Vitamin B12, Folate, and Homocysteine in Depression: The Rotterdam Study
Abstract
OBJECTIVE: The associations of vitamin B12, folate, and homocysteine with depression were examined in a population-based study. METHOD: The authors screened 3,884 elderly people for depressive symptoms. Subjects with positive screening results had psychiatric workups. Folate, vitamin B12, and homocysteine blood levels were compared in 278 persons with depressive symptoms, including 112 with depressive disorders, and 416 randomly selected reference subjects. Adjustments were made for age, gender, cardiovascular disease, and functional disability. RESULTS: Hyperhomocysteinemia, vitamin B12 deficiency, and to a lesser extent, folate deficiency were all related to depressive disorders. For folate deficiency and hyperhomocysteinemia, the association with depressive disorders was substantially reduced after adjustment for functional disability and cardiovascular disease, but for vitamin B12 this appeared independent. CONCLUSIONS: The association of vitamin B12 and folate with depressive disorders may have different underlying mechanisms. Vitamin B12 may be causally related to depression, whereas the relation with folate is due to physical comorbidity.
Folate and vitamin B12 are involved in the one-carbon metabolism necessary for the production of monoamine transmitters. Several case-control studies since the 1960s have shown high prevalences of folate and vitamin B12 deficiency in depression (1). More recently, the total plasma homocysteine level was shown to be a sensitive marker of folate and vitamin B12 deficiency, and higher concentrations of homocysteine were observed in depressed patients (2).
Thus far we know of only one population-based study; that study (3) confirmed that vitamin B12 deficiency was associated with depressive symptoms. However, folate deficiency and hyperhomocysteinemia were not related to depressive symptoms. The study was restricted to physically disabled women, and no clinical diagnosis of depression was made. In this study we examined the associations of folate, vitamin B12, and homocysteine with depression in community-dwelling elderly subjects in the Rotterdam Study who had a clinical diagnosis of depression.
Method
This study was conducted as part of the Rotterdam Study, a population-based study to which all inhabitants aged 55 years and over in a district of Rotterdam were invited in 1990–1993. In the third survey (1997–1999) we added assessments of depression to the study protocol. Of the 5,901 subjects who were invited, 4,730 participated in the home interview. Of these, 3,884 visited the research center and had overnight fasting blood samples drawn. Subjects were screened for depressive symptoms during the home interview with the Center for Epidemiology Studies Depression Scale (CES-D Scale) (4). This is a 20-item self-report measure of symptoms experienced in the last week and is scored on a scale of 0 to 3 points. Subsequently, subjects with positive results in the screening (a score of 16 or higher) had a psychiatric workup with the Present State Examination. Psychiatric disorders were classified according to DSM-IV criteria. Of the subjects who visited the research center and had blood taken, 278 (7.0%) had positive screening results, and 262 (94.2%) of these were given psychiatric workups. Of these subjects, 112 fulfilled the diagnostic criteria for depression, i.e., major or minor depression or dysthymia. The subjects with depressive symptoms and disorders were compared with 416 comparison subjects randomly selected from the subjects with negative screening results. The study was approved by the Medical Ethics Committee of Erasmus University School. After complete description of the study to the subjects, written informed consent was obtained.
Serum folate and vitamin B12 levels were measured by using an immunoassay. The total plasma homocysteine level was determined with high-performance liquid chromatography. To determine vitamin deficiency we used the following cutoffs, which correspond to the normal ranges of the assays and are described in the literature (3). Folate deficiency was considered present when the serum folate level was less than 11.4 nmol/liter and the homocysteine level was higher than 13.9 mmol/liter. Vitamin B12 deficiency was defined as a level less than 258 pmol/liter. We defined hyperhomocysteinemia as a plasma level above 15.0 mmol/liter.
The following variables were considered as confounders: age, gender, alcohol consumption, education, smoking, and cognitive function as measured by the Mini-Mental State Examination (MMSE). In additional analyses we also adjusted for functional status, blood pressure, and history of stroke and myocardial infarction. The latter were obtained through direct questioning and linkage with general practitioner records. Functional status was assessed with the Stanford Health Assessment Questionnaire (5). We used logistic regression to assess the associations of hyperhomocysteinemia, vitamin B12 deficiency, and folate deficiency with depressive disorders or symptoms. Furthermore, differences in mean levels were tested with analysis of variance. Finally, we analyzed the relation between self-reported loss of appetite and vitamin deficiencies. For this the subjects’ score on the CES-D Scale item “I did not have any appetite” was entered in an age- and gender-adjusted model.
Results
The mean age of the study sample was 72.9 years (SD=7.1), 16% were smokers, 11% had had a myocardial infarction, and 4% had had a stroke. The subjects with depressive symptoms were more likely to be female (73% versus 58%, χ2=18.2, df=1, p<0.001), had a higher age-adjusted functional disability score (mean=0.8, SD=0.6, versus mean=0.5, SD=0.4; F=47.7, df=1, 694, p<0.001), and had a lower MMSE score (mean=27, SD=2, versus mean=28, SD=3; F=22.2, df=1, 694, p<0.001) than the nondepressed subjects.
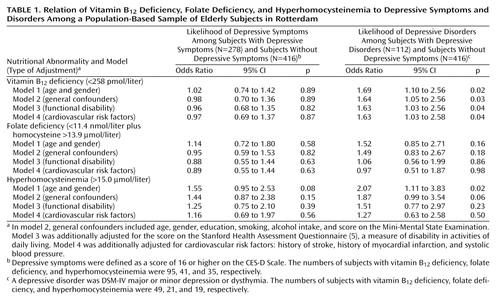
Among the 278 subjects with depressive symptoms and the 416 comparison subjects, hyperhomocysteinemia, vitamin B12 deficiency, and folate deficiency were not associated with the presence of depressive symptoms (Table 1). When we restricted the analysis to the 112 subjects fulfilling the DSM-IV criteria for depressive disorders, we found that subjects with vitamin B12 deficiency were nearly 70% more likely than the comparison subjects to have a depressive disorder (Table 1). This association was independent of cardiovascular factors and functional status. When adjusting for only age and gender, we also observed a significant association of hyperhomocysteinemia and a nonsignificant association of folate deficiency with depressive disorders. However, these associations disappeared after correction for cardiovascular factors and functional status.
No substantial differences in mean levels of vitamin B12 and folate were observed between the subjects with depressive disorders or symptoms and the reference subjects (data not shown). The subjects with depressive disorders had higher homocysteine levels (mean=11.7 μmol/liter, SD=5.9) than the reference subjects (mean=10.6, SD=3.7); the age- and gender-adjusted difference was 1.1 μmol/liter, and the 95% confidence interval (CI) was 0.3 to 2.0 (p=0.01). However, adjustment for cardiovascular risk indicators and functional disability strongly decreased this difference, to 0.2 μmol/liter (95% CI=–0.7 to 1.1, p=0.66).
Self-reported loss of appetite was not associated with vitamin B12deficiency (odds ratio=1.03 per 1-point increase in symptom score, 95% CI=0.86 to 1.24, p=0.73), whereas it was significantly related to hyperhomocysteinemia (odds ratio=1.32, 95% CI=1.03 to 1.70, p=0.03); the relationship to folate deficiency fell short of significance (odds ratio=1.24, 95% CI=0.98 to 1.58, p=0.07).
Discussion
This population-based study shows that elderly persons with vitamin B12 deficiency are more likely to have a depressive disorder. Furthermore, we observed a significant relationship of hyperhomocysteinemia and a nonsignificant relationship of folate deficiency to depression that were due to physical comorbidity and cardiovascular risk factors in subjects with depression.
Some methodological issues of the present study need to be considered. First, this was a cross-sectional study; it cannot demonstrate whether the observed association with vitamin deficiencies precedes or results from the depression. Most important, lack of appetite is a cardinal feature of depression and could explain the association. Indeed, we observed a relationship of self-reported loss of appetite with hyperhomocysteinemia and folate deficiency. However, no such relationship was found with vitamin B12 deficiency, and thus, appetite loss cannot account for the respective findings. Second, the prevalence of depressive symptoms (7.0%) was relatively low. However, it falls within the range (2.8% to 35%) reported in a review of the prevalence of depression among elderly people (6). A strength of this population-based study is the psychiatric workup of subjects who had positive screening results. Therefore, misclassification of disease is unlikely to have influenced our results.
The findings are consistent with the results of Penninx et al. (3), who reported that physically disabled women with vitamin B12 deficiency were twice as likely to have severe depressive symptoms. However, they observed no association between homocysteine or folate and depression. The present study strongly suggests that the higher rates of depressive disorders in subjects with low folate and high homocysteine levels are due to differences in cardiovascular factors and physical comorbidity. It is possible that earlier reports of a relation between folate deficiency and depression in psychiatric patients were confounded (1). The cardiovascular risk profile of the patients with depression might have been different from those of comparison subjects. On the other hand, it can be argued that adjustment for cardiovascular factors and functional disability is overcorrection. First, functional disabilities in daily activities, which include difficulties in shopping, may reduce the vitamin intake. It is plausible that depression develops thereafter as a consequence of low intake. Second, late-life depression might result from vascular diseases due to hyperhomocysteinemia. Adjustment for cardiovascular factors is appropriate only if one studies the direct effects of hyperhomocysteinemia that might be due to alterations in monoamine metabolism. In this case one should control for the fact that depression may also result from health impairment or neuronal damage in patients with vascular disease.
The effects of adjustment indicate that the associations of vitamin B12 and folate deficiency with depression have different underlying mechanisms. It has been reported (3) that serum folate is more sensitive to nutritional intake than vitamin B12. Moreover, in the present study, folate but not vitamin B12 deficiency was related to loss of appetite. In summary, this study suggests that clinicians need to be aware of possible hyperhomocysteinemia in combination with cardiovascular factors or functional disability in depressed patients. Furthermore, vitamin B12 deficiency may be causally related to depression in the elderly. In view of the possible benefits of vitamin replacement, detection of this subgroup is important.
 |
Received Jan. 22, 2002; revision received June 18, 2002; accepted June 26, 2002. From the Department of Epidemiology and Biostatistics and the Department of Psychiatry, Erasmus Medical Centre; and Numico Research, Wageningen, The Netherlands. Address reprint requests to Dr. Breteler, Department of Epidemiology and Biostatistics, Erasmus Medical Centre, P.O. Box 1738, 3000 DR Rotterdam, The Netherlands; [email protected] (e-mail). The Rotterdam Study is supported in part by the NESTOR program for geriatric research in The Netherlands (Ministry of Health and Ministry of Education), the Municipality of Rotterdam, the Netherlands Heart Foundation, and the Netherlands Organization for Scientific Research. This work has been supported by an unrestricted grant from Numico Research.
1. Alpert JE, Mischoulon D, Nierenberg AA, Fava M: Nutrition and depression: focus on folate. Nutrition 2000; 16:544-546Crossref, Medline, Google Scholar
2. Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH: Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry 2000; 69:228-232Crossref, Medline, Google Scholar
3. Penninx BWJH, Guralnik JM, Ferrucci L, Fried LP, Allen RH, Stabler SP: Vitamin B12 deficiency and depression in physically disabled older women: epidemiologic evidence from the Women’s Health and Aging Study. Am J Psychiatry 2000; 157:715-721Link, Google Scholar
4. Radloff LS: The CES-D Scale: a self-report depression scale for research in the general population. J Applied Psychol Measurement 1977; 1:385-401Crossref, Google Scholar
5. Fries JF, Spitz P, Kraines RG, Holman HR: Measurement of patient outcome in arthritis. Arthritis Rheum 1980; 23:137-145Crossref, Medline, Google Scholar
6. Beekman AT, Copeland JR, Prince MJ: Review of community prevalence of depression in later life. Br J Psychiatry 1999; 174:307-311Crossref, Medline, Google Scholar



