Lithium in the Prevention of Suicidal Behavior and All-Cause Mortality in Patients With Mood Disorders: A Systematic Review of Randomized Trials
Abstract
OBJECTIVE: Observational studies suggest that long-term lithium treatment has a strong antisuicidal effect in mood disorders, but it is uncertain whether this association is a genuine therapeutic effect or is due to confounding factors in nonrandomized studies. The authors conducted a systematic review and meta-analysis of randomized trials to investigate the effect of lithium, compared to placebo and other active treatments, on the risk of suicide, deliberate self-harm, and all-cause mortality in patients with mood disorder. METHOD: The data source was the Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register, incorporating results of searches of MEDLINE (1966–June 2002), EMBASE (1980–June 2002), CINAHL (1982–March 2001), PsycLIT (1974–June 2002), PSYNDEX (1977–October 1999), and LILACS (1982–March 2001). The Cochrane Central Register of Controlled Trials (CENTRAL) was searched with the term “lithium” for new records entered into the database from 1999 to 2003. Studies selected included randomized, controlled trials comparing lithium with placebo or all other compounds used in long-term treatment for mood disorders (unipolar depression, bipolar disorder, schizoaffective disorder, dysthymia, and rapid cycling, diagnosed according to DSM or ICD criteria). Of 727 references identified in the search, 52 articles were marked as possibly relevant on the basis of the abstract, and 32 randomized, controlled trials were eligible for inclusion in the review. Two independent reviewers extracted the data, and disagreements were resolved by consensus with a third reviewer. Methodological quality was assessed according to the criteria of the Cochrane Collaboration. When the outcomes of interest were not reported, an attempt was made to obtain the required data from the original authors. RESULTS: In 32 trials, 1,389 patients were randomly assigned to receive lithium and 2,069 to receive other compounds. Patients who received lithium were less likely to die by suicide (data from seven trials; two versus 11 suicides; odds ratio=0.26; 95% confidence interval [CI]=0.09–0.77). The composite measure of suicide plus deliberate self-harm was also lower in patients who received lithium (odds ratio=0.21; 95% CI=0.08–0.50). There were fewer deaths overall in patients who received lithium (data from 11 trials; nine versus 22 deaths; odds ratio=0.42, 95% CI=0.21–0.87). CONCLUSIONS: Lithium is effective in the prevention of suicide, deliberate self-harm, and death from all causes in patients with mood disorders.
Mood disorders are frequently recurrent and are associated with a lifetime risk of suicide that is about 15 times higher than in the general population (1). There is increasing recognition that strategies for suicide prevention should include improved treatment of mood disorders. Although evidence from randomized trials suggests that drug treatments, including antidepressants and lithium, can substantially reduce the risk of relapse in mood disorders (2, 3), the effects on suicide are uncertain because the low event rate means that individual randomized trials are invariably underpowered to investigate any potential benefit. On the basis of the existing observational and randomized evidence, however, there have been claims that lithium may substantially reduce the risk of suicide in bipolar disorder (4). Proposed mechanisms of action include a lowering of risk secondary to a reduction in risk of depressive relapse, a serotonin-mediated reduction in impulsivity or aggressive behavior, and a nonspecific benefit arising from the long-term monitoring provided during lithium therapy (4). Goodwin and colleagues (5) recently reported a large observational study from a health maintenance organization that found a 2.7-fold increase in the risk of suicide in patients prescribed divalproex, compared to patients prescribed lithium. Goodwin et al. compared active treatments and so partly controlled for some of the limitations of previous studies, including the possibilities that patients who are able to adhere to long-term lithium treatment may be less disturbed and that nonspecific effects of the close follow-up associated with lithium therapy may reduce the risk of suicide. Nonrandomized studies such as the study of Goodwin et al., however, cannot exclude fully the possibility of confounding by indication, i.e., the decision to prescribe lithium or divalproex may be influenced by other patient factors that, in turn, are associated with suicide risk (6–8).
Lithium is a drug with recognized toxicity, and suicide is just one of a number of possible causes of death in patients receiving long-term lithium treatment. It is important, therefore, to consider all-cause mortality, because of the possibility that any possible antisuicidal effect of lithium is offset by increased deaths from other causes.
To obtain an unbiased assessment of the potential antisuicidal effect—and the effects on all-cause mortality—of lithium, we conducted a systematic review and meta-analysis of the evidence from randomized trials of lithium in patients with mood disorders.
Method
Inclusion Criteria
We included randomized, controlled trials comparing lithium with placebo or all other compounds used in long-term treatment for mood disorders (unipolar depression, bipolar disorder, schizoaffective disorder, dysthymia, and rapid cycling, diagnosed according to DSM and ICD criteria). We included only long-term treatment (prophylaxis) trials. We arbitrarily defined long-term treatment as treatment with a minimum duration of 3 months.
Search Strategy
We based our search strategy on the Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register, incorporating results of group searches of MEDLINE (1966–June 2002), EMBASE (1980–June 2002), CINAHL (1982–March 2001), PsycLIT (1974–June 2002), PSYNDEX (1977–October 1999), and LILACS (1982–March 2001). We used the search term “lithium” and restricted the search from 1999 to 2003. The Cochrane Central Register of Controlled Trials (CENTRAL) was searched with the term “lithium” for new records entered into the database from 1999 to 2003.
To supplement the results from the Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register and CENTRAL database, MEDLINE (1999–2003), EMBASE (1999–2003), PsycINFO (1999–2003), and CINAHL (1999–2003) were searched by a librarian using a modified Cochrane randomized, controlled trial filter and the following terms: (lithium or lithium carbonate or calith or camcolit* or carbolit* or ceglution or duralith or durolith or eskalith or hypnorex or hynorex or hyponrex or lentolith or licab or licarb or licarbium or lidin or lilipin or li?liquid or li-liquid or lilitin or limas or liskonum or litarex or lithan or lithane or litheum or lithicarb or lithionate or lithizine or lithobid or lithocarb or lithocap or lithonate or lithosun or lithotabs or litheril or litilent or manialit* or maniprex or phanate or phasal or plenur or priadel or quilonium or quilonorm or quilonum or teralithe or theralite) AND (mood disorders or affective disorders, psychotic or bipolar disorder or cyclothymic disorder or depressive disorder or depression, involutional or dysthymic disorder or seasonal affective disorder or affective disorders or depression, reactive or dysthymic disorder or seasonal affective disorder or affective disorders, psychotic or bipolar disorder or affective disorders or bipolar disorder or cyclothymic personality or major depression or dysthymic disorder or endogenous depression or involutional depression or reactive depression or recurrent depression or treatment resistant depression or seasonal affective disorder or schizoaffective disorder or affective neurosis or depression or dysthymia or involutional depression or manic depressive psychosis or bipolar depression or schizoaffective psychosis or depress* or bipolar or schizoaffective).
In addition, other relevant articles and major textbooks that cover mood disorders were checked. The authors of significant papers, other experts in the field, and pharmaceutical companies that manufacture lithium or the comparator drugs were contacted to identify other relevant published or unpublished randomized, controlled trials.
Outcomes
The primary outcomes were suicide, deliberate self-harm (including attempted suicide), and death from all causes in patients randomly assigned to receive lithium or another compound. All-cause mortality was specified as an outcome for several reasons. First, it is free from the variations in both definition and application of the definition that limit the reliability of suicide reports, and it includes deaths from suicide that have not been correctly classified. Second, given the known toxic effects of lithium, any reduction in suicide may be offset by an increase in deaths from other causes, and this possibility would be apparent in the comparison of all-cause mortality rates. Last, all-cause mortality includes suicide plus all other deaths, and the number of deaths from all causes must be at least as large as the number of suicides.
If lithium has a specific action in preventing suicide, one would expect it to also reduce attempted suicide and deliberate self-harm. As suicide occurs too infrequently to be used as a primary outcome in individual clinical trials, use of a composite outcome of suicide plus attempted suicide/self-harm is likely to increase the event rate and, thus, power of the study (9). For example, a composite outcome of negative events, including suicide and deliberate self-harm, was used as the primary outcome in the recent International Suicide Prevention Trial, which found evidence in favor of clozapine, compared to olanzapine, in patients with schizophrenia and schizoaffective disorder (10). We therefore planned, a priori, to investigate a composite of suicide plus episodes of deliberate self-harm.
Data Extraction and Quality Assessment
Two reviewers (A.C. and J.R.G.) independently extracted data; disagreements were resolved by discussion and consensus with a third member of the team (K.H.). For each trial we identified inclusion and exclusion criteria, duration of follow-up, diagnosis, doses, and main outcome measures. We assessed the methodological quality of studies according to the criteria of the Cochrane Reviewers’ Handbook (11). Crossover studies were included, but only the first phase of such trials (before crossover) was considered. For trials in which our outcomes of interest were not reported, we attempted to obtain the required data from the original authors.
Data Analysis
Data from intention-to-treat analyses were used where possible; otherwise endpoint data for trial completers were used. Deaths and self-harm are comparatively rare in clinical trials, and data were sparse. Several trials had no such events in one or more arms. Meta-analysis of sparse data can be problematic, because some methods add continuity corrections to trial arms with zero events, and these corrections may exert a substantial effect on the overall results (12). Peto’s method was used to calculate odds ratios and 95% confidence intervals (CIs) because this method does not apply continuity corrections and has been shown to be the most reliable method when applied to data on sparse events from studies without extreme imbalances (12). Trials with no events in any treatment arm were excluded from the analyses because they are uninformative (12). Sensitivity analyses using other meta-analytic methods were done to assess the robustness of the results. Statistical heterogeneity, in which variation between the results of the individual trials is greater than can be explained by chance alone, was investigated with chi-square tests. Data were analyzed by using the metan routine in Stata (13).
For trials with more than two arms, we considered each pairwise comparison as if it were separate two-arm trials. For example, if a trial compared lithium with another active drug and with placebo, we included the lithium versus placebo arm and the lithium versus active drug arm as separate trials. This approach included the single lithium group twice in the meta-analysis. We therefore investigated the effect of this double counting by sensitivity analyses that excluded each of the two trials from the pooled analysis.
Results
Included Trials
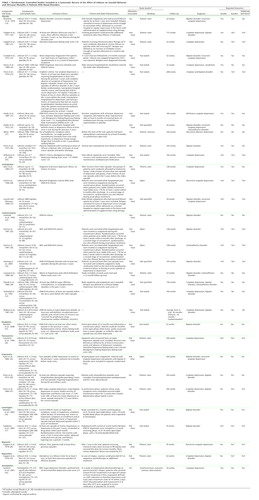
A total of 727 references were identified in the search; 52 references were marked as possibly relevant on the basis of the content of the abstract. After reading the papers and excluding duplicates, we included 32 randomized, controlled trials in the study (14–45) (summarized in Table 1; see Figure 1 for flowchart). These trials comprised 19 comparisons of lithium to placebo (14–18, 21, 23–25, 27, 31, 33–35, 37, 39–41, 45), three to amitriptyline (27, 28, 35), nine to carbamazepine (22, 28–30, 32, 36, 38, 43, 44), one to divalproex (15), one to fluvoxamine (26), three to imipramine (alone or in combination with lithium) (34, 39, 41), two to lamotrigine (16, 17), one to mianserin (20), one to maprotiline (19), and one to nortriptyline (alone or in combination) (42). There was therefore considerable clinical heterogeneity between the trials. In total, 1,389 patients were randomly assigned to receive lithium, and 2,069 were assigned to receive other compounds. The results reported here were unaffected in the sensitivity analyses that excluded trials contributing more than one comparison with lithium.
The low rates of the outcome events meant that statistical tests of heterogeneity were underpowered to detect even substantial qualitative heterogeneity. Formal statistical investigation of any heterogeneity by meta-regression was therefore not possible and was limited to sensitivity analysis and visual examination of the forest plots.
Suicide and Deliberate Self-Harm
Seven trials reported the occurrence of suicides (Figure 2) (17, 27–30, 39, 40). Two trials were informative for lithium versus placebo (39, 40), two for lithium versus amitriptyline (27, 28), two for lithium versus carbamazepine (29, 30), and one for lithium versus lamotrigine (17). Patients allocated to receive lithium were less likely to die by suicide (two suicides among lithium-treated patients versus 11 suicides among patient who received comparators) (Peto odds ratio=0.26, 95% CI=0.09–0.77, p=0.01) (χ2=1.57, df=6, p=0.95, chi-square test for heterogeneity). Despite the heterogeneous comparators, there was no evidence of statistically significant variation between the results of the individual trials. Numerically fewer suicides occurred in the lithium group in all the trials except one, in which one suicide occurred in both the lithium and comparator (carbamazepine) groups (30).
Only seven deliberate self-harm events were reported (none among the lithium-treated patients, five among the carbamazepine-treated patients, one among the lamotrigine-treated patients, and one among the patients who received placebo). The difference between groups, however, was not statistically significant because of the low event rate. When suicide and deliberate self-harm events were considered together as a composite outcome (Figure 3), fewer patients who received lithium experienced this negative outcome (Peto odds ratio=0.21, 95% CI=0.08–0.50, p=0.0005) (χ2=0.44, df=8, p=1.00, chi-square test for heterogeneity). All the trials reported fewer instances of this composite event in the lithium-treated group.
Although there was no statistically significant heterogeneity, post hoc analyses were conducted in subgroups of particular interest. No significant differences were found between the results from the placebo-controlled trials and the active comparison trials. There was no evidence of any difference between trials that included patients with unipolar depression and trials that included patients with bipolar disorder.
Mortality From All Causes
Eleven trials comprising 12 comparisons (17, 18, 24, 27–31, 39, 40, 45) contributed data that could be used in the pooled analysis of all-cause mortality. In the majority of trials there were no deaths (Figure 4). Six of the 12 trials were informative for lithium versus placebo (18, 24, 31, 39, 40, 45), three for lithium versus tricyclic antidepressants (27, 28, 39), two for lithium versus carbamazepine (29, 30), and one for lithium versus lamotrigine (17). There were fewer deaths overall among the patients who received lithium (nine versus 22, Peto odds ratio=0.42, 95% CI=0.21–0.87, p=0.02) (χ2=4.98, df=11, p=0.93, chi-square test for heterogeneity).
Therefore, despite the heterogeneous comparators, little statistical variation was found between the results of the individual trials. Numerically fewer deaths occurred in the lithium group in all trials, except three. In two of these trials (30, 45), the number of deaths was the same in both groups; in the other small trial (31), one death occurred in the lithium-treated group, and none occurred in the placebo group.
Again, post hoc analyses found no differences between the results from trials with placebo comparator and those with an active drug comparator or between the results from trials that included patients with unipolar depression and those that included patients with bipolar disorder.
Discussion
In this review and meta-analysis, we synthesized the available randomized evidence of the effect of lithium on suicide, deliberate self-harm, and all-cause mortality. This work extends the findings of previous reviews because 1) it includes only randomized trials and 2) further data on deaths and causes of death were obtained from the original authors. The effect of lithium on the prevention of symptomatic relapse was not assessed, and the present findings should be considered alongside this evidence, which we reviewed previously for bipolar disorder (3). As with all quantitative reviews, the current study is subject to a number of limitations. Publication bias—caused by the tendency for trials with negative or neutral findings not to be published—can seriously limit the reliability of meta-analyses (46). It is possible that trials that failed to demonstrate an advantage for lithium over a comparator are less likely to be published. We consider this possibility particularly unlikely in recent industry-sponsored trials that have included lithium as a comparator, because any design bias in such trials could reasonably be expected to favor the investigational drug (47). However, because of the small numbers of events and small size of the trials, only one or two moderately sized trials with neutral or negative results could materially affect the estimates.
Overall, few deaths from suicide occurred in the trials included in this meta-analysis, which perhaps reflects the fact that patients judged to be at high risk of suicide are not normally recruited to randomized trials. The low numbers of events led to substantial random error and, consequently, unstable estimates of the treatment effect with wide confidence intervals. Thus, the results must be interpreted with caution because the true effect of lithium may be either smaller or greater than we estimated. However, the evidence seems unequivocal that patients treated with lithium were much less likely to die from suicide or from any cause than patients given an alternative to lithium, whether the alternative was placebo or another compound. Lithium appears to reduce the risk of death and suicide by approximately 60% and the risk of a composite of suicide and deliberate self-harm by about 70%. This substantial effect is comparable to that reported in the recent observational study by Goodwin et al. (5), although it is less than that estimated from previous nonrandomized studies (4). To our knowledge, this study provides the first demonstration with evidence from randomized trials that any treatment can reduce suicide, specifically, and mortality, in general, in psychiatric disorders.
The trials were clinically heterogeneous in terms of patients, diagnoses, and comparators, and the small numbers of events limited the power of the analysis to detect any interaction between these factors and the treatment effect of lithium. Despite these limitations, the consistency of the results across trials may indicate that the life-preserving effect of lithium is independent of that of the comparator.
Long-term evidence for other agents in the prevention of relapse in bipolar disorder is very limited (48), and so the results of the current analysis may reflect a general superior efficacy of lithium for the treatment of mood disorder or may reflect a specific antisuicidal property. The consistency of the findings across comparators suggests that lithium prevents suicide rather than that any other drug increases the risk of suicide.
The reduction in the risk of all-cause mortality mainly reflects reduction in risk of suicide, because most of the deaths in the trials were suicides. However, the analysis of all-cause mortality avoids possible ascertainment bias (i.e., events in patients who take lithium may be more or less likely to be classified as suicides) and increases power (because more events are included, and there is less random error). The comparability in the relative risk reduction of both suicide and all-cause mortality also indicates that there was no increase in fatal events due to lithium. The composite of suicide plus deliberate self-harm also showed a similar effect and may be a more feasible primary outcome in future trials. That there were fewer cases of deliberate self-harm than of suicide overall may indicate that data on deliberate self-harm were not recorded systematically in these trials. Given the pattern for suicidal behavior in the general population (49), the number of patients with deliberate self-harm might be expected to be greater than the number of patients who die by suicide, although this pattern may not be present in the patient population represented in these trials. Clearly, however, if these events are underrecorded, this failure should be rectified in future trials.
In conclusion, this meta-analysis of randomized trials indicates that lithium reduces the risk of suicide in patients with mood disorders. Lithium remains the treatment with the most substantial evidence base for the prevention of relapse in bipolar disorder and should be a first-line therapy for patients with that disorder, including those at risk of suicidal behavior.
 |
Received Sept. 17, 2004; revision received Nov. 18, 2004; accepted Dec. 29, 2004. From the Department of Psychiatry, University of Oxford; the Department of Medicine and Public Health, Section of Psychiatry and Clinical Psychology, University of Verona, Verona, Italy; and the Centre for Suicide Research, Department of Psychiatry, University of Oxford. Address correspondence and reprint requests to Dr. Geddes, Department of Psychiatry, University of Oxford, Warneford Hospital, Oxford OX3 7JX, UK; [email protected] (e-mail). Supported by Oxfordshire Mental Healthcare National Health Service Trust (Dr. Hawton). The authors thank Dr. Bruno Mueller-Oerlinghausen, Dr. Waldemar Greil, Dr. Nikolaus Kleindienst, Dr. David Wilkinson, Dr. Jan-Otto Ottosson, Dr. Mogens Schou, Dr. Victoria Grochocinski, Dr. David Kupfer, Dr. David Dunner, Dr. Alec Coppen, Dr. Fredric Quitkin, Dr. Gary Evoniuk, Dr. Erwin Hartong, and Dr. Joseph Calabrese for providing data from their trials, Dr. Sally Burgess for help in planning and organizing the early stages of this study, Ed Juszczak for statistical advice on meta-analysis of sparse data, and Prof. Guy Goodwin for comments on the manuscript.
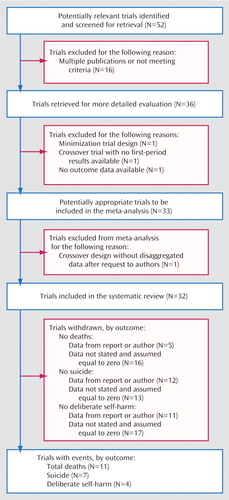
Figure 1. Flow Diagram Showing Selection of Studies Included in a Meta-Analysis of the Effect of Lithium on Suicidal Behavior and All-Cause Mortality in Patients With Mood Disorders
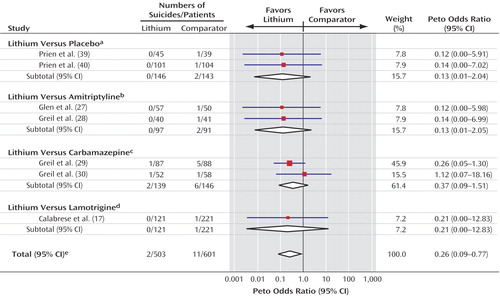
Figure 2. Forest Plot Showing Meta-Analysis of Suicides in Randomized Trials Comparing Lithium With Placebo or Active Comparators
aTest for heterogeneity: χ2<0.001, df=1, p=0.95; test for overall effect: z=1.46, p=0.15.
bTest for heterogeneity: χ2<0.001, df=1, p=0.95; test for overall effect: z=1.45, p=0.15.
cTest for heterogeneity: χ2=0.80, df=1, p=0.37; test for overall effect: z=1.38, p=0.17.
dTest for overall effect: z=0.74, p=0.46.
eTest for heterogeneity: χ2=1.57, df=6, p=0.95; test for overall effect: z=2.43, p=0.01.
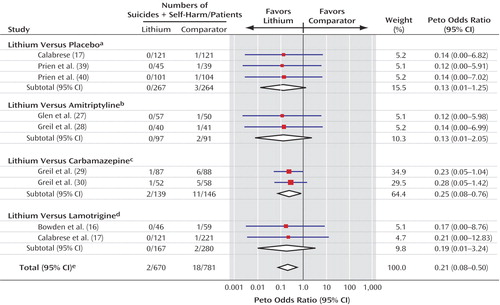
Figure 3. Forest Plot Showing Meta-Analysis of Suicides Plus Deliberate Self-Harm in Randomized Trials Comparing Lithium With Placebo or Active Comparators
aTest for heterogeneity: χ2<0.001, df=2, p=1.00; test for overall effect: z=1.77, p=0.08.
bTest for heterogeneity: χ2<0.001, df=1, p=0.95; test for overall effect: z=1.45, p=0.15.
cTest for heterogeneity: χ2=0.03, df=1, p=0.87; test for overall effect: z=2.45, p=0.01.
dTest for heterogeneity: χ2=0.01, df=1, p=0.94; test for overall effect: z=1.15, p=0.25.
eTest for heterogeneity: χ2=0.44, df=8, p=1.00; test for overall effect: z=3.48, p=0.0005.
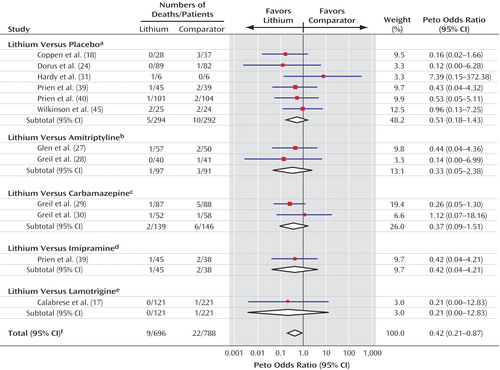
Figure 4. Forest Plot Showing Meta-Analysis of Deaths From All Causes in Randomized Trials Comparing Lithium With Placebo or Active Comparators
aTest for heterogeneity: χ2=3.60, df=5, p=0.61; test for overall effect: z=1.28, p=0.20.
bTest for heterogeneity: χ2=0.25, df=1, p=0.62; test for overall effect: z=1.10, p=0.27.
cTest for heterogeneity: χ2=0.80, df=1, p=0.37; test for overall effect: z=1.38, p=0.17.
dTest for overall effect: z=0.74, p=0.46.
eTest for overall effect: z=0.74, p=0.46.
fTest for heterogeneity: χ2=4.98, df=11, p=0.93; test for overall effect: z=2.35, p=0.02.
1. Harris EC, Barraclough B: Suicide as an outcome for mental disorders: a meta-analysis. Br J Psychiatry 1997; 170:205–228Crossref, Medline, Google Scholar
2. Geddes JR, Carney SM, Davies C, Furukawa TA, Kupfer DJ, Frank E, Goodwin GM: Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 2003; 361:653–661Crossref, Medline, Google Scholar
3. Geddes JR, Burgess S, Hawton K, Jamison K, Goodwin GM: Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry 2004; 161:217–222; correction, 161:1517Link, Google Scholar
4. Baldessarini RJ, Tondo L, Hennen J: Lithium treatment and suicide risk in major affective disorders: update and new findings. J Clin Psychiatry 2003; 64(suppl 5):44–52Google Scholar
5. Goodwin FK, Fireman B, Simon GE, Hunkeler EM, Lee J, Revicki D: Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA 2003; 290:1467–1473Crossref, Medline, Google Scholar
6. Yerevanian BI, Koek RJ, Feusner JD: Pharmacotherapy and risk of suicidal behaviors among patients with bipolar disorder (letter). JAMA 2004; 291:939Google Scholar
7. Bowden C, Fawcett J: Pharmacotherapy and risk of suicidal behaviors among patients with bipolar disorder (letter). JAMA 2004; 291:939Google Scholar
8. Johnston SC: Identifying confounding by indication through blinded prospective review. Am J Epidemiol 2001; 154:276–284Crossref, Medline, Google Scholar
9. Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C: Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA 2003; 289:2554–2559Crossref, Medline, Google Scholar
10. Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, Bourgeois M, Chouinard G, Islam MZ, Kane J, Krishnan R, Lindenmayer JP, Potkin S (International Suicide Prevention Trial Study Group): Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003; 60:82–91; correction, 60:735Crossref, Medline, Google Scholar
11. Alderson P, Green S, Higgins JP: Cochrane Reviewers’ Handbook 4.2.2 (updated December 2003), in The Cochrane Library, Issue 1, 2004. Chichester, UK, John Wiley & Sons, 2004Google Scholar
12. Sweeting MJ, Sutton AJ, Lambert PC: What to add to nothing? use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 2004; 23:1351–1375Crossref, Medline, Google Scholar
13. Stata Reference Manual: Release 7.0. College Station, Tex, Stata Corp, 2001Google Scholar
14. Baastrup PC, Poulsen JC, Schou M, Thomsen K, Amdisen A: Prophylactic lithium: double blind discontinuation in manic-depressive and recurrent-depressive disorders. Lancet 1970; 2:326–330Crossref, Medline, Google Scholar
15. Bowden CL, Calabrese JR, McElroy SL, Gyulai L, Wassef A, Petty F, Pope HG Jr, Chou JC, Keck PE Jr, Rhodes LJ, Swann AC, Hirschfeld RM, Wozniak PJ (Divalproex Maintenance Study Group): A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Arch Gen Psychiatry 2000; 57:481–489Crossref, Medline, Google Scholar
16. Bowden CL, Calabrese JR, Sachs G, Yatham LN, Asghar SA, Hompland M, Montgomery P, Earl N, Smoot TM, DeVeaugh-Geiss J (Lamictal 606 Study Group): A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry 2003; 60:392–400; correction, 2004; 61:680Google Scholar
17. Calabrese JR, Bowden CL, Sachs G, Yatham LN, Behnke K, Mehtonen OP, Montgomery P, Ascher J, Paska W, Earl N, DeVeaugh-Geiss J (Lamictal 605 Study Group): A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry 2003; 64:1013–1024Crossref, Medline, Google Scholar
18. Coppen A, Noguera R, Bailey J, Burns BH, Swani MS, Hare EH, Gardner R, Maggs R: Prophylactic lithium in affective disorders. Lancet 1971; 2:265–279Google Scholar
19. Coppen A, Montgomery SA, Gupta RK, Bailey JE: A double-blind comparison of lithium carbonate and maprotiline in the prophylaxis of the affective disorders. Br J Psychiatry 1976; 128:479–485Crossref, Medline, Google Scholar
20. Coppen A, Ghose K, Rao R, Bailey J, Peet M: Mianserin and lithium in the prophylaxis of depression. Br J Psychiatry 1978; 133:206–210Crossref, Medline, Google Scholar
21. Coppen A, Abou-Saleh MT, Milln P, Bailey J, Metcalfe M, Burns BH, Armond A: Lithium continuation therapy following electroconvulsive therapy. Br J Psychiatry 1981; 139:284–287Crossref, Medline, Google Scholar
22. Coxhead N, Silverstone T, Cookson J: Carbamazepine versus lithium in the prophylaxis of bipolar affective disorder. Acta Psychiatr Scand 1992; 85:114–118Crossref, Medline, Google Scholar
23. Cundall RL, Brooks PW, Murray LG: A controlled evaluation of lithium prophylaxis in affective disorders. Psychol Med 1972; 2:308–311Crossref, Medline, Google Scholar
24. Dorus W, Ostrow DG, Anton R, Cushman P, Collins JF, Schaefer M, Charles HL, Desai P, Hayashida M, Malkerneker U, et al: Lithium treatment of depressed and nondepressed alcoholics. JAMA 1989; 262:1646–1652Crossref, Medline, Google Scholar
25. Fieve RR, Kumbaraci T, Dunner DL: Lithium prophylaxis of depression in bipolar I, bipolar II, and unipolar patients. Am J Psychiatry 1976; 133:925–929Link, Google Scholar
26. Franchini L, Gasperini M, Smeraldi E: A 24-month follow-up study of unipolar subjects: a comparison between lithium and fluvoxamine. J Affect Disord 1994; 32:225–231Crossref, Medline, Google Scholar
27. Glen AI, Johnson AL, Shepherd M: Continuation therapy with lithium and amitriptyline in unipolar depressive illness: a randomized, double-blind, controlled trial. Psychol Med 1984; 14:37–50Crossref, Medline, Google Scholar
28. Greil W, Ludwig-Mayerhofer W, Erazo N, Engel RR, Czernik A, Giedke H, Muller-Oerlinghausen B, Osterheider M, Rudolf GA, Sauer H, Tegeler J, Wetterling T: Comparative efficacy of lithium and amitriptyline in the maintenance treatment of recurrent unipolar depression: a randomised study. J Affect Disord 1996; 40:179–190Crossref, Medline, Google Scholar
29. Greil W, Ludwig-Mayerhofer W, Erazo N, Schochlin C, Schmidt S, Engel RR, Czernik A, Giedke H, Muller-Oerlinghausen B, Osterheider M, Rudolf GA, Sauer H, Tegeler J, Wetterling T: Lithium versus carbamazepine in the maintenance treatment of bipolar disorders—a randomised study. J Affect Disord 1997; 43:151–161Crossref, Medline, Google Scholar
30. Greil W, Ludwig-Mayerhofer W, Erazo N, Engel RR, Czernik A, Giedke H, Muller-Oerlinghausen B, Osterheider M, Rudolf GA, Sauer H, Tegeler J, Wetterling T: Lithium vs carbamazepine in the maintenance treatment of schizoaffective disorder: a randomised study. Eur Arch Psychiatry Clin Neurosci 1997; 247:42–50Crossref, Medline, Google Scholar
31. Hardy BG, Shulman KI, Zucchero C: Gradual discontinuation of lithium augmentation in elderly patients with unipolar depression. J Clin Psychopharmacol 1997; 17:22–26Crossref, Medline, Google Scholar
32. Hartong EG, Moleman P, Hoogduin CA, Broekman TG, Nolen WA: Prophylactic efficacy of lithium versus carbamazepine in treatment-naive bipolar patients. J Clin Psychiatry 2003; 64:144–151Crossref, Medline, Google Scholar
33. Hullin RP, McDonald R, Allsopp MN: Prophylactic lithium in recurrent affective disorders. Lancet 1972; 1:1044–1046Crossref, Medline, Google Scholar
34. Kane JM, Quitkin FM, Rifkin A, Ramos L Jr, Nayak DD, Howard A: Lithium carbonate and imipramine in the prophylaxis of unipolar and bipolar II illness: a prospective, placebo-controlled comparison. Arch Gen Psychiatry 1982; 39:1065–1069Crossref, Medline, Google Scholar
35. Laurell B, Ottosson JO: Prophylactic lithium? Lancet 1968; 2:1245–1246Crossref, Medline, Google Scholar
36. Lusznat RM, Murphy DP, Nunn CM: Carbamazepine vs lithium in the treatment and prophylaxis of mania. Br J Psychiatry 1988; 153:198–204Crossref, Medline, Google Scholar
37. Melia PI: Prophylactic lithium: a double-blind trial in recurrent affective disorders. Br J Psychiatry 1970; 116:621–624Crossref, Medline, Google Scholar
38. Placidi GF, Lenzi A, Lazzerini F, Cassano GB, Akiskal HS: The comparative efficacy and safety of carbamazepine versus lithium: a randomized, double-blind 3-year trial in 83 patients. J Clin Psychiatry 1986; 47:490–494Medline, Google Scholar
39. Prien RF, Klett CJ, Caffey EM Jr: Lithium carbonate and imipramine in prevention of affective episodes: a comparison in recurrent affective illness. Arch Gen Psychiatry 1973; 29:420–425Crossref, Medline, Google Scholar
40. Prien RF, Caffey EM Jr, Klett CJ: Prophylactic efficacy of lithium carbonate in manic-depressive illness: report of the Veterans Administration and National Institute of Mental Health Collaborative Study Group. Arch Gen Psychiatry 1973; 28:337–341Crossref, Medline, Google Scholar
41. Prien RF, Kupfer DJ, Mansky PA, Small JG, Tuason VB, Voss CB, Johnson WE: Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders: report of the NIMH Collaborative Study Group comparing lithium carbonate, imipramine, and a lithium carbonate-imipramine combination. Arch Gen Psychiatry 1984; 41:1096–1104Crossref, Medline, Google Scholar
42. Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, Greenberg RM, Crowe RR, Cooper TB, Prudic J: Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA 2001; 285:1299–1307Crossref, Medline, Google Scholar
43. Simhandl C, Denk E, Thau K: The comparative efficacy of carbamazepine low and high serum level and lithium carbonate in the prophylaxis of affective disorders. J Affect Disord 1993; 28:221–231Crossref, Medline, Google Scholar
44. Watkins SE, Callender K, Thomas DR, Tidmarsh SF, Shaw DM: The effect of carbamazepine and lithium on remission from affective illness. Br J Psychiatry 1987; 150:180–182Crossref, Medline, Google Scholar
45. Wilkinson D, Holmes C, Woolford J, Stammers S, North J: Prophylactic therapy with lithium in elderly patients with unipolar major depression. Int J Geriatr Psychiatry 2002; 17:619–622Crossref, Medline, Google Scholar
46. Egger M, Smith GD: Bias in location and selection of studies. BMJ 1998; 316:61–66Crossref, Medline, Google Scholar
47. Montaner JS, O’Shaughnessy MV, Schechter MT: Industry-sponsored clinical research: a double-edged sword. Lancet 2002; 358:1893–1895Crossref, Google Scholar
48. Goodwin GM: Evidence-based guidelines for treating bipolar disorder: recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2003; 17:149–173Crossref, Medline, Google Scholar
49. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Web-Based Injury Statistics Query and Reporting System (WISQARS), 2005. http://www.cdc.gov/ncipc/wisqars/default.htmGoogle Scholar



