Synaptic Proteins in the Hippocampus Indicative of Increased Neuronal Activity in CA3 in Schizophrenia
Abstract
Objective:
In schizophrenia, hippocampal perfusion is increased and declarative memory function is degraded. Based on an a priori model of hippocampal dysfunction in schizophrenic psychosis, the authors postulated molecular and cellular changes in CA3 consistent with increased NMDA receptor signaling.
Method:
Postmortem hippocampal subfield tissue (CA3, CA1) from subjects with schizophrenia and nonpsychiatric comparison subjects was analyzed using Western blotting and Golgi histochemistry to examine the hypothesized outcomes.
Results:
The GluN2B-containing NMDA receptors (GluN2B/GluN1) and their associated postsynaptic membrane protein PSD95 were both increased in schizophrenia in CA3 tissue, but not in CA1 tissue. Quantitative analyses of Golgi-stained hippocampal neurons showed an increase in spine density on CA3 pyramidal cell apical dendrites (stratum radiatum) and an increase in the number of thorny excrescences.
Conclusions:
The hippocampal data are consistent with increased excitatory signaling in CA3 and/or with an elevation in silent synapses in CA3, a state that may contribute to an increase in long-term potentiation in CA3 with subsequent stimulation and “unsilencing.” These changes are plausibly associated with increased associational activity in CA3, with degraded declarative memory function, and with formation of false memories with psychotic content. The influence of these hyperactive hippocampal projections on targets in the limbic neocortex could contribute to components of schizophrenia manifestations in other cerebral regions.
The molecular and cellular underpinnings of psychosis in diseases like schizophrenia are unknown yet remain essential knowledge for rational treatment development. The hippocampus has been implicated in schizophrenia, and the repeated demonstration of changes in its structure and function have grown convincing. Studies show reduced hippocampal volume (1), abnormal in vivo function (2–4), and replicable molecular pathology (5) reliably across laboratories. Declarative memory, known to depend on the conjunctive memory function of the hippocampus, is one of the most consistently impaired cognitive functions in schizophrenia (6–9). In vivo biomarkers of hippocampal dysfunction in schizophrenia characteristically correlate with the psychotic symptomatology in individuals who are medication free (10, 11).
The study of hippocampal subfield function in schizophrenia has already proven generative (12). The subfields themselves (the dentate gyrus, the cornu ammonis [CA3, CA2, CA1], and the subiculum) have distinct and sequential functions in declarative memory formation (13) and are differentially affected in the illness (14). Excitatory projections connecting subfields have a low firing threshold, creating a unique hippocampal capacity for plasticity that advantages learning and memory (15) but that, under pathological circumstances, such as in psychosis, can be a liability. CA3 contains an extensive network of recurrent collateral connections that represent the anatomic substrate of conjunctive encoding and pattern completion processes and that create the basis for declarative memory performance (16) as well as an opportunity for pathological hyperassociation, as postulated in psychosis. In contrast, CA1 receives its strongest afferent stimulus from CA3 and shows a slower plasticity than CA3 for stabilizing place coding, while tuning multiple inputs dynamically from CA3 and the entorhinal cortex (17).
In this study, we examined CA3 tissue pathology in schizophrenia, contrasting the molecular changes in CA3 with those in CA1, postulating an increase in the specific molecular and cellular biomarkers of activity-dependent signaling in CA3. We previously articulated (18) a model of psychosis in schizophrenia based on evidence of increased neuronal excitability in the hippocampus and of a reduction in afferent stimulation to CA3 from the dentate gyrus, a state we postulated to be mediated by increased long-term potentiation in CA3 and to result in neuronal hyperactivity downstream. The characteristic molecular determinants of increased long-term potentiation are well described in basic laboratory studies as being increases in “immature” GluN2B-containing NMDA receptors and in PSD95 or SAP102, both accompanied by synaptic remodeling (increased spine number), representing synaptic strengthening (18). Specifically, based on this hippocampal psychosis model and testing the hypothesis of increased long-term potentiation in CA3, we postulated that GluN2B-containing NMDA receptors would be increased in CA3 in schizophrenia, with increased PSD95 protein, representing new synapses, along with anatomic evidence of spine proliferation, but that these would not be present in CA1, even though the increased neuronal activity generated in CA3 would be transmitted downstream to CA1. This hypothesis, if supported, would suggest increased long-term potentiation in CA3, supported molecularly and anatomically, that could plausibly be associated with hippocampal hyperactivity, mistakes of memory, and false memories with psychotic content in schizophrenia. These observations would support the concept of psychosis as a pathological alteration of hippocampal neuroplasticity resulting in alterations of normal learning and memory processes (19, 20).
Method
Postmortem Tissue
Brain tissue was collected by collaboration between the University of Texas Southwestern Medical Center (UTSW) Department of Psychiatry and the Dallas County Medical Examiner’s office with the UTSW Tissue Transplant Service; the cases and their characterization form the Dallas Brain Collection. Cases within 24 hours of death, with schizophrenia or healthy diagnoses, and without agonal duress or any other primary brain disorder diagnosis were collected, with permission from next of kin. A cohort of high-tissue-quality hippocampal cases with CA1 and CA3 enriched samples was created, including schizophrenia cases (N=21; 10 cases on antipsychotic medication at death and 11 off medication at death) and matched healthy comparison cases (N=21; none on CNS medications) (21). “Off medication at death” was confirmed by negative plasma and vitreous antipsychotic drug levels at autopsy and confirmed by family history of no recent medication use (estimated, within 2 weeks) and/or pharmacy records whenever available. Schizophrenia and healthy cases were matched based on RNA integrity number, pH, age, race, and sex (in that order) as closely as possible (see Table S1 in the data supplement that accompanies the online edition of this article); tissue from matched pairs was run together on Western blots with the within-pair identity masked until statistical analysis. Tissue methods and characteristics are detailed in the data supplement. We sought cases with a schizophrenia diagnosis, off medication, as well as in the more standard on-medication condition, in order to test disease (schizophrenia versus healthy) and medication (on- versus off-medication schizophrenia cases) effects of target protein changes in the schizophrenia and healthy cohorts.
Western Blotting
Proteins were analyzed using the usual protein blotting techniques (22). The methods are detailed in the data supplement.
Golgi Processing and Analysis
Blocks measuring approximately 1 cm2 on face and no more than 0.5 cm thick of medial temporal lobe containing the hippocampal formation were dissected from a new cohort of fresh brain tissue from seven schizophrenia and five comparison cases. Blocks were placed in 4% paraformaldehyde in phosphate-buffered saline for 3 hours and then transferred to a solution of rapid Golgi fixative (0.2% tetroxide and 2.33% potassium dichromate) at room temperature in the dark. The blocks were shipped to Yale University for further processing. After 5 days in rapid Golgi fixative, the blocks were washed several times with 0.75% silver nitrate and reacted in this same solution for 24–48 hours in the dark. The blocks were then dehydrated through increasing concentrations of ethanol, embedded in celloidin, and sectioned at 120 μm. Sections were mounted on slides with Permount, coverslipped, and air-dried on a flat surface. The slides were coded such that analysis was performed blind to diagnosis.
In each case, 10 neurons in the CA3 region of the hippocampus were selected for analysis of spine density on the apical dendritic trunk (stratum radiatum). All selected neurons had apical dendrites that extended at least 250 μm from the soma. Spine density was measured at three locations on the apical dendritic trunk: 50 μm proximal to peak density, peak density, and 50 μm distal to peak density, as described in detail in reference 23. The number of thorny excrescences, which are large outcroppings of the apical dendritic trunk thought to be the receptive zones for mossy fiber synapses, was counted along the entire length of the apical dendritic trunk. In addition, in five neurons in each case that had at least one complete basal dendritic branch, this branch was analyzed for spine density and dendritic length on branch orders 1–4 (stratum oriens). All analyses were performed on a Zeiss Axiophot microscope equipped with a Lucivid system for computer-aided analysis of dendritic morphology using Neurolucida, version 9.0 (MicroBrightfield, Williston, Vt.).
Statistical Analysis
Based on our hippocampal psychosis model and preliminary data, we hypothesized a priori that GluN2B/GluN1 would be increased in CA3, as well as concentrations of the related postsynaptic protein, PSD95. We tested the hypothesized outcomes in CA3 using paired and unpaired statistics and found the differences between groups significant in both contrasts; the outcomes of the paired statistics are presented in the text, given that the cases were not only matched by demographic characteristics but also run together on the same blots throughout the analyses. These same proteins were assessed in CA1 with no a priori hypothesis, to test the extent of any changes identified in CA3 in schizophrenia. Other proteins were tested in an exploratory fashion in CA3 and CA1 (GluN1, GluN2A, GluA1, GluA2, CREB, pCREB, SAP102, Rac1, ERK1/2, and cofilin) to detect any indication of the intracellular pathways that might be mediating changes in signaling, for future study. The comparison of each hypothesized CA3 protein between schizophrenia cases and their matched comparison cases was tested in paired two-tailed t tests with the significance threshold set at 0.05. The additional exploratory measures were analyzed with paired t tests and corrected for multiple comparisons using the Bonferroni correction, whenever significant by t test. Moreover, we contrasted the schizophrenia cases on and off antipsychotic medication to obtain any indication that the protein change might be associated with antipsychotic medication. Measures of dendritic spine density and length were also compared using two-tailed t tests, equal variances not assumed. To control for multiple comparisons in these anatomic analyses, we used a false-discovery-rate analysis with q set at 0.10 (24).
Results
Hippocampal CA3 Plasticity-Related Proteins in Schizophrenia
CA3 subfield tissue was blotted for the postulated proteins, with each schizophrenia case and its comparison case run on the same blot. The analyses showed increased concentrations of GluN2B-containing NMDA receptors (GluN2B/GluN1) in schizophrenia compared with matched comparison cases (t=2.56, df=20, p=0.018) (Figure 1A), with no difference in GluN1 in CA3 between schizophrenia and comparison cases (Figure 2A). Among schizophrenia cases, no differences were detected in GluN2B-containing NMDA receptors between those on and those off medication (Table 1), indicating that the detected increase in schizophrenia is not a medication effect. PSD95 in CA3 was increased in the schizophrenia cohort (t=2.53, df=20, p=0.02) (Figure 1B), and no differences were detected in PSD95 protein levels between schizophrenia cases on and off medication, again suggesting no influence of treatment on this protein (Table 1). No changes in GAD67 protein were detected in CA3 in the overall schizophrenia cohort (Figure 1C) or between schizophrenia cases on and off medication (Table 1).
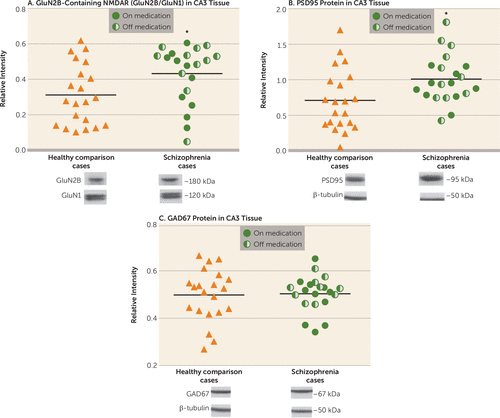
FIGURE 1. Hippocampal CA3 Plasticity-Related Proteins in Healthy Comparison Cases and Schizophrenia Casesa
a In panel A, GluN2B-containing NMDA receptors (GluN2B/GluN1) are significantly increased in the schizophrenia cases compared with healthy subjects in the whole tissue cohort (p=0.018). The horizontal bars represent the group averages. The GluN2B and GluN1 proteins are quantified by the ratio of protein immunoreactivity to β-tubulin immunoreactivity. In panel B, PSD95 protein is significantly increased in the schizophrenia cases compared with the healthy comparison cases in the whole tissue cohort (p=0.020). Protein is quantified by the ratio of PSD95 immunoreactivity to β-tubulin immunoreactivity. In panel C, GAD67 did not differ between the healthy subjects and the schizophrenia patients in the whole group (p=0.640). Protein is quantified by the ratio of GAD67 immunoreactivity to β-tubulin immunoreactivity.
*p<0.05.
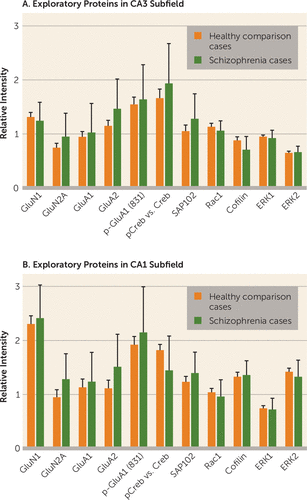
FIGURE 2. Exploratory Testing of Proteins in Hippocampal CA3 and CA1 Subfields in Healthy Comparison Cases and Schizophrenia Casesa
a Error bars represent group average with standard deviation. No protein was different between groups with Bonferroni correction for multiple testing. Proteins were quantified by the ratio of their immunoreactivity to β-tubulin immunoreactivity.
| On Medication (N=10) | Off Medication (N=11) | ||||
|---|---|---|---|---|---|
| Protein | Mean | SD | Mean | SD | p |
| GluN2B/GluN1 | 0.47 | 0.16 | 0.39 | 0.17 | 0.302 |
| PSD95 | 1.09 | 0.42 | 0.92 | 0.21 | 0.243 |
| GAD67 | 0.52 | 0.05 | 0.48 | 0.10 | 0.242 |
TABLE 1. Hippocampal CA3 Concentrations of the Hypothesized Proteins in Schizophrenia Cases On and Off Medicationa
Several other related proteins found within glutamatergic synapses were quantified in CA3 in an exploratory manner contrasting schizophrenia and comparison cases, including GluN2A, GluA1, GluA2, and synaptic remodeling proteins (Figure 2A). No differences were detected between schizophrenia and comparison cases in any of the key proteins related to glutamate receptor subunits, including GluN1, GluN2a, GluA1, and GluA2. In other signaling proteins, no differences between the schizophrenia and comparison cases could be detected in concentrations in CA3 tissue, including pCREB/CREB, SAP102, Rac1, ERK1/2, and cofilin.
Hippocampal CA1 Plasticity-Related Proteins in Schizophrenia
In order to establish whether these molecular changes in schizophrenia were confined to CA3, we examined the same proteins in CA1. No increase in the GluN2B-containing NMDA receptor (GluN2B/GluN1) protein could be detected in the overall schizophrenia cohort (Table 2), and GluN1 in CA1 did not differ between schizophrenia and comparison cases (Figure 2B). Neither were any differences detected in the GluN2B-containing NMDA receptors between the schizophrenia cases on and off medication. PSD95 in CA1 was higher in the overall schizophrenia cohort, but the difference fell short of significance (t=1.82, df=20, p=0.084) (Table 2); the effect was not maintained when matched cases were compared between schizophrenia cases off medication and comparison cases. No difference in GAD67 could be detected in CA1 between the overall schizophrenia cohort and matched comparison cases (Table 2) or between schizophrenia cases on and off medication.
| Comparison Cases (N=21) | Schizophrenia Cases (N=21) | Comparison Cases, Paired Subgroupb (N=11) | Schizophrenia Cases, Off Medication (N=11) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protein | Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | p |
| GluN2B/GluN1 | 0.56 | 0.35 | 0.65 | 0.41 | 0.34 | 0.64 | 0.37 | 0.64 | 0.28 | 0.99 |
| PSD95 | 1.46 | 1.06 | 2.05 | 0.75 | 0.084 | 1.75 | 1.24 | 1.91 | 0.73 | 0.72 |
| GAD67 | 0.86 | 0.19 | 0.83 | 0.20 | 0.70 | 0.88 | 0.18 | 0.79 | 0.28 | 0.31 |
TABLE 2. Hippocampal CA1 Concentrations of GluN2B-Containing NMDA Receptor, PSD95, and GAD Protein in Healthy Comparison Cases and Schizophrenia Casesa
The same group of postsynaptic candidate signaling proteins was tested in the CA1 tissue. In the CA1 subfield in comparison tissue compared with schizophrenia tissue, no differences could be detected in any of the key proteins related to glutamate receptor subunits, including GluA1, GluA2, and GluN1; neither were any between-group differences detected in other related signaling and structural proteins, including SAP102, Rac1, Cofilin, and ERK1/2 (Figure 2B).
Hippocampal Spine Analysis in CA3 in Schizophrenia
Based on the CA3-selective increases in known markers of synaptic strength, CA3 tissue from schizophrenia and comparison cases was collected and stained using a rapid Golgi method to determine whether the suggestive molecular changes in CA3 in schizophrenia were accompanied by morphologic changes in the number of excitatory synapses. Spine density was significantly elevated in the schizophrenia cases relative to comparison cases at the three locations (proximal, peak, and distal) measured on the apical dendritic trunk of CA3 pyramidal neurons in the stratum radiatum—specifically, “peak density” (t=4.656, df=8, p=0.001), “50 µm distal to peak” (t=3.506, df=10, p=0.006), and “50 µm proximal to peak” (t=2.951, df=10, p=0.015) were elevated in the schizophrenia tissue compared with the comparison tissue (Figures 3 and 4). The number of thorny excrescences was higher in the schizophrenia cases as well (t=2.976, df=9, p=0.016) (Table 3). Using the false-discovery-rate statistic to correct for multiple comparisons (q=0.10), all of the above contrasts remained significant. For comparison with the morphometric alterations observed on the apical dendrite (stratum radiatum), we assessed spine density and dendritic length on the basal dendrites of CA3 pyramids (stratum oriens) and found several anatomic measures possibly indicating increased synaptic strength unchanged between schizophrenia and comparison cases. Spine density on 1st- through 4th-order dendrites did not differ between groups, nor were differences in basal dendritic length detected at any branch order, or overall (Figure 3, Table 3).
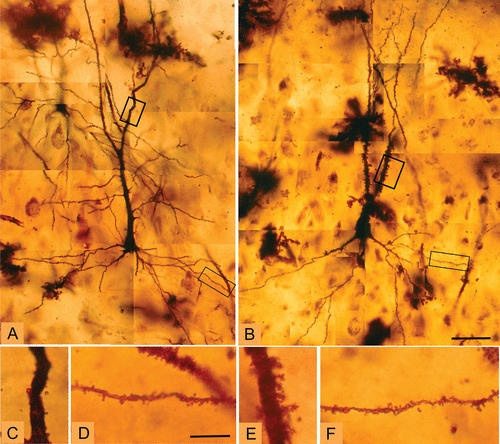
FIGURE 3. Montages of Representative CA3 Pyramidal Neurons From a Healthy Comparison Case and a Schizophrenia Casea
a Images A and B are from a healthy comparison case and from a schizophrenia case, respectively. These images further illustrate the increased spine density on the apical dendrite in schizophrenia. The boxes indicate the enlarged sections of apical and basal dendrites shown in the bottom row of images: images C and D are apical dendrite and basal dendrite, respectively, from a healthy comparison case; images E and F are apical dendrite and basal dendrite, respectively, from a schizophrenia case. Scale bars for images A and B, 50 μm; for images C–F, 10 μm.
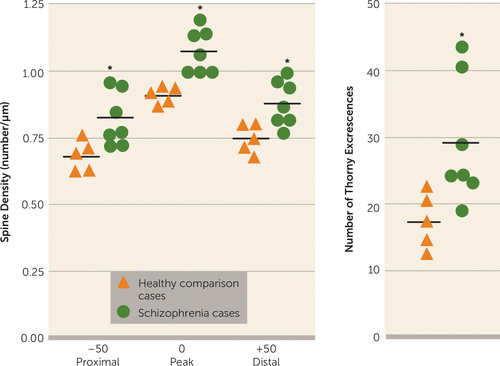
FIGURE 4. Spine Density Along the Apical Dendrite in CA3 in Healthy Comparison Cases and Schizophrenia Casesa
a Spine density along the apical dendrite (stratum radiatum) is significantly higher at three locations: the point of peak spine density and 50 μm distal and proximal to the peak. The number of thorny excrescences also shows a significant elevation in the schizophrenia cohort.
*p<0.01.
| Comparison Cases | Schizophrenia Cases, On Medication | ||||
|---|---|---|---|---|---|
| Structure | Mean | SD | Mean | SD | p |
| Spine density (spines/µm) | |||||
| Stratum radiatum | |||||
| Proximal | 0.68 | 0.06 | 0.82 | 0.10 | 0.015 |
| Peak | 0.91 | 0.03 | 1.07 | 0.08 | 0.001 |
| Distal | 0.74 | 0.05 | 0.88 | 0.08 | 0.006 |
| Number of thorny excrescences | 17.3 | 4.1 | 29.2 | 9.3 | 0.016 |
| Stratum oriens | |||||
| 1st order | 0.37 | 0.07 | 0.38 | 0.07 | 0.73 |
| 2nd order | 0.49 | 0.11 | 0.45 | 0.03 | 0.51 |
| 3rd order | 0.54 | 0.10 | 0.56 | 0.06 | 0.66 |
| 4th order | 0.56 | 0.09 | 0.61 | 0.08 | 0.30 |
| Dendritic length (µm) | |||||
| Stratum oriens | |||||
| 1st order | 29.3 | 9.5 | 28.5 | 8.4 | 0.88 |
| 2nd order | 96.0 | 26.2 | 90.9 | 26.7 | 0.75 |
| 3rd order | 163.5 | 47.3 | 172.3 | 73.5 | 0.81 |
| 4th order | 87.5 | 84.8 | 126.0 | 74.3 | 0.44 |
| Total length | 449.5 | 137.0 | 473.0 | 118.9 | 0.77 |
TABLE 3. Spine Density and Dendritic Length in Hippocampal CA3 in Healthy Comparison Cases and Schizophrenia Cases On Medicationa
Discussion
In this study, we contrasted tissue from healthy and schizophrenia cases on molecular and anatomic markers of synaptic plasticity within the CA3 hippocampal subfield, and produced evidence of altered plasticity in CA3 characterized by an increase in GluN2B-containing NMDA receptors and PSD95 protein and an increase in spine density. We did not observe changes in these cellular and molecular markers in CA1. The increases in GluN2B-containing receptors, the early developmental variant of the NMDA receptor, and in PSD95 protein occur without indication of AMPA receptor subunit alterations. The GluN2B/GluN1 increase in CA3 was present in the entire schizophrenia cohort, without any difference between cases on and off antipsychotic medication, indicating that this is a disease effect and not a chronic medication effect. These data are consistent with our previous report of increased BDNF mRNA in CA3 in schizophrenia, which also points to an increase in excitatory signaling in CA3 (20). The mossy fiber innervations in CA3 contact both the excitatory pyramidal neurons at thorny excrescences and inhibitory interneurons by way of en passant synapses, and these innervations have inverse effects on CA3 excitation (25). Thus, strengthened transmission at the pyramidal cell and reduced inhibitory control of local interneurons occur together, augmenting CA3 pyramidal cell excitation from two sites, advantaging feed-forward excitation (26). Uncontrolled feed-forward excitation may be the cerebral process that fuels hyperassociation, false memories, and psychotic mental events.
Golgi staining was used to examine the morphologic correlates of these molecular changes in pyramidal cell architecture in schizophrenia. Two distinct cellular regions of the CA3 pyramidal dendritic tree were examined: the proximal apical and basal dendrites corresponding to the CA3 stratum radiatum and the CA3 stratum oriens substrata, respectively. The anatomic changes show a clear increase in spine density limited to the stratum radiatum, at the apical trunk of pyramidal CA3 neurons, but not in the stratum oriens at the insertions of the recurrent collaterals. An increase was detected in the number of mossy fiber receptive sites, the thorny excrescences, in the stratum radiatum. The presence of greater spine density in the stratum radiatum is consistent with and could represent the morphologic manifestation of increased GluN2B-containing NMDA receptors in CA3 in schizophrenia, particularly as the GluN2B subunit advantages long-term potentiation (27, 28). Increased spine density in CA3 is also compatible with the molecular findings of elevated PSD95, as overexpression of this protein has been shown to elevate spine density in hippocampal cultures (29). Increased spine number is regularly observed following long-term potentiation-mediated increases in synaptic strength at excitatory synapses (30, 31). These changes are compatible with evidence from the electron microscopy study of Kolomeets et al. (32) showing that the specific number of mossy fiber/CA3 synapses is reduced, an effect that would lead to an increase in excitability and synaptic strength in CA3 itself through metaplasticity mechanisms. The specificity of morphometric abnormalities to sublayer components of the dendritic tree is intriguing and suggests that there may be a complex pattern of pathology in hippocampal subfields and their sublayers that could be related to afferent input patterning. Future studies may target morphometric features of the distal apical dendrites, which receive perforant path afferent input from the entorhinal cortex to further address sublayer specificity as well as extend the morphologic studies to substrata of the CA1 region.
These outcomes, including the increase in spines, an increase in PSD95, and the increase in GluN2B-containing NMDA receptors in CA3, are consistent with alterations in excitatory signaling in the hippocampus in CA3. This could represent an increase in neuronal excitability and long-term potentiation within CA3; moreover, we have previously suggested (18) this as a model of altered metaplasticity dynamics in CA3, based on reduced afferent stimulation from the dentate gyrus, elevated sensitivity, and increased direct stimulation from the entorhinal cortex. Alternatively, or in addition, there could be an increase in silent synapses in CA3, which would prime the synapse for an increase in experience-dependent plasticity with synaptic “unsilencing.” Silent synapses are identified in laboratory preparations as synapses that exhibit NMDA-receptor-mediated electrical responses but no evidence of AMPA-receptor-mediated transmission; silent synapses are important during development (33–35). Recent studies have identified the generation of silent synapses in the mature brain during salient in vivo experiences (36). It is thought that strong in vivo experiences—which, we suggest, might include acute psychotic experiences in schizophrenia—could selectively sensitize a neural circuit by generating silent synapses as a basis for robust increases in synaptic plasticity with relevant subsequent experience (36). This interpretation is consistent with the presentation of psychosis, as most individuals with schizophrenic psychosis retain a propensity to psychosis after an initial psychotic episode and many show a cyclic recurrence of psychotic manifestations. The alteration in CA3 plasticity conditions with an initial psychotic episode could create a vulnerability to new excitatory input within CA3 after a florid psychosis, which could generate “runaway” activity within the recurrent collateral projections that would diminish prediction error mechanisms, increase associations, and allow false memory formation with psychotic content. Both of these interpretations are consistent with emerging schizophrenia genetics, which converge not only on strong risk genes like NRG1 (which shows an altered NRG1 fragment in hippocampus [37]), DISC1, and CHRNA7 (38), but also on functional gene networks, including those modifying glutamate, synaptic function, and neuronal plasticity. The 15q13-14 locus, which is implicated in schizophrenia, includes the alpha-7 nicotinic receptor promoter (38); because cholinergic innervation is differentially directed to CA3 within the human hippocampus (39) and largely localized to CA3 interneurons (40), a loss of cholinergic capacity and subsequent failure of interneuronal inhibition could support CA3 hyperactivity. Also, a genetically manipulated mouse based on the 22q11.2 schizophrenia risk deletion shows an increase in hippocampal high-frequency synaptic transmission, increased long-term plasticity, and exaggerated dendritic spines (41), suggesting these as disease elements in schizophrenia.
The CA3 subfield changes in schizophrenia tissue described here may provide the molecular and cellular substrate supporting hippocampal hyperactivity in vivo that has been well described in schizophrenia patients—that is, hyperactivity represented by increased hippocampal cerebral blood flow (3, 42) and blood volume (12). These molecular alterations were not seen in CA1, suggesting that the in vivo hippocampal hyperactivity that has been reported in CA1 is propagated downstream from CA3 (12). The dynamic characteristics of NMDA receptor membrane insertion, removal, and translocation within neural synapses allow for their involvement in alterations of synaptic strength (43, 44), recognizing that the regulation of synaptic strength at the mossy fiber-CA3 pyramidal cell synapse is unique and complex (26, 33). This change could contribute to psychosis manifestations through pathological “pattern separation” (45) and/or “pattern completion” computations (46–48).
In this study, we examined NMDA receptor subunits only in hippocampal subfields. A large literature exists on these subunits in other schizophrenia-affected brain regions (49) and in whole hippocampus (50), prefrontal cortex (51), thalamus (52), cerebellum (53), and substantia nigra (54). Changes, especially in the prefrontal cortex, have been variable across studies (55–57). The relationship between the molecular changes reported here in hippocampal subfields and the neocortical anatomic and molecular neuropathology of schizophrenia reported elsewhere (58, 59) is not clear. These changes could be independent, correlated, or interdependent, a question important for future investigation. The coexistence of both hippocampal hyperactivity (plausibly associated with psychosis) and prefrontal cortical hypoactivity (plausibly associated with cognitive impairment) could be functionally linked if the hyperexcitatory efferents from the hippocampus to prefrontal cortical targets fell primarily onto the affected populations of cortical inhibitory interneurons, contributing to the dysregulation of GABA inhibitory control of prefrontal pyramidal neurons.
A classic hypothesis for hippocampal hyperactivity in schizophrenia has been disinhibition of hippocampal pyramidal cells by a reduction in the inhibitory GABA-mediated modulation of pyramidal cell firing (60). Cell-counting studies in the hippocampus contrasting schizophrenia and bipolar disorder patients with healthy subjects have reported metabolic (61) and molecular (2) alterations in bipolar disorder, largely sparing the hippocampus in schizophrenia, although previous studies found inhibitory deficits in the hippocampus in schizophrenia (62, 63). Yet, in our study, in neither CA3 nor CA1 tissue from schizophrenia cases did we detect reductions in GAD67 protein. Further research is clearly needed to examine subtle effects of changes in inhibitory GABA modulation in CA3.
In summary, these data show changes in schizophrenia in the GluN2B-containing NMDA receptor in CA3 of the hippocampus and not in CA1, along with consistent increases in PSD95 and in pyramidal cell spine number. These findings are consistent with increased neuronal excitability in CA3 and/or with an increase in silent synapses in CA3 in schizophrenia, conditions that could plausibly underlie manifestations of psychosis in the illness. The findings have implications for potential molecular markers in the illness, for novel medication targeting with further confirmation, and for use in modeling schizophrenia psychosis in animal paradigms.
1 : Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry 2005; 162:2233–2245Link, Google Scholar
2 : Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci 1998; 1:318–323Crossref, Medline, Google Scholar
3 : Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus 2001; 11:543–550Crossref, Medline, Google Scholar
4 : Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am J Psychiatry 2014; 171:549–556Link, Google Scholar
5 : The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004; 174:151–162Crossref, Medline, Google Scholar
6 : Creating a false memory in the hippocampus. Science 2013; 341:387–391Crossref, Medline, Google Scholar
7 : The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol Psychiatry 2008; 64:18–25Crossref, Medline, Google Scholar
8 : Transitive inference in schizophrenia: impairments in relational memory organization. Schizophr Res 2004; 68:235–247Crossref, Medline, Google Scholar
9 : Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science 1998; 281:1188–1191Crossref, Medline, Google Scholar
10 : Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry 2009; 66:938–946Crossref, Medline, Google Scholar
11 : Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology 2006; 31:221–230Crossref, Medline, Google Scholar
12 : Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 2013; 78:81–93Crossref, Medline, Google Scholar
13 : Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev 2003; 110:611–646Crossref, Medline, Google Scholar
14 : Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry 2004; 9:609–620, 544Crossref, Medline, Google Scholar
15 : Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 1995; 102:419–457Crossref, Medline, Google Scholar
16 : Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem 2007; 14:745–757Crossref, Medline, Google Scholar
17 : A single microcircuit with multiple functions: state dependent information processing in the hippocampus. Curr Opin Neurobiol 2012; 22:704–708Crossref, Medline, Google Scholar
18 : The hippocampal formation in schizophrenia. Am J Psychiatry 2010; 167:1178–1193Link, Google Scholar
19 : From prediction error to psychosis: ketamine as a pharmacological model of delusions. J Psychopharmacol 2007; 21:238–252Crossref, Medline, Google Scholar
20 : Glutamate dysfunction in hippocampus: relevance of dentate gyrus and CA3 signaling. Schizophr Bull 2012; 38:927–935Crossref, Medline, Google Scholar
21 : Human postmortem tissue: what quality markers matter? Brain Res 2006; 1123:1–11Crossref, Medline, Google Scholar
22 : The GABAB receptor as a target for antidepressant drug action. Br J Pharmacol 2011; 162:1–17Crossref, Medline, Google Scholar
23 : Amphetamine sensitization alters dendritic morphology in prefrontal cortical pyramidal neurons in the non-human primate. Neuropsychopharmacology 2007; 32:919–931Crossref, Medline, Google Scholar
24 : Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002; 15:870–878Crossref, Medline, Google Scholar
25 : Differential mechanisms of transmission and plasticity at mossy fiber synapses. Prog Brain Res 2008; 169:225–240Crossref, Medline, Google Scholar
26 : Interneuron diversity series: containing the detonation: feedforward inhibition in the CA3 hippocampus. Trends Neurosci 2003; 26:631–640Crossref, Medline, Google Scholar
27 : NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 2005; 48:289–301Crossref, Medline, Google Scholar
28 : NR2A−/− mice lack long-term potentiation but retain NMDA receptor and L-type Ca2+ channel-dependent long-term depression in the juvenile superior colliculus. J Neurosci 2007; 27:13649–13654Crossref, Medline, Google Scholar
29 : PSD-95 involvement in maturation of excitatory synapses. Science 2000; 290:1364–1368Crossref, Medline, Google Scholar
30 : Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 1999; 283:1923–1927Crossref, Medline, Google Scholar
31 : Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 1999; 399:66–70Crossref, Medline, Google Scholar
32 : Decreased numerical density of CA3 hippocampal mossy fiber synapses in schizophrenia. Synapse 2007; 61:615–621Crossref, Medline, Google Scholar
33 : Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci 2008; 9:813–825Crossref, Medline, Google Scholar
34 : Evidence for silent synapses: implications for the expression of LTP. Neuron 1995; 15:427–434Crossref, Medline, Google Scholar
35 : Silent synapses speak up. Neuron 1997; 19:473–476Crossref, Medline, Google Scholar
36 : In vivo cocaine experience generates silent synapses. Neuron 2009; 63:40–47Crossref, Medline, Google Scholar
37 : Differential neuregulin 1 cleavage in the prefrontal cortex and hippocampus in schizophrenia and bipolar disorder: preliminary findings. PLoS ONE 2012; 7:e36431Crossref, Medline, Google Scholar
38 : Association of the 5′-upstream regulatory region of the alpha7 nicotinic acetylcholine receptor subunit gene (CHRNA7) with schizophrenia. Schizophr Res 2009; 109:102–112Crossref, Medline, Google Scholar
39 : Cholinergic innervation in the human hippocampal formation including the entorhinal cortex. J Comp Neurol 1994; 345:321–344Crossref, Medline, Google Scholar
40 : Target-cell-dependent plasticity within the mossy fibre-CA3 circuit reveals compartmentalized regulation of presynaptic function at divergent release sites. J Physiol 2008; 586:1495–1502Crossref, Medline, Google Scholar
41 : The pattern of cortical dysfunction in a mouse model of a schizophrenia-related microdeletion. J Neurosci 2013; 33:14825–14839Crossref, Medline, Google Scholar
42 : Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry 2002; 59:521–529Crossref, Medline, Google Scholar
43 : NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 2007; 8:413–426Crossref, Medline, Google Scholar
44 : The cell biology of synaptic plasticity. Science 2011; 334:623–628Crossref, Medline, Google Scholar
45 : Pattern separation in the hippocampus. Trends Neurosci 2011; 34:515–525Crossref, Medline, Google Scholar
46 : Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem 2011; 18:15–18Crossref, Medline, Google Scholar
47 : Hippocampal activation during the recall of remote spatial memories in radial maze tasks. Neurobiol Learn Mem 2013; 106:324–333Crossref, Medline, Google Scholar
48 : A quantitative theory of the functions of the hippocampal CA3 network in memory. Front Cell Neurosci 2013; 7:98Crossref, Medline, Google Scholar
49 : Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Res 2007; 1127:108–118Crossref, Medline, Google Scholar
50 : Gene expression of PSD95 in prefrontal cortex and hippocampus in schizophrenia. Neuroreport 2000; 11:3133–3137Crossref, Medline, Google Scholar
51 : N-methyl-d-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. Am J Psychiatry 2001; 158:1400–1410Link, Google Scholar
52 : Up-regulation of NMDA receptor subunit and post-synaptic density protein expression in the thalamus of elderly patients with schizophrenia. J Neurochem 2006; 98:1114–1125Crossref, Medline, Google Scholar
53 : Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. Am J Psychiatry 2008; 165:1594–1603Link, Google Scholar
54 : Expression of the ionotropic glutamate receptor subunits and NMDA receptor-associated intracellular proteins in the substantia nigra in schizophrenia. Brain Res Mol Brain Res 2004; 121:60–69Crossref, Medline, Google Scholar
55 : Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry 2006; 11:737–747, 705Crossref, Medline, Google Scholar
56 : Cortical glutamatergic markers in schizophrenia. Neuropsychopharmacology 2005; 30:1521–1531Crossref, Medline, Google Scholar
57 : Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology 2008; 33:2175–2186Crossref, Medline, Google Scholar
58 : Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry 2012; 169:1082–1091Link, Google Scholar
59 : Perisomatic inhibition and cortical circuit dysfunction in schizophrenia. Curr Opin Neurobiol 2011; 21:866–872Crossref, Medline, Google Scholar
60 : Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci 2008; 31:234–242Crossref, Medline, Google Scholar
61 : Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry 2004; 61:300–308Crossref, Medline, Google Scholar
62 : Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA 2007; 104:10164–10169Crossref, Medline, Google Scholar
63 : Circuitry-based gene expression profiles in GABA cells of the trisynaptic pathway in schizophrenics versus bipolars. Proc Natl Acad Sci USA 2008; 105:20935–20940Crossref, Medline, Google Scholar



