining a direct link to volumetric data from which surface models and data originated. Combining functional and structural neuroimaging data using SUMA has been described in detail elsewhere (44). BOLD response values, averaged across the parcellation regions derived from FreeSurfer (39), were imported from AFNI to SPSS (IBM, Armonk, N.Y.).
Statistical Analysis
Independent-sample t tests or chi-square tests (for dichotomous variables) compared differences between groups (Table 1). Random forest classification was implemented in the R statistics package (http://cran.r-project.org; randomForest library) to predict alcohol initiator status, with missing data handled using the rfimpute function (see Table 1 for sample sizes per variable). Default parameters for the randomForest function were used, with the exception of expanding the number of trees to 2,000 (45).
Random forest classification has been described in detail elsewhere (20, 23). Briefly, random forest classification has two primary parameters: the number of trees (2,000 were used in these analyses) and the number of variables tried at each node (as recommended [20], the square root of the total number of variables). In addition, trees were grown to the highest possible number of nodes such that all participants in the bootstrap training sample were accurately classified. Variable selection was accomplished using permutation importance scores, defined as the mean decrease in model accuracy when a predictor variable’s values are randomly permuted. Specifically, the random forest algorithm was run 500 times on the entire set of possible predictors to generate stable importance scores for each predictor (based on the median score across the 500 repetitions). Because negative permutation importance scores arise from random variation around zero of the poor predictor variables (22), only variables with an importance score greater than the magnitude of the most negative score were selected as important predictors (22, 23). Notably, this technique utilizes bootstrapped cross-validation to reduce overfitting when generating permutation importance scores.
This approach is consistent with the first steps executed in Ball et al. (23). However, we do not generate and evaluate a final model using the select predictors because this results in “double dipping,” or selecting and evaluating variables from the same sample (46). Although a holdout sample could be used to circumvent this issue (i.e., splitting the sample into variable selection and final model testing subsamples), this requires a sample size of greater than 200 for sufficient power in the subsamples. Therefore, analyses are focused exclusively on variable selection to identify potentially important risk factors for adolescent alcohol use.
Three sequential models were built to compare the following sets of variables (see Table 3): demographic and behavioral variables only; demographic and behavioral and neuropsychological test variables; and demographic and behavioral, neuropsychological, and neuroimaging variables. (See the data supplement for models that were run on 26 neuropsychological and 136 neuroimaging variables separately.) The relatively wide range in baseline age (12–14 years) could have biased findings because those enrolled at age 14 survived 2 more years without initiating alcohol use; this was accounted for by including baseline and follow-up age in the models.
Results
Demographic Model
The initial variable set comprised 41 demographic and psychological variables (see Table 3) as predictors of moderate to heavy alcohol initiation. Eleven of these were identified as important: sex, age at follow-up, socioeconomic status, pubertal development, dating status, grade point average, Child Behavior Checklist total problems, Conduct Disorder Questionnaire total problems count, AEQ total score, AEQ global positive score, and AEQ social behavior change score.
Demographic and Neuropsychological Performance Model
After adding neuropsychological test variables (26 additional variables; see Table 3) to the variable set, 13 of the 67 total variables were identified as important: eight of 11 from the previous model (sex, age at follow-up, socioeconomic status, dating status, Conduct Disorder Questionnaire total problems count, AEQ total score, AEQ global positive score, and AEQ social behavior change score), and five additional variables (Digit Vigilance Test total time; the Wechsler Abbreviated Scale of Intelligence (WASI) block design, matrix reasoning, and vocabulary total raw scores; and the D-KEFS trails condition 4 [number-letter switching] time to complete).
Demographic, Neuropsychological Performance, and Neuroimaging Model
After including the neuroimaging data (cortical thickness and BOLD response for each of the 68 brain regions; see Table 3), 34 of the 203 total variables were identified as important (see Table 3 and Figure 2).
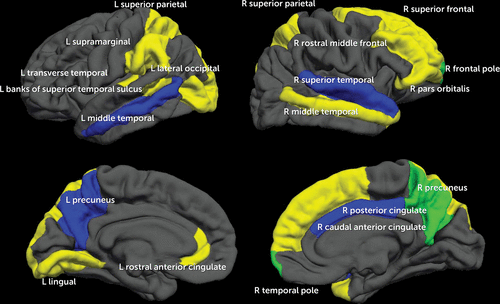
FIGURE 2. Twenty Brain Regions That Predicted Alcohol Initiation by Approximately Age 18 in a Study of Neural Predictors of Alcohol Use Initiation During Adolescencea
a Yellow indicates cortical thickness regions identified as important in model 3. Blue indicates blood-oxygen-level-dependent (BOLD) response regions included in the final model. Green (overlapping of yellow and blue regions) indicates cortical thickness and BOLD response in the same brain region. With regard to neuroimaging data, thinner cortices (in 13 of the 15 regions) and less BOLD response contrast (in all seven regions) predicted initiation of moderate to heavy drinking by approximately age 18.
Further Investigation of Model 3
The precise contribution of each variable to the outcome prediction is complex, owing to the high-order interactions critical to the success of random forests. However, main effects can be investigated straightforwardly. As shown in Table 4, alcohol initiators had less brain activation contrast between supra- and subspan conditions than continuous nonusing control participants in all seven brain regions and had thinner cortices in 13 of the 15 brain regions in the final model. The lingual gyrus and lateral occipital gyrus were thicker in future alcohol initiators. Neuropsychological test variables that contributed to predicting future initiation of drinking included faster Digit Vigilance Test time and poorer performance on the WASI block design and matrix reasoning tests. Demographic predictors of initiating alcohol use included being male, having a higher socioeconomic status, starting to date at an earlier age (by age 14), having a greater endorsement of conduct disorder–related behaviors, having higher positive alcohol expectancies (i.e., higher AEQ global positive, social behavior change, and total scores), and having more motion during the fMRI task. Table 4 lists the variables in the third model, in order of importance, and indicates which variables were statistically different between continuous nonusers and moderate to heavy alcohol initiators. Notably, while each variable by itself may not differentiate continuous controls from drinkers (as shown by the p values in Table 4), selected variables can contribute to prediction via interaction effects, supporting the importance of using statistical techniques such as random forests that can model these complex, high-order interaction terms.
| Measure | Continuous Nonusers (N=67) | Moderate to Heavy Alcohol Initiators (N=70) | p | Variable Importance | Cohen’s d | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | % | Mean | SD | % | ||||
| 1. L supramarginal cortical thickness | 2.96 | 0.14 | 2.89 | 0.13 | 0.002 | 10.01 | 0.518 | ||
| 2. Sex (female)b | 60 | 29 | >0.001 | 9.99 | 0.661 | ||||
| 3. R posterior cingulate BOLD | –0.63 | 3.90 | –2.19 | 3.60 | 0.016 | 8.84 | 0.416 | ||
| 4. R superior temporal BOLD | –1.17 | 3.60 | –1.89 | 2.83 | 0.194 | 8.45 | 0.222 | ||
| 5. Dating at baselineb | 22 | 61 | >0.001 | 8.08 | 0.860 | ||||
| 6. Socioeconomic status | 24.03 | 16.75 | 21.11 | 11.50 | 0.235 | 7.44 | 0.203 | ||
| 7. L transverse temporal cortical thickness | 2.78 | 0.21 | 2.68 | 0.21 | 0.006 | 7.37 | 0.476 | ||
| 8. R pars orbitalis cortical thickness | 3.02 | 0.22 | 2.92 | 0.19 | 0.005 | 7.28 | 0.487 | ||
| 9. WASI matrix reasoning raw score | 27.80 | 3.62 | 26.41 | 3.34 | 0.023 | 7.11 | 0.399 | ||
| 10. WASI block design raw score | 47.27 | 13.81 | 42.86 | 13.13 | 0.060 | 6.43 | 0.327 | ||
| 11. R rostral middle frontal cortical thickness | 2.53 | 0.10 | 2.49 | 0.11 | 0.058 | 6.06 | 0.381 | ||
| 12. L middle temporal BOLD | –1.11 | 2.56 | –1.62 | 2.47 | 0.238 | 5.83 | 0.203 | ||
| 13. R superior parietal cortical thickness | 2.60 | 0.12 | 2.55 | 0.10 | 0.016 | 5.76 | 0.453 | ||
| 14. L lingual cortical thickness | 2.26 | 0.16 | 2.28 | 0.13 | 0.367 | 5.62 | 0.137 | ||
| 15. R precuneus cortical thickness | 2.82 | 0.11 | 2.78 | 0.11 | 0.049 | 5.42 | 0.364 | ||
| 16. R caudal anterior cingulate BOLD | 2.40 | 4.90 | 0.43 | 4.66 | 0.017 | 5.24 | 0.412 | ||
| 17. AEQ social behavior change total scoreb | 36.92 | 7.41 | 41.23 | 7.94 | 0.001 | 5.23 | 0.561 | ||
| 18. R temporal pole cortical thickness | 3.88 | 0.30 | 3.76 | 0.37 | 0.041 | 5.18 | 0.356 | ||
| 19. R precuneus BOLD | –1.46 | 5.64 | –3.06 | 4.90 | 0.078 | 4.81 | 0.303 | ||
| 20. Repetitions excluded from functional magnetic resonance imaging series due to motion | 4.46 | 6.77 | 7.03 | 8.82 | 0.060 | 4.44 | 0.327 | ||
| 21. L lateral occipital cortical thickness | 2.45 | 0.16 | 2.46 | 0.14 | 0.774 | 4.41 | 0.067 | ||
| 22. Conduct Disorder Questionnaire total score | 0.45 | 1.08 | 1.17 | 1.88 | 0.007 | 4.04 | 0.470 | ||
| 23. R frontal pole BOLD | 2.02 | 4.74 | 0.50 | 7.54 | 0.161 | 3.91 | 0.241 | ||
| 24. R frontal pole cortical thickness | 3.10 | 0.28 | 2.99 | 0.33 | 0.038 | 3.80 | 0.360 | ||
| 25. L rostral anterior cingulate cortical thickness | 3.20 | 0.30 | 3.12 | 0.26 | 0.107 | 3.71 | 0.285 | ||
| 26. AEQ total score | 79.14 | 32.23 | 92.45 | 29.75 | 0.013 | 2.88 | 0.429 | ||
| 27. R middle temporal cortical thickness | 3.26 | 0.14 | 3.22 | 0.18 | 0.192 | 2.85 | 0.248 | ||
| 28. L banks superior temporal sulcus cortical thickness | 2.85 | 0.19 | 2.80 | 0.16 | 0.104 | 2.67 | 0.285 | ||
| 29. L precuneus BOLD | –2.03 | 5.85 | –3.46 | 5.17 | 0.130 | 2.63 | 0.260 | ||
| 30. L superior parietal cortical thickness | 2.60 | 0.12 | 2.5 | 0.11 | 0.015 | 2.55 | 0.869 | ||
| 31. AEQ global positive total score | 37.81 | 8.37 | 40.83 | 9.53 | 0.051 | 2.42 | 0.336 | ||
| 32. Digit vigilance completion time (seconds) | 225.06 | 52.80 | 219.59 | 41.46 | 0.504 | 2.36 | 0.115 | ||
| 33. Right superior frontal cortical thickness | 3.05 | 0.15 | 3.01 | 0.13 | 0.076 | 2.19 | 0.285 | ||
| 34. Follow-up age | 18.20 | 0.66 | 18.20 | 0.57 | 0.943 | 1.22 | 0.000 | ||
TABLE 4. Variables, in Order of Importance, in Model 3 (Including Demographic, Neuropsychological, and Structural and Functional Neuroimaging Data) in a Study of Neural Predictors of Alcohol Use Initiation During Adolescencea
Discussion
This study aimed to address an important public health issue by identifying variables that can generate individual-level predictions of initiating alcohol use during adolescence. This multimodal variable identification will be crucial to inform ongoing longitudinal adolescent studies (47; see also https://addictionresearch.nih.gov/abcd-study), which will have sufficient power to provide predictive accuracy estimates. The findings show that a mix of demographic, neuropsychological, and brain imaging indices are important in predicting which 12- to 14-year-olds would initiate moderate to heavy alcohol use by approximately age 18.
Specifically, being male and coming from higher socioeconomic backgrounds appear to be risk factors for initiation of drinking by age 18. Dating by age 14, reporting more externalizing behaviors, and having more positive expectations about how alcohol would affect behaviors and cognitions (particularly in social settings) appear to be risk factors for adolescent-onset alcohol initiation. In terms of neuropsychological functioning, adolescents who showed poorer performance on executive function tests and were faster on sustained attention tests (perhaps indicating impulsivity) during early adolescence had higher rates of alcohol initiation, consistent with previous findings (48). The neuroimaging features of thinner cortices and less BOLD response contrast to a cognitive challenge by age 14 also contribute to risk of moderate to heavy drinking by age 18, consistent with previous findings (5, 8, 11). Interestingly, more head movement (yet still within the acceptable limits to be included in analyses) while in the scanner was selected as an important variable, perhaps representing a phenotypic marker of impulsivity. While current substance use is highly predictive of future use (11), baseline alcohol, cigarette, and marijuana use was not selected as an important variable in our model; however, this is not surprising given that our sample was almost completely substance naive at baseline (97% had never tried alcohol). These findings build on previous reports (11) and focus on predicting patterns of more frequent and intense alcohol use as opposed to initiation alone.
Five of the 10 most important predictors were MRI and fMRI variables, suggesting that the inclusion of neuroimaging improves the accuracy of predicting future alcohol use (see Table 4). Morphometry or activation of 20 diffusely distributed brain regions substantially contributed to alcohol initiation (see Figure 1). Cortical thickness and BOLD response regions did not overlap, except in the right precuneus and right frontal pole, similar to previous studies showing that structural and functional maturation tend to show distinct developmental trajectories during early adolescence (44). More “mature” neural functioning (i.e., thinner cortices and less BOLD response contrast) was related to greater rates of transitioning to substance use, which is consistent with previous findings (8–10, 49). This “pseudomaturity” in at-risk youths has also been observed in other behavioral studies, including a 33-year longitudinal study that found that more mature behavior during childhood (based on psychiatrist ratings) predicted greater nicotine dependence in adulthood (F.X. Castellanos, personal communication, 2015). Early maturation of neural features could be considered a vulnerability for youths, increasing the likelihood of engaging in sensation-seeking behaviors at an earlier age. Neurodevelopmentally precocious youths may have a greater tendency to initiate and escalate risk-taking behaviors (e.g., early dating, substance use), relative to peers. Longitudinal studies with three or more time points will be needed to elucidate the trajectory of youths with these different outcomes.
Consistent with epidemiological data, alcohol was the most commonly used substance in this sample (1). However, significant marijuana use was reported among the moderate to heavy alcohol initiators. We chose to focus specifically on alcohol initiation because only 15% of our overall sample (29% of alcohol initiators) endorsed more than 30 lifetime occasions of marijuana use, and most alcohol initiators (80%) used alcohol before trying marijuana. However, it is likely that the reported risk factors confer risk not only to use of alcohol but also to use of marijuana and other illicit substances, and potentially to additional risky behaviors. Larger studies and additional years of follow-up will indicate the extent to which these predictive features are replicated, are predictive of substance use or problem behavior more broadly, and, as participants age, are predictive of addiction.
Strengths of this study include its extensive neuropsychological and multimodal neuroimaging data and utilization of a robust machine learning technique to identify potential risk factors for adolescent alcohol use. Limitations of this study include the lack of an independent replication sample. Nevertheless, random forests is a robust statistical technique that has been suggested to be superior to other machine learning techniques (21), and it includes bootstrapped cross-validation, with permutation importance determined based on an out-of-bag sample to reduce overfitting. Regardless, future work should seek to replicate these predictors. To this end, we are publishing the random forest script (see the online data supplement) that we used here so that other groups can try to replicate our findings using their own data sets. In random forest analyses, the contribution of each variable to the outcome prediction is complex given the high-order interactions critical to the success of this technique. While some group differences on individual variables are statistically nonsignificant or would not survive control for multiple comparisons (Table 4), each variable contributes significantly to the overall success of the model when allowed to interact with other variables. The group differences are presented to better understand the direction of the relationship. Genotyping was not included in this study. While previous findings suggest a nominal role of genetics in adolescent alcohol initiation compared with other personality and environmental factors (11), future studies should explore potential genetic risk factors associated with alcohol use and risk-taking generally. The participants in this sample came from a relatively high socioeconomic status, which may limit generalizability to low socioeconomic status youths; published scripts will allow for replication in more diverse samples (see the data supplement). A limitation inherent to fMRI is that the BOLD findings are task dependent and have sensitivity only to detect regions engaged by the task. Therefore, it is possible that functional activity in different regions would be predictive of future alcohol use if a different task were used. There is a large quantity and frequency range covered across the moderate to heavy alcohol initiator category, and predictors may vary across the severity of this continuum. While most alcohol users in this study were not drinking frequently, they tended to drink in large quantities (an average of >9 drinks on peak occasion in the past year), suggesting that we were capturing risky drinking behaviors in this group. Continued follow-up of this sample as some youths transition to alcohol use disorders will help clarify which predictors are most important in identifying problematic drinking.
The results provide evidence that interactions between multimodal neuroimaging data, neuropsychological testing, and demographic and clinical information can best predict future behaviors such as initiation of alcohol use. Understanding neurocognitive factors that predate substance use initiation is crucial to specifying the consequences of substance use on brain development, as well as identifying at-risk youths and potential targets of preventive efforts. The random forest script used in this study is now published to allow other groups to replicate findings, in the hope that a final, validated model can be used clinically to predict adolescent alcohol use.
1 : Monitoring the Future: National Survey Results on Drug Use: 1975–2014: Overview, Key Findings on Adolescent Drug Use. Ann Arbor, University of Michigan, Institute for Social Research, 2015Google Scholar
2 : Binge drinking and associated health risk behaviors among high school students. Pediatrics 2007; 119:76–85Crossref, Medline, Google Scholar
3 : The effect of alcohol use on human adolescent brain structures and systems. Handb Clin Neurol 2014; 125:501–510Crossref, Medline, Google Scholar
4 : Alcohol and drug use and the developing brain. Curr Psychiatry Rep 2016; 18:46Crossref, Medline, Google Scholar
5 : A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics 2008; 121(suppl 4):S290–S310Crossref, Medline, Google Scholar
6 : Inhibition during early adolescence predicts alcohol and marijuana use by late adolescence. Neuropsychology 2014; 28:782–790Crossref, Medline, Google Scholar
7 : Brain development in heavy-drinking adolescents. Am J Psychiatry 2015; 172:531–542Link, Google Scholar
8 : Brain response to working memory over three years of adolescence: influence of initiating heavy drinking. J Stud Alcohol Drugs 2012; 73:749–760Crossref, Medline, Google Scholar
9 : A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology (Berl) 2013; 230:663–671Crossref, Medline, Google Scholar
10 : Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug Alcohol Depend 2014; 141:51–57Crossref, Medline, Google Scholar
11 : Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature 2014; 512:185–189Crossref, Medline, Google Scholar
12 : Big data analytics and machine learning: 2015 and beyond. Lancet Psychiatry 2016; 3:13–15Crossref, Medline, Google Scholar
13 : Machine learning approaches for integrating clinical and imaging features in late-life depression classification and response prediction. Int J Geriatr Psychiatry 2015; 30:1056–1067Crossref, Medline, Google Scholar
14 : Identifying neuroanatomical signatures of anorexia nervosa: a multivariate machine learning approach. Psychol Med 2015; 45:2805–2812Crossref, Medline, Google Scholar
15 : Using structural MRI to identify individuals at genetic risk for bipolar disorders: a 2-cohort, machine learning study. J Psychiatry Neurosci 2015; 40:316–324Crossref, Medline, Google Scholar
16 : Machine learning algorithm accurately detects fMRI signature of vulnerability to major depression. Psychiatry Res 2015; 233:289–291Crossref, Medline, Google Scholar
17 : Application of machine learning algorithms for clinical predictive modeling: a data-mining approach in SCT. Bone Marrow Transplant 2014; 49:332–337Crossref, Medline, Google Scholar
18 : Machine learning classification of resting state functional connectivity predicts smoking status. Front Hum Neurosci 2014; 8:425Crossref, Medline, Google Scholar
19 : Dissecting psychiatric spectrum disorders by generative embedding. Neuroimage Clin 2013; 4:98–111Crossref, Medline, Google Scholar
20 : Random forests. Mach Learn 2001; 45:5–32Crossref, Google Scholar
21 : Evaluation of different biological data and computational classification methods for use in protein interaction prediction. Proteins 2006; 63:490–500Crossref, Medline, Google Scholar
22 : An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol Methods 2009; 14:323–348Crossref, Medline, Google Scholar
23 : Single-subject anxiety treatment outcome prediction using functional neuroimaging. Neuropsychopharmacology 2014; 39:1254–1261Crossref, Medline, Google Scholar
24 : Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav 2009; 23:715–722Crossref, Medline, Google Scholar
25 : Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol 1998; 59:427–438Crossref, Medline, Google Scholar
26 : Correlates of success following treatment for adolescent substance abuse. Appl Prev Psychol 1994; 3:61–73Crossref, Google Scholar
27 : Two-factor index of social position. New Haven, Conn, Yale University Press, 1965Google Scholar
28 : Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res 1995; 19:1018–1023Crossref, Medline, Google Scholar
29 : A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc 1988; 17:117–133Crossref, Medline, Google Scholar
30 : Manual for the ASEBA School-Age Forms & Profiles. Burlington, Vt, University of Vermont, Research Center for Children, Youth, & Families, 2001Google Scholar
31 : Conduct disorder among adolescent alcohol and drug abusers. J Stud Alcohol 1996; 57:314–324Crossref, Medline, Google Scholar
32 : Manual for the Beck Depression Inventory-2. San Antonio, Tex, Psychological Corporation, 1996Google Scholar
33 : Manual for the State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychologists Press, 1970Google Scholar
34 : The Alcohol Expectancy Questionnaire: an instrument for the assessment of adolescent and adult alcohol expectancies. J Stud Alcohol 1987; 48:483–491Crossref, Medline, Google Scholar
35 : Level of response to alcohol and brain response during visual working memory. J Stud Alcohol 2004; 65:692–700Crossref, Medline, Google Scholar
36 : The capacity of visual working memory for features and conjunctions. Nature 1997; 390:279–281Crossref, Medline, Google Scholar
37 : Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999; 9:179–194Crossref, Medline, Google Scholar
38 : Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999; 9:195–207Crossref, Medline, Google Scholar
39 : An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31:968–980Crossref, Medline, Google Scholar
40 : AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Crossref, Medline, Google Scholar
41 : Real-time 3D image registration for functional MRI. Magn Reson Med 1999; 42:1014–1018Crossref, Medline, Google Scholar
42 : Temporal dynamics of brain activation during a working memory task. Nature 1997; 386:604–608Crossref, Medline, Google Scholar
43 : SUMA. Neuroimage 2012; 62:768–773Crossref, Medline, Google Scholar
44 : BOLD response to working memory not related to cortical thickness during early adolescence. Brain Res 2013; 1537:59–68Crossref, Medline, Google Scholar
45 : Variable selection using random forests. Pattern Recognit Lett 2010; 31:2225–2236Crossref, Google Scholar
46 : Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci 2009; 12:535–540Crossref, Medline, Google Scholar
47 : The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): a multisite study of adolescent development and substance use. J Stud Alcohol Drugs 2015; 76:895–908Crossref, Medline, Google Scholar
48 : Weaknesses in executive functioning predict the initiating of adolescents’ alcohol use. Dev Cogn Neurosci 2015; 16:139–146Crossref, Medline, Google Scholar
49 : Brain volume reductions in adolescent heavy drinkers. Dev Cogn Neurosci 2014; 9:117–125Crossref, Medline, Google Scholar



