Association of Diabetes Mellitus With Use of Atypical Neuroleptics in the Treatment of Schizophrenia
Abstract
OBJECTIVE: The development of both type I and type II diabetes after initiation of some atypical neuroleptics has been reported, primarily in studies involving small series of patients. This study used administrative data from a large national sample of patients with a diagnosis of schizophrenia to compare the prevalence of diabetes mellitus in patients receiving prescriptions for atypical and typical neuroleptics. METHOD: All outpatients with schizophrenia treated with typical and atypical neuroleptics over 4 months in 1999 in the Veterans Health Administration of the Department of Veterans Affairs (VA) were included in this study. Patients treated with atypical neuroleptics were those who received prescriptions for clozapine, olanzapine, risperidone, or quetiapine. Patients with a diagnosis of diabetes were also identified by using ICD-9 codes in VA administrative databases. The prevalence of diabetes mellitus across age groups and among patients receiving prescriptions for different atypical neuroleptics was examined with multiple logistic regression. RESULTS: A total of 38,632 patients were included in the study: 15,984 (41.4%) received typical neuroleptics and 22,648 (58.6%) received any atypical neuroleptic (1,207 [5.3%] received clozapine; 10,970 [48.4%], olanzapine; 955 [4.2%], quetiapine; and 9,903 [43.7%], risperidone; 387 patients received prescriptions for more than one atypical neuroleptic). When the effects of age were controlled, patients who received atypical neuroleptics were 9% more likely to have diabetes than those who received typical neuroleptics, and the prevalence of diabetes was significantly increased for patients who received clozapine, olanzapine, and quetiapine, but not risperidone. However, for patients less than 40 years old, all of the atypical neuroleptics were associated with a significantly increased prevalence of diabetes. CONCLUSIONS: In this large group of patients with schizophrenia, receipt of a prescription for atypical neuroleptics was significantly associated with diabetes mellitus.
Atypical neuroleptics such as clozapine (1), olanzapine (2), quetiapine (3), and risperidone (4) have demonstrated efficacy in the treatment of schizophrenia with fewer extrapyramidal side effects than other neuroleptics (5). However, other side effects have been described. The use of olanzapine, for example, has been associated with weight gain (6), exacerbation of previously well controlled diabetes (7), and onset of type I and type II diabetes mellitus (7–9), although a recent report failed to demonstrate an association between weight gain and development of hyperglycemia (10). Clozapine use has also been associated with weight gain in several reports (11–14). Although an association between clozapine-induced weight gain and the development of either hyperglycemia or diabetes mellitus has not been demonstrated (14), there have been several reports of hyperglycemia and diabetes mellitus (both type I and type II) directly associated with clozapine use (9, 14–21). There is also a single case report of quetiapine-associated new-onset diabetes mellitus (22).
The degree to which patients who receive prescriptions for atypical neuroleptics are at risk for development of diabetes mellitus cannot be ascertained definitively from the reports thus far. To our knowledge, nearly all of the studies of this issue to date are case reports. There have been exceptions, including two uncontrolled studies (14, 21) and one study (20) that used an unmatched comparison group of patients treated with typical neuroleptics. A naturalistic study of clozapine treatment reported that 30% of patients received a diagnosis of type II diabetes during the 5-year follow-up (14). This study did not include a comparison group. In addition, a comparison of patients randomly assigned to receive olanzapine or haloperidol and followed over several years failed to demonstrate a difference in the incidence of nonfasting glucose levels equal to 160 mg/dl (10); the rate of high nonfasting glucose level was 4.6% after 2.54 years in the olanzapine-treated patients and 5.0% after 1.15 years the haloperidol-treated patients. However, this analysis failed to stratify the data for age, which might have obscured important differences between the two groups—especially in younger patients, for whom the baseline rate of diabetes is not as high as in older patients. The authors were also silent about family history of diabetes in these patients and about ethnicity. However, the two groups did not differ significantly in baseline body mass index. A well-matched comparison group is particularly important, as patients with schizophrenia, regardless of treatment, have been reported to have a higher rate of diabetes than the general population (23, 24). For example, the rate of diabetes mellitus in the general U.S. male population age 20–39 years is 1.1% (25); in patients with schizophrenia age 18–44 years, rates of 5.6%–6.7% have been reported (23).
Although no pathophysiologic mechanism connecting atypical neuroleptic treatment and the development of diabetes mellitus has been delineated to date, olanzapine prescription has been associated with weight gain, hyperlipidemia, hyperinsulinemia, and insulin resistance (21), suggesting both direct and indirect mechanisms by which the medication may be involved in dysregulation of relevant metabolic pathways. Clozapine has also been found to induce insulin resistance (26). With regard to weight gain, a review has estimated that after 10 weeks of treatment a patient receiving clozapine would gain an average of 3.99 kg; a patient receiving olanzapine, an average of 3.51 kg; and a patient receiving risperidone, an average of 2 kg; and after 6 weeks of treatment, a patient receiving quetiapine would gain an average of 2.18 kg.
Thus, although researchers have reported that some patients treated with the atypical neuroleptics clozapine, olanzapine, and quetiapine develop diabetes mellitus that is sometimes, but not always, reversible, these studies have involved relatively small groups of patients. Studies have also demonstrated that prescription of olanzapine and clozapine can result in the development of metabolic abnormalities associated with diabetes mellitus (21, 26). In these studies, as well, the numbers of patients studied has been small. In summary, although the association between atypical neuroleptic prescription and development of diabetes mellitus has been observed and some potential pathophysiologic mechanisms have been elucidated, the risk of developing diabetes mellitus for patients who receive prescriptions for atypical neuroleptics has not been well studied.
This study used data for a comprehensive national sample of patients treated in the Veterans Health Administration of the Department of Veterans Affairs (VA) who had a diagnosis of schizophrenia and received prescriptions for either typical or atypical neuroleptics to test the hypothesis that prescription of atypical neuroleptics is associated with an increased prevalence of diabetes. Although the study design precluded an assessment of either a causal relationship or a mechanism of action, it provided a means for determining the potential public health risk. The large number of patients with schizophrenia treated within the VA system also allowed for examination of the prevalence of diabetes associated with each of the atypical neuroleptics available at the time of study—clozapine, olanzapine, quetiapine, and risperidone.
Method
Patients
All patients with a diagnosis of schizophrenia during fiscal year 1999 (October 1, 1998, to September 30, 1999) were identified through VA workload databases. A diagnosis of schizophrenia was operationally defined as having at least two outpatient encounters in a specialty mental health outpatient clinic for which either the primary or secondary diagnosis was schizophrenia (corresponding to ICD codes 295.00–295.99).
Data describing patient characteristics such as age, income, gender, ethnicity, receipt of VA compensation or pension, comorbid medical and psychiatric diagnoses, hospital utilization, and zip code of residence were also obtained from the VA workload databases. By using the zip code and data from the American Hospital Association annual survey, the distances from the centrum of the district designated by the patient’s zip code to the nearest VA and non-VA hospitals were calculated (27). We controlled for distance as a proxy measure for access, since the likelihood of receiving a diagnosis of diabetes mellitus might increase with easier access to care.
For all patients identified as having a diagnosis of schizophrenia, records of all medications prescribed between June (when data first became available) and September (the end of the fiscal year) of 1999 were obtained from the VA Drug Benefit Management System. For each patient, the last neuroleptic prescription written between June and September 1999 was identified as the index prescription. All prescriptions written for neuroleptics in the previous week were also identified. If any of the prescriptions written during that 7-day period were for clozapine, olanzapine, quetiapine, or risperidone, then the patient was entered into the atypical neuroleptic group, regardless of whether the patient was also prescribed typical neuroleptics at the same time. If a patient received only typical neuroleptics during that week, the patient was entered into the typical neuroleptic group. If a patient had no neuroleptic prescription written for the entire 4-month period, the patient was not included in this analysis. Within the atypical group, 94% of patients received the same atypical neuroleptic for the entire 4-month period and 8.9% also received a prescription for a typical neuroleptic (28).
For the group of patients identified as having a diagnosis of schizophrenia and receiving neuroleptic medications, computerized administrative data were examined for record of a diagnosis of diabetes mellitus. A patient was operationally defined as having a diagnosis of diabetes mellitus if the patient had at least one outpatient encounter or inpatient stay with either a primary or secondary diagnosis of diabetes mellitus (corresponding to ICD codes 250.00–250.99) during the 4-month period for which the prescribing data were available.
Analysis
Because of the strong relationship between age and development of diabetes (25), the patients in both the atypical and typical neuroleptic groups were stratified into five age groups: under 40, 40–49, 50–59, 60–69, and over 70 years. Within each age division, the percentages of patients with a diagnosis of diabetes in the atypical and typical neuroleptic groups were compared with chi-square tests.
To address potentially confounding factors, we used logistic regression analysis to calculate odds ratios for the association of atypical neuroleptic prescription and the diagnosis of diabetes within each of the age strata, controlling for the effects of the demographic, diagnostic, and treatment factors listed in Table 1.
These analyses were then repeated to examine the prevalence of diabetes mellitus associated with each atypical neuroleptic. A series of four dichotomous variables was included in these analyses to represent each of the atypical medications available at the time of the study.
In a final analysis the five age groups were combined in a single analysis with age as a covariate to determine the population-wide prevalence of diabetes in association with atypical neuroleptic prescription and in association with each particular atypical medication.
Results
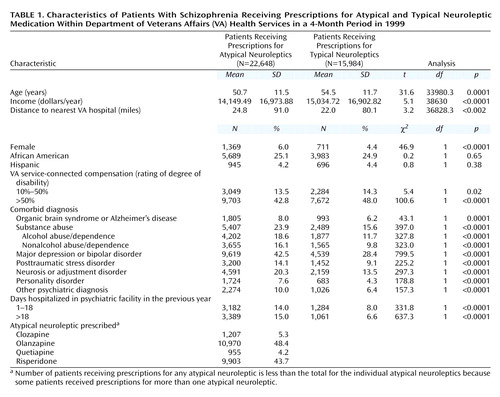
In the 4-month study period, 38,632 patients identified with schizophrenia received a prescription for a neuroleptic. Of those patients, 22,648 (58.6%) received an index prescription for an atypical neuroleptic and 15,984 (41.4%) received an index prescription for typical neuroleptics alone. A comparison of the two groups is presented in Table 1. Significant differences were examined by using t tests for continuous variables and chi-square tests for dichotomous variables.
Compared to the patients who received prescriptions for typical neuroleptics, the patients who received prescriptions for atypical neuroleptics were significantly younger and had less income. They were significantly more likely to be female and have another psychiatric diagnosis, including organic brain syndrome or Alzheimer’s dementia, substance abuse, major depression or bipolar disorder, posttraumatic stress disorder, neurosis or adjustment disorder, personality disorder, or other, unspecified, psychiatric disorder. Patients in the atypical group lived significantly further from the nearest VA hospital and were significantly more likely to have been hospitalized. Patients in the typical neuroleptic group were more likely to be receiving service-connected compensation from the VA.
Patients who received atypical neuroleptic medications demonstrated a statistically significant higher rate of diagnosis of diabetes in the three youngest age strata (Figure 1). Among patients less than 40 years old, for example, 8.75% of those receiving an atypical neuroleptic had a diagnosis of diabetes mellitus, compared to 6.43% of those receiving typical neuroleptics (χ2=7.24, df=1, p=0.007). This relationship was also observed in the 40–49-year age group (15.89% versus 13.93%) (χ2=9.81, df=1, p=0.002) and in the 50–59-year age group (22.73% versus 20.56%) (χ2=8.53, df=1, p=0.003). No significant association was observed in either the 60–69-year group or the group age 70 and older. Comparing across all ages, 18.84% of the patients who received a prescription for an atypical neuroleptic and 18.64% of those who received a prescription for a typical neuroleptic also had a diagnosis of diabetes, a nonsignificant difference.
The adjusted odds ratios for the entire group and each of the age groups are presented in Table 2. For the entire group (all ages), the odds of having a diagnosis of diabetes mellitus were significantly greater for patients receiving any atypical neuroleptic and, specifically, for patients receiving clozapine, olanzapine, and quetiapine, but not risperidone. In the under-40-year age group, the odds were significantly greater for all of the atypical neuroleptics. In the 40–49-year-old age group, a prescription for clozapine, olanzapine, or quetiapine was associated with significantly increased odds for having a diagnosis of diabetes mellitus, but a risperidone prescription was not. In the 50–59-year age group, only olanzapine and risperidone were associated with a significantly increased prevalence of a diagnosis of diabetes. In the 60–69-year age group, clozapine was associated with a significantly decreased prevalence.
Discussion
This study demonstrated, overall, a significant association between prescription of clozapine, olanzapine, and quetiapine, but not risperidone, and diagnosis of diabetes mellitus, compared with prescription of typical neuroleptics. In analyses that controlled for age, the strongest effect was observed in patients less than 40 years old (odds ratio=1.63, 95% CI=1.23–2.16), and each of the atypical neuroleptics was associated with an increased prevalence of diabetes mellitus in at least two of the age groups. These results are consistent with recent reports of significantly higher serum glucose levels in patients randomly assigned to olanzapine, compared to those receiving haloperidol (10), even without adjustments for age.
The observation that there are no overall differences in the rates of diabetes mellitus in the older age groups suggests that those with the predilection to develop diabetes in these age groups have already done so, and those left, lacking the diathesis for its development, will not develop diabetes despite being exposed to atypical neuroleptics. Thus the increased prevalence associated with atypical antipsychotics may be thought of hastening the onset of diabetes rather than precipitating it de novo. The observation of no difference at older ages may also suggest that, in those groups, age may be a more important risk factor for comorbid diabetes than the neuroleptic prescribed.
Both the typical and atypical treated groups demonstrated very high rates of diabetes mellitus. The rate of diabetes mellitus in the general U.S. male population age 20–39 years is 1.1% (25), whereas we observed rates of 6.2%–8.7%. These findings are consistent with previous reports of rates of 5.6%–6.7% in U.S. patients with schizophrenia age 18–44 years (23).
Several methodological limitations deserve mention. First, although this study examined data from more than 38,000 patients with a diagnosis of schizophrenia who received prescriptions for neuroleptics, the narrow time frame of 4 months yielded a virtual cross-sectional sample, precluding determination of the temporal relationship between the prescription of neuroleptics and the development of diabetes mellitus. It is possible, therefore, that patients may have been switched from an atypical to either a typical neuroleptic or another atypical secondary to development of diabetes before the 4-month prescription window. Such a switch would have the practical consequence of underestimating the risk for those medications more likely to cause diabetes and overestimating the risk for those medications less likely to cause the disease.
Second, data on changes in weight in patients who received prescriptions for atypical neuroleptics were also unavailable. These data might have clarified one potential mechanism of action for the development of diabetes.
Third, the patients who received prescriptions for typical neuroleptics may have been less likely to take the medications because of their side effect profiles than were those with prescriptions for the atypical neuroleptics. Thus, even if both groups of neuroleptics were equally likely to cause diabetes, more cases of diabetes might be seen in the atypical group if patients were taking more of their prescribed medication. We think, however, that this explanation is unlikely because empirical data show that, although patients taking atypical neuroleptics are more likely to continue on those medications for an extended period of time, they are no more likely to take the prescribed dosage than patients who take typical neuroleptics (29).
Fourth, this study relied on screening an administrative database for appropriate ICD-9 codes to identify cases of diabetes mellitus. Although the validity of this method has not been established empirically, there is no reason to believe that this method would result in any bias in the assignment of cases of diabetes to either the typical or atypical neuroleptic groups.
Finally, several possible alternative explanations for our observation deserve comment. First, it is possible that patients with preexisting diabetes mellitus were selectively switched to atypical neuroleptics. This explanation is unlikely, since the literature has implicated atypical neuroleptics in potentially inducing or exacerbating diabetes mellitus. It is more likely that clinicians, aware of case reports asserting a connection between some atypical neuroleptics and diabetes mellitus, might have avoided these medications in patients with diabetes, artificially decreasing our ability to detect an association. It is also possible, however, that clinicians who were aware of the potential risks could have chosen to monitor blood sugars more carefully in patients taking atypical neuroleptics, thereby identifying additional cases of diabetes and inflating the effect size.
A second alternative interpretation is that both atypical neuroleptic prescription and receipt of a diagnosis of diabetes could be associated with more severe forms of schizophrenia. If more severe cases of schizophrenia have a higher risk of diabetes mellitus, then more symptomatic patients may be both more likely to receive atypical antipsychotics and to be at greater risk for diabetes, although the atypical neuroleptics are not the causal agent. Although we cannot rule out these potentially confounding associations, we have attempted to adjust for them by covarying the effect of several measures of severity in our analyses.
Third, it is possible that receipt of atypical neuroleptics and having a diagnosis of diabetes both reflect a higher overall quality of medical care, including careful medical monitoring and state-of-the-art psychopharmacology.
Thus, although this study in a large national sample has demonstrated a substantial and statistically significant association between atypical neuroleptic prescription and diabetes mellitus, it did not definitively establish a causal relationship. The results are, however, strongly suggestive of such a relationship and demonstrate both the potential magnitude and public health importance of this association, as well as the potential utility of monitoring patients who receive atypical neuroleptics for signs and symptoms of diabetes mellitus.
In a recent comprehensive review of the effectiveness of atypical neuroleptics, Geddes et al. (30) concluded that the primary benefit of atypical antipsychotics was in reducing side effects. If it is established that these medications reduce the risk of extrapyramidal symptoms but increase the risk of diabetes, the risk/benefit ratio of these medications might be significantly altered.
 |
 |
Presented in part at the 19th annual meeting of the Department of Veterans Affairs Health Services Research and Development, Washington, D.C., Feb. 14–16, 2001, and the 154th annual meeting of the American Psychiatric Association, New Orleans, May 5–10, 2001. Received March 15, 2001; revisions received June 22 and July 27, 2001; accepted Aug. 24, 2001. From the Psychiatry Service, VA Connecticut Healthcare System; VA Mental Illness Research, Education, and Clinical Center, and Department of Psychiatry, Yale University School of Medicine, New Haven, Conn.; VA Northeast Program Evaluation Center, New Haven, Conn.; Psychiatry Service, Atlanta VA Medical Center, and Department of Psychiatry, Emory University School of Medicine, Atlanta; Psychiatry Service, VA New Jersey Healthcare System, and Department of Psychiatry, University of Medicine and Dentistry of New Jersey, New Jersey Medical School and Robert Wood Johnson School of Medicine, East Orange, N.J. Address reprint requests to Dr. Sernyak, Psychiatry Service, 116A, VA Connecticut Healthcare System, West Haven Campus, 950 Campbell Ave., West Haven, CT, 06516; [email protected] (e-mail). Supported by the Department of Veterans Affairs Veterans Integrated Service Network 1 Mental Illness Research, Education, and Clinical Center. The authors thank the Drug Benefit Management System, Hines, Ill., for supplying the prescription drug information.
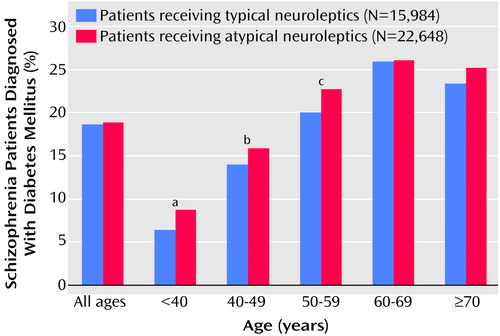
Figure 1. Percentage of Patients With Schizophrenia Receiving Prescriptions for Atypical and Typical Neuroleptic Medication Who Also Had a Diagnosis of Diabetes Mellitus
aχ2=7.24, df=1, p<0.07.
bχ2=9.81, df=1, p=0.002.
cχ2=8.53, df=1, p=0.003.
1. Kane J, Honigfeld G, Singer J, Meltzer H (Clozaril Collaborative Study Group): Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789-796Crossref, Medline, Google Scholar
2. Tollefson GD, Beasley CM Jr, Tran PV, Street JS, Krueger JA, Tamura RN, Graffeo KA, Thieme ME: Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry 1997; 154:457-465Link, Google Scholar
3. Small J, Hirsch S, Arvanitis L, Miller B, Link C: Quetiapine in patients with schizophrenia: a high- and low-dose double-blind comparison with placebo. Arch Gen Psychiatry 1997; 54:549-557Crossref, Medline, Google Scholar
4. Marder SR, Meibach RC: Risperidone in the treatment of schizophrenia. Am J Psychiatry 1994; 151:825-835Link, Google Scholar
5. Stahl SM: Selecting an atypical antipsychotic by combining clinical experience with guidelines from clinical trials. J Clin Psychiatry 1999; 60(suppl 10):31-41Google Scholar
6. Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ: Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999; 156:1686-1696Abstract, Google Scholar
7. Ober SK, Hudak R, Rusterholtz A: Hyperglycemia and olanzapine (letter). Am J Psychiatry 1999; 156:970Link, Google Scholar
8. Fertig MK, Brooks VG, Shelton PS, English CW: Hyperglycemia associated with olanzapine. J Clin Psychiatry 1998; 59:687-689Crossref, Medline, Google Scholar
9. Wirshing DA, Spellberg BJ, Erhart SM, Marder SR, Wirshing WC: Novel antipsychotics and new onset diabetes. Biol Psychiatry 1998; 44:778-783Crossref, Medline, Google Scholar
10. Kinon BJ, Basson BR, Gilmore JA, Tollefson GD: Long-term olanzapine treatment: weight change and weight-related health factors in schizophrenia. J Clin Psychiatry 2001; 62:92-100Crossref, Medline, Google Scholar
11. Cohen S, Chiles J, MacNaughton A: Weight gain associated with clozapine. Am J Psychiatry 1990; 147:503-504Link, Google Scholar
12. Lamberti JS, Bellnier T, Schwarzkopf SB: Weight gain among schizophrenic patients treated with clozapine. Am J Psychiatry 1992; 149:689-690Link, Google Scholar
13. Bustillo JR, Buchanan RW, Irish D, Breier A: Differential effect of clozapine on weight: a controlled study. Am J Psychiatry 1996; 153:817-819Link, Google Scholar
14. Henderson DC, Cagliero E, Gray C, Nasrallah RS, Hayden DL, Schoenfeld DA, Goff DC: Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: a five-year naturalistic study. Am J Psychiatry 2000; 157:975-981Link, Google Scholar
15. Kamran A, Doraiswamy PM, Jane JL, Hammett EB, Dunn L: Severe hyperglycemia associated with high doses of clozapine (letter). Am J Psychiatry 1994; 151:1395Medline, Google Scholar
16. Koval MS, Rames LJ, Christie S: Diabetic ketoacidosis associated with clozapine treatment (letter). Am J Psychiatry 1994; 151:1520-1521Medline, Google Scholar
17. Peterson GA, Byrd SL: Diabetic ketoacidosis from clozapine and lithium cotreatment (letter). Am J Psychiatry 1996; 153:737-738Medline, Google Scholar
18. Kostakoglu AE, Yazici KM, Erbas T, Guvener N: Ketoacidosis as a side-effect of clozapine: a case report. Acta Psychiatr Scand 1996; 93:217-218Crossref, Medline, Google Scholar
19. Popli AP, Konicki PE, Jurjus GJ, Fuller MA, Jaskiw GE: Clozapine and associated diabetes mellitus. J Clin Psychiatry 1997; 58:108-111Crossref, Medline, Google Scholar
20. Hagg S, Joelsson L, Mjorndal T, Spigset O, Oja G, Dahlqvist R: Prevalence of diabetes and impaired glucose tolerance in patients treated with clozapine compared with patients treated with conventional depot neuroleptic medications. J Clin Psychiatry 1998; 59:294-299Crossref, Medline, Google Scholar
21. Melkersson KI, Hulting AL, Brismar KE: Elevated levels of insulin, leptin, and blood lipids in olanzapine-treated patients with schizophrenia or related-psychoses. J Clin Psychiatry 2000; 61:742-749Crossref, Medline, Google Scholar
22. Sobell M, Jaggers ED, Franz MA: New-onset diabetes mellitus associated with the initiation of quetiapine treatment. J Clin Psychiatry 2000; 60:556-557Crossref, Google Scholar
23. Dixon L, Weiden P, Delahanty J, Goldberg R, Postrado L, Lucksted A, Lehman A: Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000; 26:903-912Crossref, Medline, Google Scholar
24. Mukherjee S, Decina P, Bocola V, Saraceni F, Scapicchio P: Diabetes mellitus in schizophrenic patients. Compr Psychiatry 1996; 37:68-73Crossref, Medline, Google Scholar
25. Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer H, Byrd-Holt D: Prevalence of diabetes, impaired fasting glucose tolerance in US adults: the Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care 1998; 21:518-524Crossref, Medline, Google Scholar
26. Melkersson KI, Hulting A, Brismar KE: Different influences of classical antipsychotics and clozapine on glucose-insulin homeostasis in patients with schizophrenia or related psychoses. J Clin Psychiatry 1999; 60:783-791Crossref, Medline, Google Scholar
27. Rosenheck R, Stolar M: Access to public mental health services: determinants of population coverage. Med Care 1998; 36:503-512Crossref, Medline, Google Scholar
28. Rosenheck R, Leslie D, Sernyak M: From clinical trials to real-world practice: use of atypical antipsychotic medication nationally in the Department of Veterans Affairs. Med Care 2001; 39:302-308Crossref, Medline, Google Scholar
29. Rosenheck R, Chang S, Choe Y, Cramer J, Xu W, Thomas J, Henderson W, Charney D: Medication continuation and compliance: a comparison of patients treated with clozapine and haloperidol. J Clin Psychiatry 2000; 61:382-386Crossref, Medline, Google Scholar
30. Geddes J, Freemantle N, Harrison P, Bebbington P: Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. Br Med J 2000; 321:1371-1376Crossref, Medline, Google Scholar



