Hypoxic-Ischemia-Related Fetal/Neonatal Complications and Risk of Schizophrenia and Other Nonaffective Psychoses: A 19-Year Longitudinal Study
Abstract
OBJECTIVE: Epidemiologic evidence linking obstetric complications to schizophrenia has been positive but inconclusive. One reason for the lack of conclusive evidence may be the inconsistency in measuring disturbances of fetal/neonatal brain development based on general obstetric markers of maternal health. The authors used data from the National Collaborative Perinatal Project to examine the relationship between schizophrenia and other nonaffective psychoses and a theoretically derived measure of hypoxic-ischemia-related fetal/neonatal complications. METHOD: Six hundred ninety-three men and women (average age 23) born to a community sample of women between 1959 and 1966 were followed up an average of 19 years after early childhood assessments. Subjects with DSM-IV schizophrenia and other nonaffective psychoses were identified using the Diagnostic Interview Schedule and best-estimate consensus diagnoses. RESULTS: Hypoxic-ischemia-related fetal/neonatal complications were associated with a doubling of the risk of developing a psychotic disorder, compared with no relevant complications (6.9% versus 1.4%). When mood disorders were excluded from the group of psychotic diagnoses, the risk of schizophrenia and other nonaffective psychoses associated with hypoxic-ischemia-related fetal/neonatal complications was strikingly elevated, compared with no relevant complications (5.75% versus 0.39%). Nonpsychotic mood disorders were unrelated to these fetal/neonatal complications. Schizophrenia and other nonaffective psychoses were most strongly associated with hypoxic-ischemia-related fetal/neonatal complications of disordered growth and development. CONCLUSIONS: The data show a strikingly elevated, graded, independent risk of schizophrenia and other nonaffective psychoses associated with this classification of antecedent hypoxic-ischemia-related fetal/neonatal complications.
By the early 1900s, schizophrenia (“dementia praecox”) had been distinguished from mood disorders (“manic depressive insanity”) (1) and had been attributed to abnormal early brain development (2). Although genetic influences appear to be the primary etiology of schizophrenia, other factors may contribute to the causal process (3–5). Among adverse conditions suggesting disordered early brain development, obstetric complications have been considered the second most important category of risk factors for schizophrenia (6).
Evidence that obstetric complications relate somehow to schizophrenia and other nonaffective psychoses is supported by recent findings from four longitudinal studies (7–10), one adoption study (11), and six case-control studies (12–17). However, several negative studies are inconsistent with these findings, in particular two case-control studies (18, 19), and one longitudinal study (20). Examining potential indices of chronic hypoxia or oxygen deprivation (12) as a hypothetical pathway of risk, one longitudinal study found a nonsignificant doubling of the risk for schizophrenia (21), although findings from other investigations were not significant at the 5% level (9, 12).
One reason for the inconclusive findings may be the inconsistency inherent in measuring abnormal brain development based on obstetric markers of maternal health. General obstetrical conditions are heterogeneous in etiology and often are only indirectly related to fetal brain development. Consequently, we derived measures of fetal and neonatal complications that linked more explicitly neurological abnormalities of mild-to-moderate severity (22). We hypothesized that certain indices of threatened fetal development and neonatal neurological abnormalities that have been associated clinically with hypoxic ischemic encephalopathy in full-term neonates (23), but that are not necessarily diagnostic of this syndrome, may represent a more homogeneous etiologic pathway for schizophrenia and other nonaffective psychoses (22). Thus we classified study participants as evidencing hypoxic-ischemia-related fetal/neonatal complications if they were born at 37 weeks or more of gestation (to exclude prematurity, which is associated with intraventricular hemorrhage) (24) and demonstrated either certain patterns of complications associated with abnormal fetal development or the equivalent of “soft signs” on neonatal neurological examination.
Furthermore, we identified two subsets of hypoxic-ischemia-related fetal/neonatal complications: 1) conditions of compromised fetal growth and development (22, 23) and 2) nonspecific neonatal signs of mild-to-moderate neurological dysfunction and other complications within the hypoxic-ischemia-related group (25). This subclassification was based on the premise that certain conditions associated with disordered fetal growth and development, such as severe pre-eclampsia, intrauterine growth retardation, and dysmaturity (26), may be related to a genetically mediated subgroup that is more relevant to schizophrenia than are more apparently direct neonatal indicators of risk to brain development or oxygenation such as hyperexcitability or meconium (22). In the higher risk subgroup, therefore, we included infants who were small for gestational age (representing intrauterine growth retardation), exposed to severe pre-eclampsia (26), and post-term, or dysmature, which is now thought to be also related to fetal constitutional factors (27). We report here on the association between this novel classification of hypoxic-ischemia-related fetal/neonatal complications and subsequent schizophrenia and other nonaffective psychoses among a group of adult offspring born to a community sample of women between 1959 and 1966 and followed up an average of 19 years after early childhood assessments.
METHOD
Participants
The background for the study has been described previously (21). Briefly, the Providence, R.I., cohort of the National Collaborative Perinatal Project includes 4,140 individuals born to a community sample of 3,078 women whose pregnancies were studied between 1959 and 1966 (28). Buka and colleagues (21) followed up a subsample of adult offspring with and without general types of pregnancy/delivery complications matched on race, gender, date of birth, maternal age, parity, and education. Of the 928 eligible participants, 693 (75%) were located. After fully explaining all research procedures, we obtained written informed consent from all 693 offspring, and they were interviewed. Of those 693 subjects, 373 had complications at birth. The remaining 320 were normal comparison subjects. Fieldwork was carried out between February 1984 and March 1990. The mean age of the interviewed sample was 23.0 years (SD=2, range=18–27).
Classification of Complications
Subjects were included in the hypoxic-ischemia-related fetal/neonatal complication group if they had an abnormal neonatal neurological examination and/or a pattern of adverse conditions suggesting compromise to intrauterine growth and development. Participants belonging in the lowest 10th percentile of birth weight for each week of gestational age were classified as small for gestational age. Individuals born at 42 or more weeks of gestational age were defined as post-term. We classified participants as evidencing hypoxic-ischemia-related fetal/neonatal complications if they were born at 37 weeks or more of gestation and met at least one of four criteria in two major groups. The first group—patterns of disordered growth and development during a pregnancy with complications—included two criteria 1) small for gestational age (16) (with evidence of pre-eclampsia [10, 12], meconium staining of the amniotic fluid [23], or uterine bleeding [8]), and 2) post-term birth (13) (with evidence of pre-eclampsia [10, 12]). The second group of criteria—neonatal neurological abnormalities—included 3) hyperexcitability (25) and 4) suspected (not definite) hypotonia (25).
Severity codes of 2,1,or 0 were assigned to the subjects in the hypoxic-ischemia-related fetal/neonatal complication group. Severity code 2 was used for conditions of disordered growth and development, including being small for gestational age, post-term, or exposed to severe pre-eclampsia. Severity code 1 was used for all other obstetrical and neonatal abnormalities in the hypoxic-ischemia-related fetal/neonatal complication group. Severity code 0 was used when no hypoxic-ischemia-related fetal/neonatal complications were found.
Adult Psychiatric Diagnoses
Psychiatric diagnoses were based on information from direct, face-to-face interview or, for 15% of subjects, from telephone interviews. The interviews included administration of version III of the National Institute of Mental Health Diagnostic Interview Schedule (DIS) (29), a completely structured instrument designed to be used by nonclinicians. Two clinician raters (G.L.Z. and Jill M. Goldstein) reviewed together all diagnostic information for subjects who endorsed at least one psychotic symptom; the raters were blind to subjects’ perinatal status and family history. The raters made best-estimate, consensus diagnoses using DSM-IV criteria.
Diagnoses were classified using the following categories. The category of all psychotic disorders comprised the subcategories of DSM-IV schizophrenia and other nonaffective psychoses and of DSM-IV mood disorders with psychotic features. The subcategory of schizophrenia and other nonaffective psychoses included schizophrenia, schizophreniform disorder, schizoaffective disorder, delusional disorder, and psychotic disorder not otherwise specified. The subcategory of mood disorders with psychotic features included bipolar I disorder with psychotic features, bipolar II disorder with psychotic depression, and major depressive disorder with psychotic features. The category of nonpsychotic mood disorders included bipolar I disorder without psychotic features, bipolar II disorder without psychotic features, and major depressive disorder without psychotic features.
Control Variables
The six control variables evaluated in this study were familial risk, gender, age, race, prenatal care, and socioeconomic status at birth. Familial risk was classified according to the presence of major psychiatric disorders (psychotic disorders, major depression, and mania) occurring ever in the lifetime of all first-degree relatives (30). Interrater reliability was evaluated by two psychiatrists (G.L.Z. and Jordan W. Smoller); the unweighted kappa value for each diagnostic classification was 1.00 for psychosis, 0.80 for major depression, and 0.60 for mania. The mean kappa across familial diagnoses was 0.80. Reduced prenatal care was defined as an initial prenatal visit after the 23rd week of gestation. Socioeconomic status was assigned a single, continuous score for education, occupation, and family income according to the system used for the United States Bureau of the Census (31).
Statistical Analysis
Unadjusted relative risk and 95% confidence intervals (CIs) were calculated as the ratio of the lifetime prevalence of psychiatric diagnosis in the hypoxic-ischemia-related fetal/neonatal complication group to the lifetime prevalence in the comparison group. Distributions of risk factors were calculated for the two groups. The bivariate relations of hypoxic-ischemia-related fetal/neonatal complications to psychiatric diagnoses were examined using exact inference on the logistic regression model with two aims: 1) to estimate odds ratios as approximations of relative risks for each diagnostic outcome conditioned on potential confounders and 2) to test for linear trend for the three groups classified by level of severity of fetal/neonatal complications coded as 0, 1, and 2 (32). This model was also used to test for possible interactions between hypoxic-ischemia-related fetal/neonatal complications and the control variables listed above. All tests were two-tailed.
RESULTS
Characteristics of Subjects
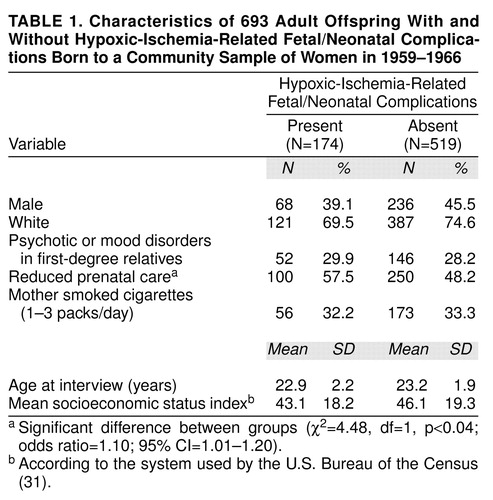
Characteristics of the subjects according to hypoxic-ischemia-related fetal/neonatal complication classification status are summarized in table 1. The majority of the sample was white. One hundred seventy-four of the 693 subjects who were interviewed had hypoxic-ischemia-related fetal/neonatal complications. There was a weak positive association between reduced prenatal care and hypoxic-ischemia-related fetal/neonatal complications (table 1).
Familial risk of psychosis and mood disorders was not significantly associated with hypoxic-ischemia-related fetal/neonatal complications nor with schizophrenia and other nonaffective psychoses among the study subjects, but was related to lifetime prevalence of bipolar disorder (Fisher’s exact test, p<0.00001, two-tailed) and nonpsychotic mood disorders (χ2=23.14, df=1, p=0.001). There was a trend toward an association between familial risk and all psychoses (both nonaffective and affective) (χ2=3.39, df=1, p=0.07).
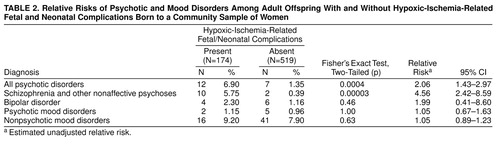
Of 693 offspring, 19 (2.7%) had a lifetime diagnosis within the all psychotic disorders category; 12 (1.7%) were diagnosed with schizophrenia and other nonaffective psychoses, and seven (1.0%) with psychotic mood disorders. Fifty-seven (8.2%) were diagnosed with nonpsychotic mood disorders. These percentages are within the reported ranges of the distribution of expected lifetime prevalences (33, 34).
Risk of Adult Psychiatric Diagnosis
As shown in table 2, there was a two-fold risk of being diagnosed with a psychotic disorder in the group with hypoxic-ischemia-related fetal/neonatal complications, compared with the group without these complications. We also examined the relationship of hypoxic-ischemia-related fetal/neonatal complications and other psychiatric diagnoses. These complications were associated with more than a four-fold risk of schizophrenia and other nonaffective psychoses, compared with no complications. There were no differences between groups with and without hypoxic-ischemia-related fetal/neonatal complications in the rates of mood disorders with psychotic features and of nonpsychotic mood disorders.
Risk Adjusted for Potential Confounders
Six exact logistic regression models were used to compute the odds ratios for risk of all psychotic disorders conditioned on potentially confounding control variables. Model 1 was unadjusted for confounders, model 2 was conditioned on familial risk of major psychiatric disorders, model 3 was conditioned on socioeconomic status, model 4 was conditioned on race, model 5 was conditioned on gender, and model 6 was conditioned on reduced prenatal care.
The unadjusted and adjusted results were similar for all six models. The unadjusted risk of developing any psychotic disorder associated with hypoxic-ischemia-related fetal/neonatal complications was 5.41 (95% CI=1.93–16.49, exact p=0.0004, N=19). The risk of developing any psychotic disorder was 5.34 (95% CI=1.90–16.34) conditioned on familial risk, 5.62 (95% CI=1.99–17.26) conditioned on socioeconomic status, 5.23 (95% CI=1.86–15.95) conditioned on race, 5.26 (95% CI=1.87–16.04) conditioned on gender, and 4.80 (95% CI=1.66–14.91) conditioned on reduced prenatal care. Because of the low base rates of psychotic disorders and the small number of psychotic subjects with no relevant hypoxic-ischemia-related fetal/neonatal complications, the odds ratios and 95% confidence intervals were greater than those of the relative risks, but remained consistent.
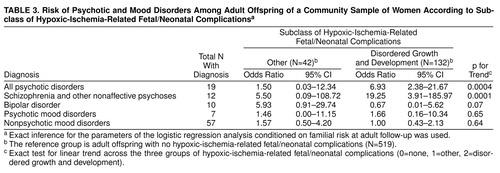
We examined the relative strength of the association between psychosis and hypoxic-ischemia-related fetal/neonatal complications that reflected disordered growth and development versus other heterogeneous conditions within the hypoxic-ischemia-related classification. Tests of trend comparing the relative risks for psychiatric disorders associated with these different classes of complications are presented in table 3. Conditioned on familial risk, a significantly greater relation was observed between hypoxic-ischemia-related fetal/neonatal complications associated with disordered growth and development, compared with all other hypoxic-ischemia-related fetal/neonatal complications, and the risk of psychosis, particularly schizophrenia and other nonaffective psychoses.
To examine further the independence of the association between hypoxic-ischemia-related fetal/neonatal complications and the risk of nonaffective psychoses as well as affective psychoses, we included an interaction term in these logistic regression models for hypoxic-ischemia-related fetal/neonatal complications and each variable listed in table 1. For schizophrenia and other nonaffective psychoses, there were no significant interactions between hypoxic-ischemia-related fetal/neonatal complications and familial risk, sex, age, race, amount of prenatal care, cigarette smoking, and socioeconomic status. There was one trend toward significance for risk of schizophrenia and other nonaffective psychoses: the presence of familial risk was associated with an exact p for trend=0.11 (N=12). On further stratification, the risk of schizophrenia and other nonaffective psychoses was 12.17 (95% CI=0.83–178.25) in the presence of familial risk and 1.89 (95% CI=0.96–3.74) in the absence of familial risk, suggesting the possibility of a quantitative interaction between these fetal/neonatal conditions and familial risk. For all other psychiatric diagnoses, including all psychotic disorders, psychotic mood disorders, and nonpsychotic mood disorders, no interaction terms were statistically significant.
DISCUSSION
We found a significant, independent, graded association between fetal/neonatal complications associated with vulnerability to hypoxic ischemic compromise to brain development and lifetime risk of schizophrenia and other psychoses. The association with hypoxic-ischemia-related fetal/neonatal complications was attributed to the large magnitude of risk for early-onset schizophrenia and other nonaffective psychoses in this 19-year follow-up study. Correspondingly, these complications were not associated with early-onset mood disorders.
The strikingly large magnitude of the relation between hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses is consistent broadly with other research findings (10, 16, 35). These results are concordant also with a report finding ischemic brain injury in newborns to be associated with long-term neurobehavioral sequelae (36). The importance of this theoretically derived classification partly stems from the shift in focus away from general obstetrical factors or “optimality” of the pregnancy (37) and to adverse fetal/neonatal conditions associated with a more homogeneous, predefined etiologic pathway of risk. Although the findings are based on a small number of individuals who developed psychotic disorders, the diagnostic specificity lends further support to the association between hypoxic-ischemia-related fetal/neonatal complications and early-onset schizophrenia and other nonaffective psychoses.
Identification and definition of these complications was based on a synthesis of clinical observations tempering the interpretation of evidence in the research literature on schizophrenia (1, 23–23, 26). There is evidence that control of developmental and reproductive problems such as abnormal fetal growth rates, prematurity, recurrent spontaneous abortion, and severe pre-eclampsia may be largely genetic in origin (26, 38). These conditions appear to be related in some way to maternal and fetal major histocompatibility genotypes, suggesting that complex genetic mechanisms may underlie a group of disorders of growth, development, and reproduction (26), which may in turn interact with other genes that contribute to the schizophrenia disease process.
This study demonstrates an association, but not necessarily a direct causal relation between hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia. Typically, researchers have assumed obstetrical risk factors to represent extrinsic brain injury separate from genetic factors (6). This assumption, however, may not reflect the complete picture (12, 22). Taken together with earlier findings of a heightened susceptibility to schizophrenia related to obstetrical complication in the presence of genetic risk (39) and developmental anomalies (40), the data presented here provide intriguing clues suggesting that certain genetically mediated developmental processes associated with vulnerability to environmental intrauterine insults may predispose to later schizophrenia.
Rather than simply suggesting a “continuum of reproductive casualty” of extrinsic insults to the fetal brain surrounding gestation (41), our findings provide further support for a continuum of genetic risk interacting with a spectrum of environmental risk for schizophrenia (22). It is possible that genes involved in disordered development may amplify the effects of genes that may primarily mediate liability to schizophrenia. The etiology of schizophrenia may depend on the nature of the interaction between combinations of genetic factors and extrinsic insults that affect brain development before the first psychotic break (42). In keeping with such a gene-environment view, extrinsic risk factors such as traumatic brain injury (9, 43), severe hypoxic insult (17, 21) or viral infection (43) could heighten the genetic vulnerability to schizophrenia.
Limitations
Five limitations of this study should be acknowledged. First, the expected low base rate of psychotic disorders in this small cohort reduces the stability of the findings, as reflected in the wide confidence intervals for the risk ratios and the inability to control simultaneously for multiple confounders. However, we used conservative statistical methods for the logistic regression analyses conditioned on major potential confounders, and the results were consistent. Nevertheless, uncontrolled confounding remains a possibility.
Second, a systematic study of psychiatric disorders in first-degree relatives and genetic studies of the candidate genes would be necessary to assess adequately an interaction with genetic risk for schizophrenia. An underestimate of familial risk would leave the results vulnerable to residual confounding by genetic factors. However, a graded, specific relation of this magnitude between hypoxic-ischemia-related fetal/neonatal complications and early onset schizophrenia and other nonaffective psychoses due to residual confounding is unlikely.
Third, the time to follow-up is too short to ascertain comparable lifetime rates of mood disorders, which have an age at onset and period of greatest risk later than schizophrenia. The short follow-up period could result in an underestimate of the risk of mood disorders, relative to the risk of schizophrenia, that is associated with these hypoxic-ischemia-related fetal/neonatal complications. Nonetheless, general obstetrical complications have been associated with mood disorders of early onset (44), and other fetal/neonatal complication categories may be more relevant to mood disorders. The hypoxic-ischemia-related classification is one of a number of hypothetical pathways of neurodevelopmental risk for schizophrenia (22, 42). Likewise, because of the relatively young age of the subjects at follow-up, individuals with later-onset psychoses are not be represented in the findings. In addition, the study sample may not be representative of individuals of other ethnic backgrounds or of individuals in different time periods or different regions.
Finally, cross-sectional diagnostic interviews by nonclinicians may underestimate the prevalence of psychotic disorders (45), which would attenuate the true risk of schizophrenia. To identify diagnoses of schizophrenia accurately, structured clinical interviews should be conducted on follow-up using two-stage ascertainment with supplementation by hospital records over a defined time period. However, the frequencies of diagnoses identified in this study are in accord with findings from published reports (33, 34). Also, the diagnostic reliability was high due to the agreement achieved through best-estimate, consensus diagnoses.
Despite these limitations, this study presents an important step forward in classifying obstetrical complications along targeted pathways of fetal/neonatal risk. The indices of the hypoxic-ischemia-related fetal/neonatal complication classification presented in this paper need to be refined through iterative assessments linked directly to brain structure and function. Because effects that have been attributed to hypoxic-ischemia-related fetal/neonatal complications may be at least partly genetically mediated, a revised approach to systematic genetic analyses that includes evaluation of the role of fetal-maternal compatibility may prove informative (22, 26, 38). Successive refinements of these measures based on findings from neuroimaging and genetic studies will need to be incorporated into longitudinal research of schizophrenia.
Presented at the American Psychiatric Association Research Colloquium for Junior Investigators, National Institutes of Health, Bethesda, Maryland, May 16, 1999. Received Dec. 30, 1998; revision received May 26, 1999; accepted May 28, 1999. From the Departments of Epidemiology and Maternal and Child Health, Harvard School of Public Health; the Brockton/West Roxbury Veterans Affairs Medical Center; the Harvard Institute of Psychiatric Epidemiology and Genetics; and the Department of Psychiatry, Harvard Medical School at the Massachusetts Mental Health Center. Address reprint requests to Dr. Zornberg, 11 Muzzey Street, Lexington, MA 02421; [email protected] (e-mail). Supported by Department of Health and Human Services Public Health Service Award through NIMH Training in Psychiatric Epidemiology and Biostatistics grant MH-17119 to Drs. Zornberg and Tsuang, NIMH grants MH-50647 and MH-43518 to Dr. Tsuang, and a grant from the Stanley Foundation to Dr. Buka. The authors thank Dr. Lee-Jen Wei for assistance with data analyses; Drs. Jill M. Goldstein, Larry J. Seidman, and Jordan W. Smoller for their contributions to diagnostic evaluations; Lynda Jacobs for encoding the data; Anne Marie Peres, Lisa Denny, and Lisa Quinn for help with data collection; and Dr. Douglas K. Richardson for his thoughtful comments on an earlier version of the manuscript.
 |
 |
 |
1. Kraepelin E: Dementia Praecox and Paraphrenia. Translated by Barclay RM; edited by Robertson GM. Edinburgh, E & S Livingstone, 1919, pp 234–235Google Scholar
2. Southard EE: On the topographical distribution of cortex lesions and anomalies in dementia praecox, with some account of their functional significance. Am J Insanity 1915; 71:603–671Abstract, Google Scholar
3. Tsuang MT, Faraone SV: Epidemiology and behavioral genetics of schizophrenia, in Biology of Schizophrenia and Affective Disease. Edited by Watson SJ. New York, Raven Press, 1996, pp 163–195Google Scholar
4. Murray RM, Lewis SW, Reveley AM: Towards an aetiological classification of schizophrenia. Lancet 1985; 1:1023–1026Google Scholar
5. Weinberger DR: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
6. McNeil TF: Perinatal risk factors and schizophrenia: selective review and methodological concerns. Epidemiol Rev 1995; 17:107–112Crossref, Medline, Google Scholar
7. Parnas J, Schulsinger F, Teasdale TW, Schulsinger H, Feldman PM, Mednick SA: Perinatal complications and clinical outcome within the schizophrenia spectrum. Br J Psychiatry 1982; 140:416–420Crossref, Medline, Google Scholar
8. Sacker A, Done JD, Crow TJ, Golding J: Antecedents of schizophrenia and affective illness: obstetric complications. Br J Psychiatry 1995; 166:734–741Crossref, Medline, Google Scholar
9. Jones PB, Rantakallio P, Hartikainen A-L, Isohanni M, Sipila P: Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: a 28-year follow-up of the 1966 North Finland general population birth cohort. Am J Psychiatry 1998; 155:355–364Link, Google Scholar
10. Dalman C, Allebeck P, Cullberg J, Grunewald, Koster M: Obstetric complications and the risk of schizophrenia. Arch Gen Psychiatry 1999; 56:234–240Crossref, Medline, Google Scholar
11. Jacobsen B, Kinney DK: Perinatal complications in adopted and non-adopted schizophrenics and their controls: preliminary results. Acta Psychiatr Scand 1980; 62:337–346Crossref, Google Scholar
12. McNeil TF, Kaij L: Obstetric factors in the development of schizophrenia: complications in the birth of preschizophrenics and in reproduction by schizophrenic parents, in Schizophrenia: New Approaches to Research and Treatment. Edited by Wynne LC, Cromwell RL, Matthysse S. New York, John Wiley & Sons, 1978, pp 401–429Google Scholar
13. Gillberg C, Wahlström J, Forsman A, Hellgren L, Gillberg IC: Teenage psychoses—epidemiology, classification and reduced optimality in the pre-, peri- and neonatal periods. J Child Psychol Psychiatry 1986; 27:87–98Crossref, Medline, Google Scholar
14. Eagles JM, Gibson I, Bremner MH, Clunie F, Ebmeier KP, Smith NC: Obstetric complications in DSM-III schizophrenics and their siblings. Lancet 1990; 335:1139–1141Google Scholar
15. Gunther-Genta F, Bovet P, Hohlfeld P: Obstetric complications and schizophrenia: a case-control study. Br J Psychiatry 1994; 164:165–170Crossref, Medline, Google Scholar
16. Hultman CM, Öhman A, Cnattingius S, Wieselgren IM, Lindström LH: Prenatal and neonatal risk factors for schizophrenia. Br J Psychiatry 1997; 170:128–133Crossref, Medline, Google Scholar
17. Kendell RE, Juszcak E, Cole SK: Obstetric complications and schizophrenia: a case control study based on standardised obstetric records. Br J Psychiatry 1996; 168:556–561Crossref, Medline, Google Scholar
18. Cantor-Graae MA, McNeil TF, Sjöstrom K, Nordstrom LG, Rosenlund T: Obstetric complications and their relationship to other etiological risk factors in schizophrenia. J Nerv Ment Dis 1994; 182:645–650Crossref, Medline, Google Scholar
19. McCreadie RG, Hall DJ, Berry IJ, Robertson LJ, Ewing JI, Geals MF: The Nithsdale schizophrenia surveys, X: obstetrical complications, family history and abnormal movements. Br J Psychiatry 1992; 161:799–805Crossref, Google Scholar
20. Done DJ, Johnstone EC, Frith CD, Golding J, Shepherd PM, Crow TJ: Complications of pregnancy and delivery in relation to psychosis in adult life: data from the British perinatal mortality survey sample. Br Med J 1991; 302:1576–1580Google Scholar
21. Buka SL, Tsuang MT, Lipsitt LP: Pregnancy/delivery complications and psychiatric diagnosis. Arch Gen Psychiatry 1993; 50:151–156Crossref, Medline, Google Scholar
22. Zornberg GL: Fetal and Neonatal Complications and Risk of Psychoses and Mood Disorders (doctoral dissertation). Boston, Mass, Harvard University, School of Public Health, Department of Epidemiology, 1997Google Scholar
23. Amiel-Tyson C: Clinical assessment of the infant nervous system, in Fetal and Neonatal Neurology and Neurosurgery. Edited by Levene MI, Lilford RJ. New York, Churchill, Livingstone, 1995, pp 83–104Google Scholar
24. Brann AW, Schwartz JF: Birth injury, in Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant. Edited by Fanaroff AA, Martin R. St Louis, Mosby, 1992, pp 703–718Google Scholar
25. Sarnat HB, Sarnat MS: Neonatal encephalopathy following fetal distress: a clinical and encephalographic study. Arch Neurol 1976; 33:696–705Crossref, Medline, Google Scholar
26. Gill TJ: Reproductive immunology and immunogenetics, in The Physiology of Reproduction, 2nd ed. Edited by Knobil E, Neill JD. New York, Raven Press, 1994, pp 783–812Google Scholar
27. Kochenour NK: Postterm pregnancy, in Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant. Edited by Fanaroff AA, Martin R. St Louis, Mosby, 1992, pp 230–234Google Scholar
28. Niswander KR, Gordon M: The Women and Their Pregnancies. Washington, DC, US Government Printing Office, 1972Google Scholar
29. Robins LN, Helzer JE, Croughan J (eds): National Institute of Mental Health Diagnostic Interview Schedule, version III: PHS Publication ADM-T-42-3. Rockville, Md, NIMH, 1981Google Scholar
30. Robins LN: St Louis Health Study: Wave II. Baltimore, Survey Research Associates, 1982Google Scholar
31. Myrianthopoulos NC, French KS: An application of the US Bureau of the Census socioeconomic index to a large, diversified patient population. Soc Sci Med 1968; 2:283–299Crossref, Medline, Google Scholar
32. Mehta CR, Patel NR, Senchaudhuri P: Exact stratified linear rank tests for ordered categorical and binary data. J Computational and Graphical Statistics 1992; 1:21–40Google Scholar
33. Robins LN, Helzer JE, Weissman MM, Orvaschel H, Gruenberg E, Burke JD Jr, Regier DA: Lifetime prevalence of specific psychiatric disorders in three sites. Arch Gen Psychiatry 1984; 41:949–958Crossref, Medline, Google Scholar
34. Parnas J, Cannon TD, Jacobsen B, Schulsinger H, Schulsinger F, Mednick SA: Lifetime DSM-III-R diagnostic outcomes in the offspring of schizophrenic mothers: results from the Copenhagen study. Arch Gen Psychiatry 1993; 50:707–714Crossref, Medline, Google Scholar
35. Geddes JR, Lawrie SM: Obstetrical complications and schizophrenia: a meta-analysis. Br J Psychiatry 1995; 167:786–793Crossref, Medline, Google Scholar
36. Whitaker AH, Van Rossem R, Feldman JF, Schonfeld IS, Pinto-Martin JA, Torre C, Shaffer D, Paneth N: Psychiatric outcomes in low-birth-weight children at age 6 years: relation to neonatal cranial ultrasound abnormalities. Arch Gen Psychiatry 1997; 54:847–856Crossref, Medline, Google Scholar
37. Prechtl HFR: The optimality concept. Early Hum Dev 1980; 4:201–205Crossref, Medline, Google Scholar
38. Ober C: The maternal-fetal relationship in human pregnancy: an immunogenetic perspective. Exp Clin Immunogenet 1992; 9:1–14Medline, Google Scholar
39. Cannon TD, Mednick SA: The schizophrenia high-risk project in Copenhagen: three decades of progress. Acta Psychiatr Scand 1993; Suppl 370:33–47Google Scholar
40. Waddington JL, Buckley PF, Scully PJ, Lane A, O’Callaghan EO, Larkin C: Course of psychopathology, cognition and neurobiological abnormality in schizophrenia: developmental origins and amelioration by antipsychotics? J Psychiatr Res 1998; 32:179–189Google Scholar
41. Pasamanick B, Rogers ME, Lilienfeld AM: Pregnancy experience and the development of behavior disorder in children. Am J Psychiatry 1956; 112:613–618Link, Google Scholar
42. Benes FM: A neurodevelopmental approach to the understanding of schizophrenia and other mental disorder, in Developmental Psychopathology, Vol. I: Theory and Methods. Edited by Ciccetti DJ, Cohen DJ. New York, John Wiley & Sons, 1995, pp 227-253Google Scholar
43. Torrey EF, Bowler AE, Taylor EH, Gottesman II: Schizophrenia and Manic-Depressive Disorder. New York, Basic Books, 1994, pp 79–101Google Scholar
44. Guth C, Jones P, Murray R: Familial psychiatric illness and obstetric complications in early-onset affective disorder. Br J Psychiatry 1993; 163:492–498Crossref, Medline, Google Scholar
45. Pulver AE, Carpenter WT: Lifetime psychotic symptoms assessed with the DIS. Schizophr Bull 1983; 9:377–382Crossref, Medline, Google Scholar



