Predictors of Spontaneous and Systematically Assessed Suicidal Adverse Events in the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) Study
Abstract
Objective: The authors sought to identify predictors of self-harm adverse events in treatment-resistant, depressed adolescents during the first 12 weeks of treatment. Method: Depressed adolescents (N=334) who had not responded to a previous trial with an SSRI antidepressant were randomized to a switch to either another SSRI or venlafaxine, with or without cognitive behavior therapy. Self-harm events, i.e., suicidal and non-suicidal self-injury adverse events were assessed by spontaneous report for the first 181 participants, and by systematic weekly assessment for the last 153 participants. Results: Higher rates of suicidal (20.8% vs. 8.8%) and nonsuicidal self-injury (17.6% vs. 2.2%), but not serious adverse events (8.4% vs. 7.3%) were detected with systematic monitoring. Median time to a suicidal event was 3 weeks, predicted by high baseline suicidal ideation, family conflict, and drug and alcohol use. Median time to nonsuicidal self-injury was 2 weeks, predicted by previous history of nonsuicidal self-injury. While there were no main effects of treatment, venlafaxine treatment was associated with a higher rate of self-harm adverse events in those with higher suicidal ideation. Adjunctive use of benzodiazepines, while in a small number of participants (N=10) was associated with higher rate of both suicidal and nonsuicidal self-injury adverse events. Conclusions: Since predictors of suicidal adverse events also predict poor response to treatment, and many of these events occurred early in treatment, improving the speed of response to depression, by targeting of family conflict, suicidal ideation, and drug use may help to reduce their incidence. The relationship of venlafaxine and of benzodiazepines to self-harm events requires further study and clinical caution.
Depression is the single most significant psychiatric risk factor for adolescent suicidal behavior. While antidepressants have been shown to be efficacious in the treatment of adolescent depression, one potentially serious effect of their use is an increased risk for spontaneously reported suicidal events (1) . However, little is known about the predictors and clinical significance of these events, nor about the relationship of spontaneously reported events to those that are systematically assessed.
Contemporaneous with safety concerns, there has been both a decline in the prescription rate for antidepressants and a reversal of the decade-long decline in the adolescent suicide rate in the United States (2 , 3) . The identification of predictors of suicidal events in depressed patients could be helpful in providing informed consent, identifying those patients at highest risk, and for designing preventive interventions.
Predictors of suicidal events in treated, depressed samples include a past suicide attempt and high baseline levels of suicidal ideation, agitation, and anger (4 – 6) . However, none of the above-noted studies have simultaneously examined the effects of both demographic and clinical predictor variables, treatment, and their interactions. Moreover, aside from an unpublished Food and Drug Administration report that suggests a tendency toward an increased risk of nonsuicidal self-injury with antidepressant medication versus placebo, the occurrence of nonsuicidal self-injury has not been examined in pediatric clinical trials (7) .
Predictors of suicidal events, namely high levels of suicidal ideation or a recent suicide attempt are also common reasons for initiating antidepressant treatment in the community, and for excluding such patients from clinical trials (8) . In order to be informative to community practice, we report on the predictors of suicidal and nonsuicidal self-injury adverse events in the Treatment of SSRI-Resistant Adolescent Depression (TORDIA) study, in which nearly 60% of participants who entered this clinical trial had clinically significant suicidal ideation and over one-third had a previous history of nonsuicidal self-injury (9) . In TORDIA, depressed adolescents who did not respond to an adequate trial with an SSRI were randomly assigned, in a 2-by-2 balanced factorial design, to one of four groups: switch to another SSRI, switch to venlafaxine, switch to another SSRI plus cognitive behavior therapy (CBT), or switch to venlafaxine plus CBT. In the first 12 weeks, one-fifth of participants experienced a self-harm (either a suicidal or nonsuicidal self-injury) adverse event, but there were no differential treatment effects (9) .
In this article, we examine the predictors and moderators of treatment effects on the occurrence of suicidal and nonsuicidal self-injury adverse events. Moreover, we capitalize on a natural experiment. During the first half of the study, participants were monitored for self-harm adverse events by spontaneous report, similar to previous studies (1 , 10) . However, in response to concerns raised by the FDA in 2003–04 (11) , participants enrolled in the latter half of the recruitment period were monitored by systematic, proactive assessment of suicidal ideation and behavior and nonsuicidal self-injury, thus allowing for a comparison of the frequency and severity of suicidal events as assessed by spontaneous report and by systematic evaluation.
Method
Participants
Participants were adolescents ages 12–18 years with moderate to severe DSM-IV (12) major depressive disorder, despite being in active treatment with an SSRI of at least 8 weeks duration, the last four of which were at a dosage equivalent to at least 40 mg of fluoxetine. Specific severity entry criteria were a Child Depression Rating Scale-Revised (CDRS-R) (13) total score ≥40 and a Clinical Global Impression-Severity (CGI-S) Subscale ≥4 (at least moderate severity) (14) . Excluded were individuals with bipolar spectrum disorder, psychosis, pervasive developmental disorder or autism, eating disorders, substance abuse or dependence, or hypertension.
This study was reviewed by each site’s local institutional review board; all subjects gave informed assent (and consent after they turned age 18), and parents gave written informed consent in accordance with local institutional review board regulations.
Random Assignment
Subjects were randomly assigned to one of four conditions in a 2-by-2 factorial design: change to another SSRI, change to venlafaxine, change to another SSRI plus CBT, or change to venlafaxine plus CBT. Subjects in the SSRI switch cells who were initially treated with citalopram, sertraline, or fluvoxamine were randomly assigned to either fluoxetine or paroxetine; if they were initially treated with fluoxetine, they were switched to paroxetine and vice versa. Midway through the study (after 181 of 334 subjects had been enrolled), one of the treatment options in the SSRI cell was changed from paroxetine to citalopram owing to concerns about the efficacy and safety of paroxetine (11) . Subjects were assigned to treatment using a variation of Efron’s biased coin toss (15) , balancing both across and within sites with respect to incoming treatment medication, comorbid anxiety, chronic depression (duration ≥24 months), and BDI item 9 (suicidal ideation) ≥2.
Interventions
Medication taper
Subjects were tapered to discontinuation from their initial medication by decreasing dosages over a period of 2 weeks, except for those who entered the study on fluoxetine, for whom the medication was simply discontinued, due to fluoxetine’s long half-life.
Pharmacotherapy
Medication sessions were 30–60 minutes in duration, and included assessment of vital signs, side effects, safety, and symptomatic response. Subjects were seen weekly for the first 4 weeks and biweekly for the next 6 weeks. Medication dose was titrated to either 20 mg of fluoxetine, citalopram, or paroxetine, or 150 mg of venlafaxine, with an option at week 6 to increase to either 40 mg of an SSRI or 225 mg of venlafaxine if insufficient improvement was observed (CGI-I≥3). By 12 weeks, the average doses of study medication were 33.8 mg (95% CI: 32–35.6) for the SSRI condition, and 205.4 mg (95% CI: 199–211.7) for the venlafaxine condition.
Cognitive behavior therapy (CBT)
CBT drew upon the manuals that emphasized cognitive restructuring and behavior activation, emotion regulation, social skills and problem-solving, and parent-child sessions to improve support, decrease criticism, and improve family communication and problem-solving (9) . These different modules were flexibly applied on the basis of the clinical needs of the participant and family with a review of case formulations in a bi-weekly CBT conference call. The protocol called for up to 12 60–90 minute sessions of CBT, during the first 12 weeks, but on average, 8.3 (SD=3.6) sessions were delivered, with no differences between medication groups or across sites. Review of psychotherapy tapes showed a high level of adherence to the treatment model.
Adjunctive medications
Participants on stimulants were able to continue this regimen. Adjunctive treatments for sleep and anxiety were also allowed. These interventions occurred in a minority of subjects (4%–21.5%) and were evenly distributed across treatment cells.
Assessments
Diagnostic assessment and clinical assessment
Diagnostic information was obtained by the School Age Schedule for Affective Disorders and Schizophrenia for School-Aged Children-Present and Lifetime Version (K-SADS-PL) (16) at baseline. Interview-rated overall severity, functional impairment, and severity of depression were rated by the Clinical Global Improvement Severity Subscale (CGI-S) (14) , Child Global Assessment Scale (C-GAS) (17) , and the Child Depression Rating Scale-Revised (CDRS-R) (13) , respectively. Self-rated depression, hopelessness, and suicidal ideation were assessed using the Beck Depression Inventory (BDI) (18) , Beck Hopelessness Scale (19) , and Suicide Ideation Questionnaire—JR (20) , respectively. History of physical or sexual abuse was obtained from the trauma section of the K-SAD-PL. Adolescent- and parent-rated parent-child conflict were rated using the Conflict Behavior Questionnaire—Adolescent and Parent Reports (21) . Alcohol and drug use were rated using the self-report questionnaire, the Drug Use Screening Inventory (22) .
Primary outcome
The primary outcome for this report was the occurrence of a suicidal adverse events, defined as the onset of new, or worsening suicidal ideation, a suicidal threat, or a suicide attempt with the first 12 weeks of the trial. A suicide attempt was defined as “self-destructive behavior with at least implied intent to die (23) .” An additional secondary outcome, nonsuicidal self-injury, was assessed, defined as self-injurious behavior resulting in physical damage with no explicit or implicit intent to die; instead, the motivation for engaging in this behavior was to achieve relief from negative emotion or to obtain social reinforcement (23 , 24) . A “serious suicidal or nonsuicidal self-injury adverse event” was an event that led to hospitalization, was life-threatening, disabling, or resulted in death (25) . All suicidal and nonsuicidal self-injury adverse events were discussed on weekly conference calls with study investigators and classified by consensus. All clinical raters were blind to medication, but not CBT status. For the first 181 participants, suicidal events were based upon spontaneous report. For the last 153 subjects, subjects were monitored weekly by clinicians for suicidal ideation and suicidal behavior using the Brief Suicide Severity Rating Scale (23) . This scale consists of two items: 1) a rating of suicidal ideation ranging from 0 to 5 (no ideation to suicidal ideation with intent and a clear plan) and 2) a rating of suicidal behavior, ranging from 0 to 5 (no behavior to multiple attempts during the assessment period) using the Columbia Classification Algorithm of Suicide Assessment (23) . The Brief Suicide Severity Rating Scale was used to determine if a suicidal adverse event had occurred; we required a two-point change on either the ideation or behavior scale. Inter-rater reliability was assessed on 49 cases and found to be excellent for both ideation (ICC=0.9, p<0.001) and behavior (100% agreement).
Statistical Analyses
Parallel analyses were conducted for suicidal and for nonsuicidal self-injury events. The frequency, severity, and characteristics of participants who experienced at least one event under spontaneous reporting and systematic monitoring were assessed using standard univariate statistics. Times to event under the two methods of assessment were compared using Kaplan-Meier analyses. The most parsimonious set of predictors was selected using logistic regression. Each variable that was selected as a predictor of events was then added to a logistic regression that also included terms for medication and CBT treatment effects and all two way interactions, to learn if there were conditions under which treatments were more or less likely to result in an event. Similarly, predictors of time to event were assessed using the method of Cox proportional hazards. These procedures were repeated with the outcome being serious suicidal or nonsuicidal self-injury adverse events; because the two sets of findings were similar, only the results for adverse events are reported.
Results
Number and types of self-harm adverse events
There were 108 incidents of self-harm adverse events in 68 participants: 58 suicidal adverse events in 48 participants, and 50 nonsuicidal self-injury events in 31 participants, 11 of whom also had a suicidal adverse event. Of the 48 participants who experienced at least one suicidal adverse event, 17 made suicide attempts (asphyxiation [N=3], cutting or stabbing [N=6], or overdose [N=8]), but none completed suicide. There were 50 nonsuicidal adverse events in 31 participants. The most common methods were cutting (N=28), scratching (N=1), both (N=4), and burning (N=2). There were 26 serious self-harm adverse events, of which 24 were suicidal adverse events (92.3%).
Rate of Events Before and After Systematic Monitoring
More suicidal (20.9% versus 8.8%, χ 2 =9.18, df=1, p=0.002) and nonsuicidal self-injury adverse events (17.6% versus 2.2%, χ 2 =23.47, df=1, p<0.001) were detected after systematic monitoring, with no difference in the rate of serious suicidal or nonsuicidal self-injury adverse events (8.4% versus 7.3%, χ 2 =0.03, df=1, p=0.87). Suicide attempts tended to be more frequent with systematic monitoring (6.5% versus 3.9%, χ 2 =1.22, df=1, p=0.27). Time to onset for either type of event was earlier under conditions of systematic monitoring (median time 2 versus 5 weeks, χ 2 =8.41, df=1, p=0.004).
Characteristics of Those With Suicidal and Non-Suicidal Self-Injury Adverse Events
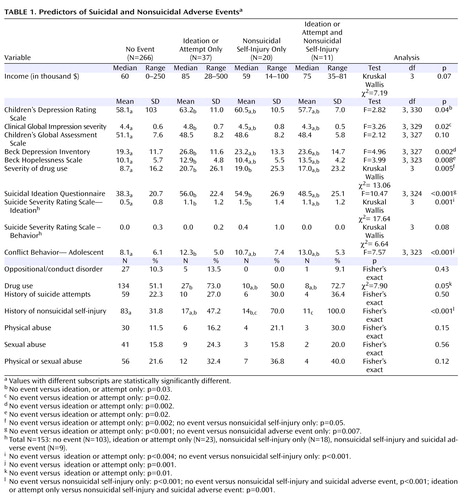
Baseline characteristics of those with no event, suicidal adverse events only, nonsuicidal self-injury only, or both were compared. There were overall differences among these four groups for most measures of clinical or symptomatic severity, adolescent reported family conflict, and history of nonsuicidal self-injury (see Table 1 ). Pairwise contrasts showed significant differences between those with suicidal adverse events without nonsuicidal self-injury compared to those without events for severity of interview-rated and self-rated depression, hopelessness, drug use, suicidal ideation, and family conflict. The two groups with nonsuicidal self-injury events were each more likely to have had a history of nonsuicidal self-injury, compared to those with no events. Also, those with nonsuicidal self-injury only compared to those with no events had higher suicidal ideation (54.9 [26.9] versus 38.3 (20.7), t=2.64 , df=19.6, p=0.02) and greater impairment from drug and alcohol use (19.0 [25.3] versus 8.7 [16.2], Mann-Whitney U=2017, p=0.05).
Baseline Predictors of Occurrence and Time to Suicidal Events
The median time to a suicidal adverse event was 3 weeks (interquartile range=5 weeks), whereas the median time to a suicidal serious suicidal or nonsuicidal self-injury adverse event was 5 weeks (interquartile range=5 weeks). Those who experienced multiple events were not different from those with a single event. Logistic regression identified self-rated suicidal ideation (odds ratio=1.02, 95% CI=1.01–1.04), family conflict (odds ratio=1.1, 95% CI=1.03–1.16), and drug or alcohol use (odds ratio=1.9, 95% CI=0.9–3.9) as the best predictors of suicidal adverse events study (Hosmer-Lemeshow χ 2 =11.75, df=8, p=0.16), with similar predictors for time to suicidal events using a backward stepwise Cox regression: suicidal ideation (z=2.99, p=0.003), family conflict (z=2.91, p=0.004), and drug and alcohol use (z=1.87, p=0.06).
Baseline Predictors of Onset and Time to Nonsuicial Self-Injury Events
Logistic regression identified a history of nonsuicidal self-injury (odds ratio=9.6, 95% CI=3.5-26.1) as the best single predictor of nonsuicidal self-injury events. The median time to a nonsuicidal self-injury event was 2 weeks (interquartile range=4 weeks), with the best predictor of time to event being a history of nonsuicidal self-injury (z=4.40, p<0.001).
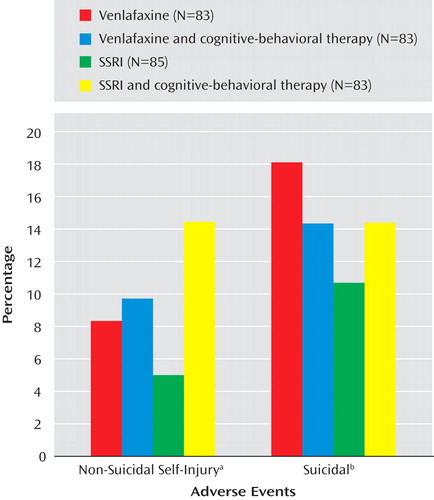
Impact of Treatment on Suicidal and Nonsuicidal Adverse Events
There were no statistically significant treatment effects with regard to the occurrence of suicidal or nonsuicidal self-injury events adverse events (see Figure 1 ). There were no treatment effects for time to suicidal adverse events, but receipt of CBT was related to earlier onset of an nonsuicidal event (hazard ratio=3.3, z=2.05, p=0.04). (See Figure 2 ). After controlling for a history of nonsuicidal event (hazard ratio=7.7, z=4.5, p<0.001), the relationship between CBT and time to nonsuicidal event was no longer significant (hazard ratio=2.0, z=1.81, p=0.07).
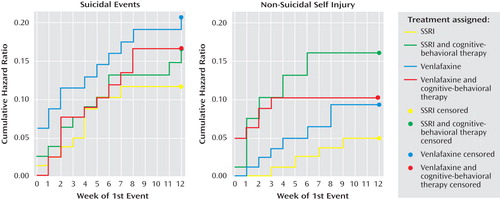
a χ 2 =4.84, df=3, p=0.18.
b χ 2 =1.91, df=3, p=0.59
Only one of the above-noted baseline predictor variables was found to moderate treatment effects with respect to onset or time to either suicidal or nonsuicidal event adverse events. Cox regression, with all main effects and two-way interactions in the model showed a significant interaction between medication and suicidal ideation (z=3.10, p=0.002) with respect to occurrence of any self-harm adverse event, meaning that participants with higher than median baseline suicidal ideation (Suicide Ideation Questionnaire score ≥35) were more likely to experience a self-harm event if they were treated with venlafaxine than with an SSRI (37.2% versus 23.3%, χ 2 =3.83, df=1, p=0.05).
Relationship of Treatment Course, Treatment Response, and Occurrence of Suicidal Events
Participants with a suicidal adverse event had a lower rate of treatment completion (41.7% versus 73.8%, χ 2 =19.87, df=1, p<0.001), treatment response (defined as CGI improvement rating ≤2 and ≥50% decrease in CDRS-R from baseline; 27.1% versus 51.0%, χ 2 =9.46, df=1, p=0.002), and a tendency toward lower attendance at pharmacotherapy sessions (7.3 [2.3] versus 7.9 [2.2], t=1.87, df=332, p=0.06), but did not attend fewer CBT sessions (7.7 [3.4] versus 8.4 [3.6], t=0.93, df=164, p=0.35). There was no relationship between experience of a nonsuicidal event and rate of treatment completion rate (58.1% versus 70.3%, χ 2 =1.97, df=1, p=0.16), or with treatment response (35.5% versus 48.8%, χ 2 =2.01, df=1, p=0.16), number of pharmacotherapy (7.8 [1.8] versus 7.8 [2.3], t=0.48, df=332, p=0.96) or CBT sessions attended (7.8 [3.2] versus 8.4 [3.6], t=0.66, df=164, p=0.51).
The trajectory of those who experienced a serious suicidal adverse event, a “non-serious” suicidal adverse event, and those who never experienced any suicidal events with respect to course on the Suicide Ideation Questionnaire and CDRS-R showed significant effects for time, event status (highest baseline symptoms associated with serious suicidal or nonsuicidal self-injury adverse event’s), but no event by time interaction, with similar findings for those with and without nonsuicidal adverse events (see Figure 3 ).
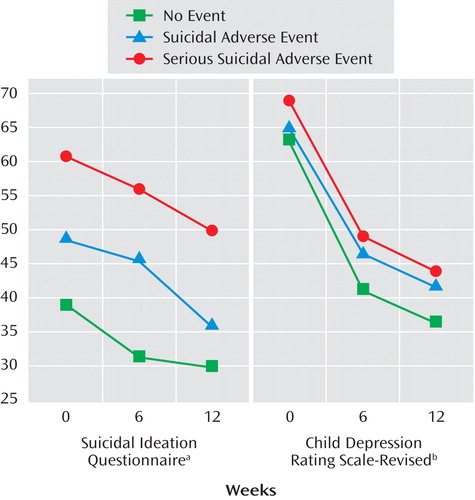
a LogTime: p<0.001; Event (suicidal adverse event): p=0.03; Event (serious suicidal adverse event): p<.001; Event (suicidal adverse event) *LogTime: p=0.92; Event (serious suicidal adverse event) *LogTime: p=0.50
b LogTime: p<0.001; Event (suicidal adverse event): p=0.49; Event (serious suicidal adverse event): p=0.02; Event (suicidal adverse event) *LogTime: p=0.20; Event (serious suicidal adverse event) *LogTime: p=0.44
Relationship of Adjunctive Treatment to Suicidal Adverse Events
There was no association between either adjunctive use of sleep medications or stimulants and suicidal events. Treatment with benzodiazepines was associated with suicidal adverse events (6 of 10 [60.0%] versus 42 of 324 (13.0%), Fisher’s exact test, p<0.001). The majority of benzodiazepine use took place at one site (8 of 10), but the relationship between benzodiazepine use and suicidal events persisted when the analyses were restricted to that site (4 of 8 [50.0%] versus 9 of 84 [10.7%], Fisher’s exact test, p=0.01). The use of a benzodiazepine was associated with a faster time to a suicidal event (z=3.27, p=0.001), even after control was added for baseline differences in self-rated ideation (z=2.18, p=0.03), family conflict (z=3.02, p=0.003), and drug or alcohol use (z=2.13, p=0.03).
Relationship of Adjunctive Treatment to Nonsuicidal Self-Injury
There was no association between the use of stimulants and nonsuicidal self-injury events. There was a much higher rate of nonsuicidal self-injury events in those treated with benzodiazepines (4 of 10 [40.0%] versus 27 of 324 [8.3%], Fisher’s exact test, p=0.009) and in those who received treatment for sleep problems (10 of 58 [17.2%] versus 21/276 [7.6%], χ 2 =5.28, df=1, p=0.02). After controlling for a history of nonsuicidal self-injury, the use of benzodiazepine was still associated with a faster time to a suicidal event (z=2.96, p=0.003), whereas use of sleep medication was not (z=0.83, p=0.41).
Discussion
During the first 12 weeks of treatment of adolescents with treatment-resistant depression, the incidence of suicidal and nonsuicidal self-injury adverse events were 14.3% and 9.3%, respectively. The rate of all events, although not serious adverse events, was higher when assessed systematically than when obtained by spontaneous report. The strongest predictors of suicidal events were high baseline suicidal ideation, family conflict, and drug or alcohol use, whereas a previous history of nonsuicidal adverse events was the best predictor of nonsuicidal adverse events. There were no main effects of treatment on the frequency of either type of self-harm adverse events, but CBT was associated with an earlier onset of nonsuicidal adverse events. Participants who experienced a suicidal adverse event, but not those with a nonsuicidal adverse event, were less likely to respond treatment. In participants with high suicidal ideation, treatment with venlafaxine was associated with an increased rate of self-harm events compared to those treated with an SSRI. Participants who received an anti-anxiety medication were more likely to experience both suicidal and nonsuicidal self-injury adverse events.
Reliance on spontaneous report of suicidal adverse events will underestimate the rate of events compared to systematic assessment. However, the frequency of serious suicidal or nonsuicidal self-injury adverse events was similar under both conditions. Because the TORDIA trial was not placebo-controlled, these results cannot be directly compared to the placebo-controlled trials in the FDA analysis (1 , 10) . The FDA analysis showed some divergence between measures of systematically assessed suicidal ideation and spontaneous reporting, although other studies have found similar patterns for suicidal events under both conditions (4 , 10 , 26) .
The predictors of a suicidal adverse event were high baseline suicidal ideation and depression, self-reported family conflict, and drug or alcohol use, consistent with other reports in the literature (4 – 6) . Although the clinical characteristics of those with suicidal and nonsuicidal adverse events overlap (27) , the clinical implications of suicidal adverse events were more serious, insofar as the occurrence of suicidal, but not nonsuicidal self-injury, adverse events was associated with a poorer response to treatment. This may be because the above-noted predictors of suicidal adverse events overlap with those that predict poorer response to treatment in depressed adolescents (28 – 33) . Participants who experienced suicidal events showed a similar slope of decline in their suicidal and depressive symptoms, but because they started at a higher level, they were at greater risk for an event over time than those who entered at lower levels of symptoms. Interventions that speed the relief of depression and help to inhibit acting on suicidal urges could reduce the risk of the occurrence of a suicidal event.
In those participants with high suicidal ideation, treatment with venlafaxine, compared to treatment with an SSRI, was associated with a higher rate of either a suicidal or nonsuicidal self-injury event. Like some studies, but in contrast to others, there was no protective effect of CBT on the occurrence of suicidal adverse events (4 , 26 , 34) . Given that the median time to a suicidal event was 3 weeks, participants could not have received an adequate “dose” of CBT before many of these events occurred. Since high ideation, drug and alcohol use, and family conflict predict early onset of suicidal adverse events, these domains should be targeted early in these patients. The association of CBT with earlier onset of nonsuicidal adverse events was probably due to increased contact and monitoring. Nevertheless, CBT treatment failed to attenuate the risk for self-injury, perhaps because contextual and behavioral functions of nonsuicidal adverse events were not emphasized in this treatment model (35) .
The relationship between the use of benzodiazepines and the occurrence of self-harm events must be interpreted cautiously because of the small number involved, the heavy representation of just one site, and nonrandom assignment. Meta-analyses do not find such an association, although some clinical studies do (36 – 38) . Possible explanations could include cognitive effects of benzodiazepines resulting in increased risk-taking and disinhibition (39) .
This study is limited by sample size, the relatively small number of events, and, with regard to the relationship between an event and the use of benzodiazepines, the small number of participants using these nonrandomly assigned agents. On the basis of these findings, especially careful monitoring of more severely depressed patients with high suicidal ideation and family conflict is warranted. Treatments that directly target family conflict and emotion regulation early in the course of treatment may be successful in reducing the occurrence of suicidal adverse events. Although no single study can be definitive, especially for post-hoc analyses, these findings suggest the need to re-evaluate the risks and benefits of venlafaxine and of anti-anxiety agents in treatment-resistant depressed adolescents at high suicidal risk.
1. Bridge J, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus H, Ren L, Brent D: Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA 2007; 297:1683–1696Google Scholar
2. Libby AM, Brent DA, Morrato EH, Orton HD, Allen R, Valuck RJ: Decline in treatment of pediatric depression after FDA advisory on risk of suiciality with SSRIs. Am J Psychiatry 2007; 164:884–891Google Scholar
3. Gibbons RD, Brown CH, Hur K, Marcus SM, Bhaumik DK, Erkens JA, Herings RMC, Mann JJ: Early evidence on the effects of regulators’ suiciality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry 2007: 64: 1356–1363Google Scholar
4. Emslie G, Kratochvil C, Vitiello B, Silva S, Mayes T, McNulty S, Weller E, Waslick B, Casat C, Walkup J, Pathak S, Rohde P, Posner K, March J: Treatment of Adolescents With Depression Study (TADS): Safety results. J Am Acad Child Adolesc Psychiatry 2006; 45:1440–1455Google Scholar
5. Apter A, Lipschitz A, Fong R, Carpenter DJ, Krulewizc S, Davies JT, Wilkinson C, Perera P, Metz A: Evaluation of suicidal thoughts and behaviors in children and adolescents taking paroxetine. J Child Adolesc Psychopharmacology 2006: 16–77Google Scholar
6. Perlis RH, Beasley CM Jr, Wines JD Jr, Tamura RN, Cusin C, Shear D. Amsterdam J, Quitkin F, Strong RE, Rosenbaum JF, Fava M: Treatment associated suicidal ideation and adverse effects in an open multicenter trial of fluoxetine for major depressive episodes. Psychother Psychosom 2007: 76:40–46Google Scholar
7. Hammad T: A relational between psychotropic drugs and pediatric suicidality. US Food and Drug Administration. Aug. 16, 2004, p. 19Google Scholar
8. Simon GE, Savarino J, Operskalski B, Wang PS: Suicide risk during antidepressant treatment. Am J Psychiatry 2006: 164:41–47Google Scholar
9. Brent DA, Emslie GJ, Clarke GN, Wagner KD, Asarnow J, Keller MB, Vitiello B, Ritz L, Iyengar S, Abebe K, Birmaher B, Ryan N, Kennard B, Hughes C, Debar L, McCracken J, Strober M, Suddath RL, Spirto A, Leonard H, Melhem MH, Porta G, Onorato M, Zelazny J: Switching to venlafaxine or another SSRI with or without cognitive behavioral therapy for adolescents with SSRI-resistant: The TORDIA Randomized Control Trial. JAMA 2008: 901–913Google Scholar
10. Hammad TA, Laughren T, Racoosin J: Suicidality in pediatric patients treated with antidepressant drugs. Arch of Gen Psychiatry 2006; 63: 332–339Google Scholar
11. US Food and Drug Administration. FDA statement regarding the antidepressant paxil for pediatric depression. FDA Talk Paper. June 19, 2003. Available at http://www.fda.gov/bbs/topics/ANSWERS/2003;ANS01230.htmlGoogle Scholar
12. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Health-IV. Washington, DC, American Psychiatric Association, 1994Google Scholar
13. Poznanski EO, Freeman LN, Mokros HB, Children’s Depression Rating Scale—Revised. Psychopharmacol Bull 1984; 21:979–989Google Scholar
14. Guy W: Clinical Global Improvement Scale: Assessment Manual of Psychopharmacology 76, 1976. Rockville, Md, National Institute of Mental HealthGoogle Scholar
15. Begg CB, Iglewicz B: A treatment allocation procedure for sequential clinical trials. Biometrics 1980; 36:81–90Google Scholar
16. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): reliability and validity data. J Am Acad Child Adolescent Psychiatry 1997; 36:908–988Google Scholar
17. Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H: A Children’s Global Assessment Scale (C-GAS). Arch Gen Psychiatry 1983; 40: 1228–1231Google Scholar
18. Beck AT, Steer RA, Garbin MG: Psychometric properties of the Beck Depression Inventory: Twenty-five years of Evaluation. Clinical Psychology Rev 1998; 8:77–100Google Scholar
19. Beck AT, Weissman A, Lester D, Trexler: The measurement of pessimism: The Hopelessness Scale. J Consult Clin Psychol 1974; 42:861–865Google Scholar
20. Reynolds WM, Mazza JJ: Assessment of suicidal ideation in inner-city children and young adults. Reliability and validity of the Suicidal Ideation Questionnaire-JR. School of Psychology Rev 1999; 28:17–30Google Scholar
21. Robin AL, Foster SL: The Conflict Behavior Questionnaire, in Dictionary of Behavorial Techniques. Edited by Hersen M, Bellack AS. New York, Pergamon, 1995, pp 148–150Google Scholar
22. Kirisci L, Mezzich AC, Tarter RE: Norms and sensitivity of the adolescent version of the Drug Use Screening Inventory. Addictive Behaviors 1995; 20:149–157Google Scholar
23. Posner K, Oquendo MA, Stanley B, Davies M, Gould M: Columbia Classification Algorithm of Suicide Assessment (C-CACA): Classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry 2007; 164:1035–1043Google Scholar
24. Coding Symbols for Thesaurus of Adverse Reaction Terms (COSTART) Terminology. http://www.fda.gov/medwatch/report/meddra.htm.Google Scholar
25. Nock MK, Prinstein MJ: A functional approach to the assessment of self-mutilative behavior. J Consult Clin Psychol 2004; 72: 885–890Google Scholar
26. The TADS Team: The Treatment for Adolescents With Depression Study (TADS): Long-term effectiveness and safety outcomes. Arch Gen Psychiatry 2007: 64:1132–1144Google Scholar
27. Nock MK, Joiner Jr, TE, Gordon K, Lloyd-Richardson E, Prinstein MJ: Non-suicidal self-injury among adolescents: Diagnostic correlates and relation to suicide attempts. Psychiatry Res 2006; 144:65–72Google Scholar
28. Birmaher B, Brent DA, Kolko D, Baugher M, Bridge J, Iyengar S, Ulloa RE: Clinical outcome after short-term psychotherapy for adolescents with major depressive disorder. Arch Gen Psychiatry 2000; 57:29–36Google Scholar
29. Brent DA, Kolko D, Birmaher B. Baugher M, Bridge J, Roth C, Holder D: Predictors of treatment efficacy in a clinical trial of three psychosocial treatments for adolescent depression. J Am Acad Child Adolesc Psychiatry 1998: 37:906–914Google Scholar
30. Clarke G, Hops H, Lewinsohn PM, Andrew J, Williams J: Cognitive-behavorial group treatment of adolescent depression: Prediction of outcome. Behav Ther 1992; 23:341–354Google Scholar
31. Curry J, Rohde P, Simons S, Silva S, Vitello B, Kratochvil C, Reinecke M, Feeney N, Wells K, Pathak S, Weller E, Rosenberg D, Kennard B, Robins M, Ginsburg G, March J: Predictors and moderators of acute outcome in the Treatment for Adolescents With Depression Study (TADS). J Am Acad Child Adolesc Psychiatry 6: 45:1427–1439Google Scholar
32. Emslie GJ, Rush AJ, Weinberg WA, Kowatch RA, Carmody T, Mayes TL: Fluoxetine in child and adolescent depression: acute and maintenance treatment. Depression and Anxiety 1998; 7:32–39Google Scholar
33. Asarnow J, Brent D, Emslie GJ, Clarke GN, Keller MB, Wagner KD: Treatment-resistant depression in adolescents: predictors and moderators of treatment response. J Am Acad Child Adolesc Psychiatry (in press)Google Scholar
34. Goodyer I, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, Breen S, Ford C, Barrett B, Leech A, Rothwell J, White L, Harrington R: Selective serotonin reuptake inhibitors (SSRIs) and routine specialist care with and without cognitive behaviour therapy in adolescents with major depression: randomised controlled trial. Br Med J 2007; 335:106–107Google Scholar
35. Nock MK, Prinstein MJ: Contextual features and behavioral functions of self-mutilation among adolescents. J Abnorm Psychol 2005; 114:140–146Google Scholar
36. Khan A, Leventhal RM, Khan S, Brown WA: Suicide risk in patients with anxiety disorders: a meta-analysis of the FDA database. J Affect Disord 2002; 68:183–190Google Scholar
37. Neutel CI, Patten SB: Risk of suicide attempts after benzodiazepine and/or antidepressant use. Annals of Epidemiology 1997; 7:568–574Google Scholar
38. Xiang YT, Weng YZ, Leung CM, Tang WK, Ungvari GS: Socio-demographic and clinical correlates of lifetime suicide attempts and their impact on quality of life in Chinese schizophrenia patients. J Psychiat Res 2007; 42:495–502Google Scholar
39. Deakin JB, Aitken MR, Dowson JH, Robbins TW, Sahakian BJ: Diazepam produces disinhibitory cognitive effects in male volunteers. Psychopharmacology 2008; 173:88–97Google Scholar