Sex Differences in Extrastriatal Dopamine D2-Like Receptors in the Human Brain
Abstract
OBJECTIVE: The study examined gender differences in extrastriatal dopamine D2-like receptor levels in the human brain in vivo. METHOD: [11C]FLB 457, a high-affinity radioligand for extrastriatal D2-like receptors, and a three-dimensional positron emission tomography system were used to measure D2-like receptor binding potentials in frontal cortex, temporal cortex, and thalamus in 12 healthy men and 12 healthy women. RESULTS: Women had higher D2-like receptor binding potentials than men in the three brain regions studied, and the difference in the frontal cortex was statistically significant. In a more detailed regional analysis, the difference between the sexes was most pronounced for the left and right anterior cingulate cortex. CONCLUSIONS: This study provides in vivo evidence for a gender difference in dopamine D2-like receptor levels, which could be reflected in gender-associated differences in clinical disorders linked to the dopamine system.
Numerous gender-associated structural and functional differences have been described for the normal human brain (1). Certain neuropsychiatric disorders with a known or proposed association with dopaminergic neurotransmission exhibit gender differences on a clinical level. Schizophrenia, major depression, Parkinson’s disease, Tourette’s syndrome, neuroleptic-induced parkinsonism, and tardive dyskinesia all show gender differences in their incidence, prevalence, clinical course, or treatment response (2–5). The gender differences in these disorders may reflect underlying biological differences in the dopamine systems of the brain. The proposed underlying differences could be disease related but could also represent gender differences of the normal brain.
Dopamine D2-like receptors are often a principal target for pharmacotherapy in the disorders of the dopamine system. D2-like receptors have a high density in the striatum, where they have been successfully quantified in vivo in humans with positron emission tomography (PET) and single photon emission tomography by using various radioligands (6). However, since the D2-like receptor density outside the striatum is much lower than in the striatum, there has been a lack of suitable ligands for imaging extrastriatal regions (7). [11C]FLB 457 is a new radioligand with high affinity for extrastriatal dopamine D2 and D3 receptor subtypes (7). In addition, the radioligand provides a signal that is sufficient for quantification of receptor binding in neocortical regions with minute densities of D2-like receptors (8).
In the study reported here, we used [11C]FLB 457 as the radioligand and a PET system in three-dimensional mode to determine whether there are gender differences in the binding potential of D2-like receptors in extrastriatal regions. Differences in extrastriatal D2-like receptors could reflect sexually dimorphic differences in mesocortical and mesolimbic dopamine systems.
Method
We examined 24 healthy subjects without any history or signs of neurologic or psychiatric disease (12 men, mean age=57.1 years, SD=12.5, range=36–72; and 12 women, mean age=55.0, SD=10.6, range=33–74, three of whom were under the age of natural menopause [9]) by using a GE Advance PET scanner (General Electric Medical Systems, Milwaukee) in high-sensitivity three-dimensional mode (10). We used [11C]FLB 457 (7, 11) as tracer, prepared essentially as described by Lundkvist et al. (12). The mean injected level of radioactivity and the mean amount of [11C]FLB 457 used were 224 MBq (SD=39) (6.05 mCi, SD=1.05) and 1.56 μg (SD=0.54) for men, and 218 MBq (SD=37) (5.89 mCi, SD=1.00) and 1.61 μg (SD=0.5) for women, respectively. All subjects gave their written informed consent after procedures had been fully explained.
The radioactivity was measured in a consecutive series of 16 frames (3 × 60 seconds, 4 × 180 seconds, 9 × 360 seconds) with a total duration of 69 minutes. To rule out structural lesions and to provide anatomical reference, we obtained a magnetic resonance imaging (MRI) brain scan for all subjects by using a 1.5-T Siemens Magnetom (Erlangen, Germany). With the MRI as reference (13), regions of interest were delineated for eight brain regions outside the striatum in each hemisphere. Cortical trace regions of interest (0.5–1 × 2–5 cm) were delineated on three to five consecutive MRI planes, and the surface extension of the Brodmann’s areas was guided by anatomical landmarks provided by an anatomical brain atlas (14). Regions of interest were copied from MRI planes to the corresponding PET planes. Regions of interest were not delineated for the striatum, since the duration of [11C]FLB 457 PET examination is too short to obtain equilibrium conditions and a reliable determination of the density of D2-like receptors in this region (11). The binding potential (Bmax/Kd) was calculated by using the cerebellum as a reference tissue in the full reference tissue model developed for analysis of reversible radioligand binding to receptors. This method has recently been cross-validated for [11C]FLB 457 (8) with good reproducibility (15). For the statistical analysis, the regions were grouped into three major areas: the frontal cortex, the temporal cortex, and the thalamus. The frontal cortex included the anterior cingulate (Brodmann’s areas 24 and 32), the prefrontal (areas 9 and 10) and the dorsolateral prefrontal cortices (area 46). The temporal cortex included the medial and lateral temporal cortices, and the thalamus included the medial and lateral thalamus. The mean volumes of the three combined regions for all subjects were: 6840 mm3 (SD=1200) for the frontal cortex, 5000 mm3 (SD=980) for the temporal cortex, and 2190 mm3 (SD=350) for the thalamus.
The binding potentials in men and women were compared by using one-way analysis of variance, with age and volume of the region of interest as covariates. To control for the effect of three comparisons, Bonferroni corrections were used. Subregions were studied if the result in the Bonferroni-corrected test was significant. Differences in the mean level of corresponding measurements on opposite hemispheres were tested by using a matched pairs t test. P values less than 0.05 were interpreted as statistically significant.
Results
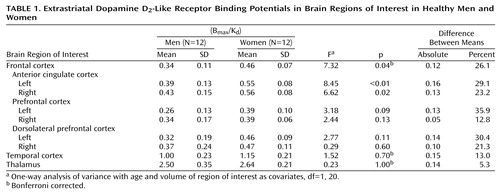
The binding potentials in the three major brain regions we studied were higher in women than in men. As Table 1 shows, the difference in the frontal cortex was statistically significant. The differences in the temporal cortex and in the thalamus were not significant. When the subregions of the frontal cortex were studied, women had significantly higher values in two regions: the left anterior cingulate cortex and the right anterior cingulate cortex. No significant interhemispheric differences were detected in men or women.
Discussion
The study results indicate a general gender difference in dopamine D2-like receptor binding potentials in brain regions outside the striatum, particularly in the frontal cortex. Since an MRI reference was used, structural degeneration, which has been reported to be more prominent in men than in women (16), was controlled for and is not a likely explanation for the difference seen in D2-like receptor binding potentials. A difference in binding potential may represent either a change in receptor density or affinity (or both). In monkeys, [11C]FLB 457 binding to D2-like receptors in extrastriatal regions seems to be sensitive to changes in the concentration of endogenous dopamine (17).
In earlier PET studies that used the radioligand raclopride and focused on the striatal receptors, no gender differences were seen in D2-like receptor binding potentials or densities (18, 19). One study reported a gender difference in the affinity of the D2-like receptors in the left striatum, with a generally lower D2-like receptor affinity in women than in men (19). Notably, a gender difference in the striatal D2-like receptor affinity has been seen only in older age groups (18, 19). One interpretation is that sex steroid changes can influence the dopamine-binding characteristics of the brain. In the rat brain, estradiol treatment increases D2-like receptor density in the striatum (2). However, human PET studies investigating a possible variation in striatal D2-like receptors that is dependent on the menstrual cycle have produced controversial results (20, 21). In the study reported here, only three of the 12 female subjects were under the mean age of natural menopause. On the basis of animal studies, a decline of estrogen in menopause would produce a decrease in extrastriatal D2-like receptor density rather than a high level, which was seen in the present study. It is thus not likely that the present finding is a phenomenon linked solely to menopause. Further studies are underway to investigate whether the demonstrated gender difference is present at all ages.
Epidemiological clinical findings that remain unexplained are the three to four times higher incidence of major depression in women compared to men during midlife (age 45–55 years) (5), and the second peak of onset of schizophrenia around menopause in women (2). A gender difference in D2-like receptors, or in endogenous dopamine levels, could be related to gender differences in the vulnerability to certain neuropsychiatric disorders. In schizophrenia, a gender related D2-like receptor asymmetry has been demonstrated in some (22, 23) but not all studies (18), with higher binding in the left striatum in male patients only. Although our results indicate a gender difference in extrastriatal dopaminergic neurotransmission, it should be noted that the present study was carried out with healthy subjects, who normally show considerable variance in receptor density measured with PET (18). Any conclusions regarding pathology should therefore be drawn with caution.
 |
Received April 11, 2000; revision received Sept. 11, 2000; accepted Sept. 29, 2000. From the Departments of Neurology and Psychiatry, University of Turku and Turku PET Centre, Turku, Finland; and the Department of Clinical Neuroscience, Karolinska Hospital, Stockholm. Address reprint requests to Dr. Kaasinen, Department of Neurology, University of Turku, P.O. Box 52, FIN-20521 Turku, Finland; [email protected] (e-mail). Supported by the Finnish Parkinson Foundation and by the Päivikki and Sakari Sohlberg Foundation. The authors thank the staff of the Turku PET Centre.
1. de Courten-Myers GM: The human cerebral cortex: gender differences in structure and function. J Neuropathol Exp Neurol 1999; 58:217–226Crossref, Medline, Google Scholar
2. Di Paolo T: Modulation of brain dopamine transmission by sex steroids. Rev Neurosci 1994; 5:27–42Crossref, Medline, Google Scholar
3. Kuopio AM, Marttila RJ, Helenius H, Rinne UK: Changing epidemiology of Parkinson’s disease in southwestern Finland. Neurology 1999; 52:302–308Crossref, Medline, Google Scholar
4. Brown AS, Gershon S: Dopamine and depression. J Neural Transm Gen Sect 1993; 91:75–109Crossref, Medline, Google Scholar
5. Epperson CN, Wisner KL, Yamamoto B: Gonadal steroids in the treatment of mood disorders. Psychosom Med 1999; 61:676–697Crossref, Medline, Google Scholar
6. Farde L: Brain imaging of schizophrenia: the dopamine hypothesis. Schizophr Res 1997; 28:157–162Crossref, Medline, Google Scholar
7. Halldin C, Farde L, Högberg T, Mohell N, Hall H, Suhara T, Karlsson P, Nakashima Y, Swahn C-G: Carbon-11-FLB 457: a radioligand for extrastriatal D2 dopamine receptors. J Nucl Med 1995; 36:1275–1281Google Scholar
8. Olsson H, Halldin C, Swahn C-G, Farde L: Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab 1999; 19:1164–1173Google Scholar
9. Luoto R, Kaprio J, Uutela A: Age at natural menopause and sociodemographic status in Finland. Am J Epidemiol 1994; 139: 64–76Google Scholar
10. Lewellen TK, Kohlmyer SG, Miyaoka RS, Kaplan MS, Stearns CW, Schubert SF: Investigation of the performance of the General Electric ADVANCE positron emission tomograph in 3D mode. IEEE Trans Nucl Sci 1996; 43:2199–2206Google Scholar
11. Farde L, Suhara T, Nyberg S, Karlsson P, Nakashima Y, Hietala J, Halldin C: A PET- study of [11C]FLB 457 binding to extrastriatal D2-dopamine receptors in healthy subjects and antipsychotic drug-treated patients. Psychopharmacology (Berl) 1997; 133:396–404Crossref, Medline, Google Scholar
12. Lundkvist C, Sandell J, Någren K, Pike VW, Halldin C: Improved synthesis of the PET radioligands, [11C]FLB 457, [11C]MDL 100907 and [11C]β-CIT-FE, by the use of [11C]methyl triflate. J Labelled Compounds & Radiopharmaceuticals 1998; 41:545–556Crossref, Google Scholar
13. Kaasinen V, Någren K, Hietala J, Oikonen V, Vilkman H, Farde L, Halldin C, Rinne JO: Extrastriatal dopamine D2 and D3 receptors in early and advanced Parkinson’s disease. Neurology 2000; 54:1482–1487Google Scholar
14. Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical Publishers, 1988Google Scholar
15. Vilkman H, Kajander J, Någren K, Oikonen V, Syvälahti E, Hietala J: Measurement of extrastriatal D2-like receptor binding with [11C]FLB 457: a test-retest analysis. Eur J Nucl Med (in press)Google Scholar
16. Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE: Sex differences in aging of the human frontal and temporal lobes. J Neurosci 1994; 14:4748–4755Google Scholar
17. Chou Y-H, Halldin C, Farde L: Effect of amphetamine on extrastriatal D2 dopamine receptor binding in the primate brain: a PET study. Synapse 2000; 38:138–143Crossref, Medline, Google Scholar
18. Farde L, Hall H, Pauli S, Halldin C: Variability in D2-dopamine receptor density and affinity: a PET study with [11C]raclopride in man. Synapse 1995; 20:200–208Crossref, Medline, Google Scholar
19. Pohjalainen T, Rinne JO, Någren K, Syvälahti E, Hietala J: Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry 1998; 155:768–773Abstract, Google Scholar
20. Wong DF, Broussolle EP, Wand G, Villemagne V, Dannals RF, Links JM, Zacur HA, Harris J, Naidu S, Braestrup C, Wagner HN Jr, Gjedde A: In vivo measurement of dopamine receptors in human brain by positron emission tomography: age and sex differences. Ann NY Acad Sci 1988; 515:203–214Crossref, Medline, Google Scholar
21. Nordström AL, Olsson H, Halldin C: A PET study of D2 dopamine receptor density at different phases of the menstrual cycle. Psychiatry Res 1998; 83:1–6Crossref, Medline, Google Scholar
22. Acton PD, Pilowsky LS, Costa DC, Ell PJ: Multivariate cluster analysis of dynamic iodine-123 iodobenzamide SPET dopamine D2 receptor images in schizophrenia. Eur J Nucl Med 1997; 24:111–118Crossref, Medline, Google Scholar
23. Schröder J, Bubeck B, Silvestri S, Demisch S, Sauer H: Gender differences in D2 dopamine receptor binding in drug-naive patients with schizophrenia: an [123I]iodobentzamide single photon emission computed tomography study. Psychiatry Res 1997; 75:115–123Crossref, Medline, Google Scholar



