Cholinesterase Inhibitors: A New Class of Psychotropic Compounds
Abstract
OBJECTIVE: This article reviews evidence indicating that acetylcholinesterase inhibitors have psychotropic properties. METHOD: The author reviewed the English-language literature pertinent to the response of neuropsychiatric symptoms in Alzheimer’s disease and related conditions to cholinergic agents. RESULTS: The cholinergic system originates in the basal forebrain and projects diffusely to the cerebral cortex; the limbic and paralimbic regions receive the most abundant cholinergic projections. The basal forebrain nuclei are positioned at the interface of the limbic system and cerebral cortex, where they play a role in mediating emotional responses. The basal forebrain nuclei are atrophic in Alzheimer’s disease, leading to a widespread cholinergic deficit. The cholinergic disturbance may contribute to neuropsychiatric manifestations of the disease. The treatment of patients with Alzheimer’s disease with acetylcholinesterase inhibitors reduces neuropsychiatric symptoms, particularly apathy and visual hallucinations. In some studies, a variety of other neuropsychiatric symptoms have been reported to respond to treatment with acetylcholinesterase inhibitors. Response profiles vary among acetylcholinesterase inhibitors. CONCLUSIONS: Acetylcholinesterase inhibitors have psychotropic effects and may play an important role in controlling neuropsychiatric and behavioral disturbances in patients with Alzheimer’s disease. These agents also may contribute to the management of other disorders with cholinergic system abnormalities and neuropsychiatric symptoms. The beneficial response is most likely mediated through limbic cholinergic structures.
Acetylcholinesterase inhibitors have recently been introduced as cognition-enhancing agents in the treatment of patients with mild to moderate Alzheimer’s disease. Tacrine (1–3) and donepezil (4–6) have been approved by the U.S. Food and Drug Administration (FDA); metrifonate (7, 8), and rivastigmine (9–11) are under review as possible marketable compounds, and long-acting physostigmine (12), eptastigmine (13), huperzine (14), galantamine (15), and velnacrine (16) have been investigated as acetylcholinesterase inhibitors with potential therapeutic benefit in Alzheimer’s disease. These agents exert their beneficial effect on intellectual functioning by blocking acetylcholinesterase and enhancing cholinergic function. They have been developed to improve the neuropsychological deficits of Alzheimer’s disease, as shown in most clinical trials by improved scores or superior performance compared to patients receiving placebo on the cognitive subscale of the Alzheimer’s Disease Assessment Scale (17), the Mini-Mental State examination (18), and a global scale such as the Clinician Interview-Based Impression (19).
Evidence has accrued that acetylcholinesterase inhibitors ameliorate behavioral disturbances as well as enhance cognition. These observations have implications for understanding the pathophysiological basis of neuropsychiatric symptoms in Alzheimer’s disease (20, 21) and for developing therapeutic options for clinicians involved in managing behavioral alterations in patients with the disease. This review presents the available data concerning the psychotropic effects of acetylcholinesterase inhibitors and describes the neurobiological basis for these neuropsychiatric alterations.
CENTRAL CHOLINERGIC SYSTEMS
Anatomy of the Cholinergic System
Acetylcholinesterase, the substrate of acetylcholinesterase inhibitors, is located in the synaptic space (soluble form) and in the synaptic membranes (membrane-bound form) of the neurons of the cholinergic system (22, 23). Thus, the anatomy of the cholinergic system determines the pharmacoanatomy of the response to acetylcholinesterase inhibitors.
Mesulam (24) identified eight cholinergic cell groups that form the origins of projections to other central nervous system (CNS) structures. The medial septal nucleus and the vertical nucleus of the diagonal band constitute the major cholinergic projections to the hippocampal formation, cingulate cortex, olfactory bulb, and hypothalamus. The horizontal limb of the diagonal band nucleus projects to the olfactory bulb, whereas the nucleus basalis of Meynert provides the major innervation of the cerebral cortex and amygdala (figure 1). The pedunculopontine nucleus and the laterodorsal tegmental nucleus of the brainstem project to the thalamus; the medial habenula innervates the interpeduncular nucleus; and the para-bigeminal nucleus projects to the superior colliculus. Cholinergic neurons manufacture choline acetyltransferase, which is transported to projection targets, where it catalyzes the synthesis of acetylcholine. All of the cholinergic innervation of the human cerebral cortex and thalamus arises from these extrinsic cholinergic sources.
All layers of the cerebral cortex receive cholinergic innervation; the density of cholinergic projections is highest in layers 1 and 2 and the upper regions of layer 3. Muscarinic cortical input disinhibits the cortical pyramidal cells that enhance the intralaminar transfer of information between cortical columns; in contrast, nicotinic input augments inhibition (25). There is regional variability in the cholinergic innervation of the human cortex (26): limbic areas that include the amygdala and hippocampus have the highest density of cholinergic axons; paralimbic regions have the next highest density of cholinergic fibers; the unimodal and heteromodal association cortices have intermediate densities of cholinergic innervation; and the primary visual cortex has the least abundant cortical cholinergic projections.
The neurons of the nucleus basalis and basal forebrain complex do not receive reciprocal projections from many of the cortical regions that they innervate. The afferent input to the nucleus basalis is largely from limbic brain regions, including the prepyriform cortex, orbitofrontal cortex, anterior insula, temporal pole, medial temporal cortex, entorhinal cortex, septal nuclei, nucleus accumbens, and hypothalamus (27). The virtually unique limbic origin of afferents to the nucleus basalis, coupled with its widespread cortical, limbic, and paralimbic projections, position this structure to determine the emotional valence of stimuli, influence the impact of emotionally relevant information on cortical function, and play a major role in emotionally relevant brain function (20, 27).
Cholinergic Receptors
Two classes of cholinergic receptors are recognized on the basis of their responses to specific agonists and antagonists (28)—muscarinic and nicotinic. Three types of muscarinic receptors have been identified pharmacologically, and five types have been shown to exist on the basis of molecular cloning experiments. Two major nicotinic receptor types have been identified in the CNS by using α-bungarotoxin and neuronal bungarotoxin (29). Muscarinic receptors use G proteins for signal transduction (28) and are metabotropic; nicotinic receptors are ionotropic and use ligand-gated ion channels for signal transduction (28, 30).
The M1 receptor is the most common muscarinic receptor subtype in the cerebral cortex (figure 2). Its highest concentrations are found in the dentate gyrus, hippocampus, anterior olfactory nucleus, cerebral cortex, olfactory tubercle, and nucleus accumbens. Moderate concentrations are found in the olfactory bulb and amygdala. The M2 receptor is found in brain areas with abundant cholinergic neurons, including the interpeduncular nucleus and basal forebrain. The M2 receptor is a presynaptic autoreceptor that governs cholinergic release (31). M3 receptors are concentrated primarily in the diencephalic and brainstem regions, and M4 receptors are found mainly in the striatum and olfactory tubercle (32). Nicotinic receptors are most abundant in the thalamus, periaqueductal gray, and substantia nigra (figure 3). Intermediate levels are found in the cerebral cortex and striatum. Relatively low levels are found in the hippocampus and amygdala (33).
Acetylcholinesterase
There are two cholinesterases in the CNS—acetylcholinesterase and butyrylcholine esterase. The latter is found also in the liver and plasma. The active site of acetylcholinesterase resides in a deep, narrow gorge within the three-dimensional structure of the enzyme (34, 35). Acetylcholinesterase inhibitors interact with the anionic or the esteratic (catalytic) site of the enzyme. Monomeric, dimeric, and tetrameric molecular forms of acetylcholinesterase arise from the posttranslational modification of the expressed protein. A single gene at chromosomal location 7q23 encodes acetylcholinesterase in humans (30, 34). The membrane-bound tetrameric form and the soluble monomeric form are the predominant enzyme species in humans (22, 23, 36).
CHOLINERGIC ABNORMALITIES IN ALZHEIMER’S DISEASE
Alzheimer’s disease is a complex neurodegenerative disease with characteristic histological changes, including neuritic plaques, neurofibrillary tangles, and a variety of neurochemical deficits that affect the serotonergic, noradrenergic, and cholinergic systems (37). The cholinergic deficit of Alzheimer’s disease is well documented; there is a marked loss of neurons in the basal forebrain nuclei that usually exceeds 75% of the total neuronal population at the time of an autopsy (38, 39). There are abundant neurofibrillary tangles in the remaining neurons of the basal nucleus (40). The death of cholinergic neurons leads to reductions in choline acetyltransferase of 80%–90% in the hippocampus and temporal cortex and 40%–75% in the parietal cortex and frontal convexity (figure 4 and figure 5). Less marked reductions occur in the mammillary bodies, cingulate gyrus, priming motor and sensory cortices, and caudate. Normal levels are present in the pons, midbrain, thalamus, hypothalamus, nucleus accumbens, and cerebellum (41, 42).
M1 receptors are preserved or modestly reduced in Alzheimer’s disease, presynaptic M2 autoreceptors are decreased, and M3 receptor levels are normal or up-regulated (31, 43). The decreased M1 receptor immunoreactivity suggests that the M1 receptor function may be altered despite near-normal receptor numbers (31). Nicotinic receptors also are decreased in Alzheimer’s disease (44). Studies of acetylcholinesterase reveal that there is a preferential loss of the tetrameric membrane-bound form compared to the monomeric soluble form (36).
CLASSES OF CHOLINESTERASE INHIBITORS
Table 1 provides comparative information on the principal acetylcholinesterase inhibitors that are currently approved or under investigation. These drugs represent different classes of agents: physostigmine is a carbamate, tacrine and velnacrine are acridines, donepezil is a piperidine, rivastigmine and eptastigmine are carbamates, metrifonate is an organophosphate, and galantamine is a phenanthrene alkaloid. They differ principally in the type of bond they form with acetylcholinesterase. Tacrine, velnacrine, donepezil, and huperzine are high-affinity, noncovalent inhibitors; metrifonate forms an irreversible covalent bond with the substrate. Tacrine and velnacrine are noncompetitive inhibitors, donepezil has noncompetitive and competitive properties, galantamine is a competitive inhibitor, and metrifonate begins with competitive inhibition and becomes a noncompetitive inhibitor over time (34). Differences in the duration of action and metabolism determine the dosing regimen and the likelihood of drug interactions. Galantamine is an acetylcholinesterase inhibitor and an allosteric modulator of nicotinic cholinergic receptors (45). The last activity may increase acetylcholine release by activating presynaptic nicotinic receptors. Tacrine and velnacrine are associated with a high frequency of hepatotoxicity; eptastigmine produces neutropenia (46).
BEHAVIORAL RESPONSES TO CHOLINESTERASE INHIBITORS
Table 2 summarizes the available information derived from standardized rating scales on the neuropsychiatric changes observed after treatment with acetylcholinesterase inhibitors.
Physostigmine
Physostigmine was the acetylcholinesterase inhibitor most studied in the early phases of the development of antidementia drugs. Many studies did not include behavioral measures, but Harrell and colleagues (47) included the Sandoz Clinical Assessment—Geriatric Scale (48) and reported that in the group of patients who had responded to physostigmine, as evidenced by cognitive improvement or correct identification of group membership by the patient’s family or physician, there was significantly greater behavioral improvement than in patients receiving placebo or those designated as nonresponders. Schwartz and Kohlstaedt (49) reported that four of 11 patients who received intramuscular physostigmine had dramatic behavioral improvements lasting up to 3 days after drug administration. There was decreased restlessness and depression and improved sociability and initiative. In contrast, Wirkowski and colleagues (50) reported two cases of “physostigmine syndrome” in which patients became anxious, irritable, and uncooperative and one experienced extreme fatigue. The doses in this study exceeded considerably those administered by Schwartz and Kohlstaedt (49).
Four studies have specifically investigated the neuropsychiatric effects of physostigmine. Molchan and colleagues (51) used a double-blind, placebo-controlled, single-case study design to assess changes in psychiatric symptoms produced by physostigmine in a patient with Alzheimer’s disease who exhibited psychotic symptoms. During the period of drug administration, there was a marked diminution in the occurrence of hallucinations and delusions and a modest increase in depressive symptoms. My colleagues and I (52) used a double-blind, active-comparator study design to investigate the relative effects of physostigmine and haloperidol in two patients with Alzheimer’s disease and psychosis. Physostigmine reduced delusions and hallucinations in both patients without effects on their mood. We also performed a double-blind crossover trial of haloperidol and physostigmine (53) in 13 patients with advanced Alzheimer’s disease and behavioral disturbances. Psychosis and agitation scores on the Behavioral Pathology in Alzheimer’s Disease Rating Scale (54) declined comparably in response to treatment with the two agents. In a study of long-acting, controlled-release physostigmine, Thal and colleagues (12) found that agitation was observed in 50% fewer patients treated with the active agent than with placebo.
Tacrine
The noncognitive portion of the Alzheimer’s Disease Assessment Scale (17) was used in three pivotal double-blind, placebo-controlled studies of tacrine (1–3). This scale includes a variety of items, including questions regarding mood, psychosis, appetite, and tremor. No statistically significant effects on the total score on this scale were observed in any of the studies, although nonsignificant trends in favor of tacrine were reported by both Farlow and colleagues (2) and Davis et al. (1). A meta-analysis of all of the randomized, double-blind, placebo-controlled trials of tacrine revealed a significant but small beneficial effect on behavior (55). In a post hoc analysis of the patient group studied by Knapp and colleagues (3), Raskind et al. (56) found that a significantly larger percentage of the patients who received tacrine had improvement or stabilization on scores for three of the scale items: cooperation, delusions, and pacing. This difference in outcome conclusions on the basis of total score compared to individual item analysis suggests that investigating the effects of acetylcholinesterase inhibitors on individual symptoms and syndromes is important; the analysis of total rating scale scores may obscure important effects. In an open-label study, my colleagues and I (57), using the Neuropsychiatric Inventory (58), documented statistically significant improvements in anxiety and disinhibition. In a follow-up study with a larger study group (59), significant changes were seen in total Neuropsychiatric Inventory scores, as well as reductions in apathy, disinhibition, and aberrant motor behavior.
Velnacrine
Antuono et al. (16) observed that the patients who received placebo had increasing symptoms on the Relative’s Assessment of Global Symptomatology (60) (which rates 21 psychiatric symptoms and behavioral disturbances), whereas those treated with high doses (225 mg/day) of velnacrine did not. The patients who received velnacrine also required less caregiving time, a measure that was significantly correlated with the noncognitive score on the Alzheimer’s Disease Assessment Scale. Finally, patients treated with velnacrine were less likely to have emergent periods of agitation in the course of the 24-week study (1% versus 4% for patients who received placebo).
Donepezil
There have been few studies of the behavioral effects of donepezil in Alzheimer’s disease. Kaufer and coworkers (61) reported a significant reduction in total Neuropsychiatric Inventory scores in a group of 40 patients with Alzheimer’s disease who were capable of walking and were enrolled in an open-label study. My colleagues and I (62) found no statistically significant effect of donepezil on the items assessed by the Neuropsychiatric Inventory; two subgroups of patients were identified in post hoc analyses—one that improved and one that had no changes in response to treatment. Small et al. (63) reported that compared to patients not treated with donepezil, those receiving donepezil were significantly less likely to be administered antidepressant, antipsychotic, and sedative medications. Shea and colleagues (64) observed behavioral improvement in eight of nine patients with dementia with Lewy bodies, a syndrome closely related to Alzheimer’s disease (65). Aarsland et al. (66) described a patient with dementia with Lewy bodies whose psychosis responded to treatment with donepezil, and Kaufer et al. (67) reported two patients with dementia with Lewy bodies who had beneficial responses.
Metrifonate
In a randomized, double-blind, parallel-group, placebo-controlled, safety and cognitive efficacy study that lasted 26 weeks (8), patients who were treated with metrifonate manifested significant improvement or significantly less behavioral deterioration as shown by differences between metrifonate and placebo in total Neuropsychiatric Inventory scores and hallucination subscores. In a pooled analysis (68) of three prospective multicenter, randomized, double-blind, parallel-group, placebo-controlled trials of metrifonate that resulted in a total study group of 2,218 (411 patients taking placebo and 1,807 taking metrifonate), there were statistically significant differences between the placebo and active agent groups in favor of metrifonate on the total Neuropsychiatric Inventory score and on the scores for the hallucinations and apathy. There was a trend toward improvement in depression, anxiety, and aberrant motor behavior. Metrifonate improved or prevented the worsening of psychiatric and behavioral symptoms in 60% of the symptomatic patients. More than 50% of the patients with symptoms at baseline had clinically relevant (e.g., at least 30%) reductions in depression, anxiety, and apathy subscale scores. Raskind and colleagues (69) found that treatment with metrifonate (50 mg/day) reduced agitation and aberrant motor behavior as measured by the Neuropsychiatric Inventory; no statistically significant change was found in the same population by using the noncognitive portion of the Alzheimer’s Disease Assessment Scale. Similarly, Becker and colleagues (70, 71) found no behavioral effects of metrifonate in studies using the noncognitive portion of the Alzheimer’s Disease Assessment Scale or the Brief Psychiatric Rating Scale (BPRS) (72).
Eptastigmine
Imbimbo and colleagues (13) assessed the safety and efficacy of two doses (30 mg/day and 45 mg/day) of eptastigmine in a double-blind, placebo-controlled study. Using the Spontaneous Behavior Interview (73), a caregiver-based instrument that assesses a wide range of neuropsychiatric symptoms, they documented significant behavioral improvement in those who received 30 mg/day.
Rivastigmine
Jan and McKeith (74) reported that Neuropsychiatric Inventory scores that reflected the presence of psychosis were reduced by treatment with rivastigmine.
DISCUSSION
The principal conclusion of this review is that there is substantial and growing evidence that acetylcholinesterase inhibitors exert beneficial psychotropic effects in patients with Alzheimer’s disease. Changes in neuropsychiatric symptoms should be among the clinical outcomes assessed in clinical trials of cholinergic agents, and clinicians should monitor psychiatric and behavioral responses in patients as an indication of drug effect when prescribing acetylcholinesterase inhibitors. Limbic and paralimbic cortices normally receive robust cholinergic innervation and have cholinergic deficits in Alzheimer’s disease. Restoration of function in these brain regions that are critical to the mediation of emotion may underlie the behavioral response to acetylcholinesterase inhibitors.
Visual hallucinations and apathy are the most predictably responsive symptoms in most investigations. Anxiety, disinhibition, agitation, depression, delusions, and aberrant motor behavior have improved in some studies but not in others. The observation that several acetylcholinesterase inhibitors have similar effects on behavior suggests that this may be a class effect that reflects cholinergic enhancement in behaviorally relevant areas of the brain. However, acetylcholinesterase inhibitors may differ in their neuropsychiatric potency, and assessments of the psychotropic effects of individual agents are necessary.
Neurobiological Basis of the Response to Cholinergic Agents
Similarities between the neuropsychiatric symptoms of Alzheimer’s disease and anticholinergic toxicity, the response of these symptoms to acetylcholinesterase inhibitors in conditions with cholinergic deficits, and the anatomic distribution of the cholinergic deficits all link cholinergic abnormalities to neuropsychiatric disturbances (20).
Anticholinergic agents induce changes in mental status that are similar to the neuropsychiatric symptoms of Alzheimer’s disease, including thought disorganization, visual hallucinations, and variable mood changes. Patients with Alzheimer’s disease are unusually sensitive to the adverse effects of anticholinergic compounds (75). Neuropsychiatric symptoms induced by anticholinergics can be ameliorated by acetylcholinesterase inhibitors, including tacrine and physostigmine (76).
Patients with neurologic disorders with concomitant cholinergic deficits have been reported to respond to acetylcholinesterase inhibitors with reduced neuropsychiatric symptoms. Patients with dementia with Lewy bodies improve behaviorally in response to treatment with acetylcholinesterase inhibitors (64, 66, 67), and patients with Parkinson’s disease and dementia with delusions and hallucinations may exhibit a beneficial neuropsychiatric response to therapy with acetylcholinesterase inhibitors (77). Cholinergic disturbances are present in the limbic and paralimbic cortices, which mediate functions critical to emotion (24, 27, 41) this regional deficiency may provide the substrate for some of the emotional disturbances of Alzheimer’s disease and their response to cholinergic therapy.
Neurobiological Basis of Variations in Response to Cholinergic Therapy
Variations in the cholinergic deficit may account for some of the observed neuropsychiatric heterogeneity of Alzheimer’s disease and the differences in response to treatment with cholinergic agents. A few patients with histopathological changes typical of Alzheimer’s disease do not show a loss of neurons in the nucleus basalis or a cortical cholinergic deficit at autopsy (78). Some investigators have found greater preservation of the nucleus basalis neurons in patients with onset of the disease after age 65—the most typical age at onset—than in those whose symptoms began earlier in life (79). Davis and colleagues (80) reported that cortical cholinergic deficits were not present in elderly patients with mild to moderate Alzheimer’s disease, although they were notable in patients with advanced disease. Patients with the apolipoprotein E-4 genotype have less brain choline acetyltransferase and nicotinic receptor binding than patients with the E-3 or E-2 genotype (81, 82). In one study (83), women with Alzheimer’s disease had more cytoskeletal changes in the nucleus basalis than men with Alzheimer’s disease. Moreover, women who receive estrogen replacement therapy and those without the E-4 genotype have been shown to respond most favorably to tacrine (84, 85). Thus, differences in age, disease severity, or genotype may influence the cholinergic deficit and the response to therapy with acetylcholinesterase inhibitors.
Additional variability in the behavioral symptoms of Alzheimer’s disease and the responsiveness to cholinergic therapy may reflect dynamic interactions between the cholinergic changes and other transmitter systems involved in Alzheimer’s disease (20, 37). The cholinergic system interacts with a variety of other transmitters or neuromodulators, including norepinephrine, dopamine, serotonin, γ-aminobutyric acid, opioid peptides, galanin, substance P, and angiotensin II (86).
Relationship of Behavioral and Cognitive Changes
Acetylcholinesterase inhibitors were developed to enhance cognition in patients with Alzheimer’s disease. In patients with mild to moderate cognitive impairment, they temporarily improve, stabilize, or reduce the rate of decline in memory and other intellectual functions relative to the results with placebo. A few studies have investigated the relationship between cognitive and behavioral responses. My colleagues and I (57), in a study of the neuropsychiatric effects of tacrine, noted that of 10 subjects who had at least a 3-point improvement in Mini-Mental State examination scores, all also had an improvement in neuropsychiatric symptoms as reflected by lower scores on the Neuropsychiatric Inventory. Of the nine patients in the study who met the stringent criteria of at least a 9-point reduction in scores on the Neuropsychiatric Inventory, six met the criteria for improved cognitive function; three patients showed a substantial behavioral benefit from therapy without a coincident improvement in cognition. In a follow-up article (59), we noted that patients with moderate cognitive impairment (Mini-Mental State examination scores between 11 and 20) had the most consistent improvement in behavior; mildly affected patients exhibited fewer behaviors and had less robust treatment effects; and patients with severe dementia (Mini-Mental State examination scores of 10 or lower) exhibited an improvement in some symptoms (delusions, anxiety, apathy, and disinhibition) and a worsening of others (hallucinations, agitation, dysphoria, euphoria, irritability, and aberrant motor behavior). The available data suggest that behavioral improvement is not contingent upon cognitive changes, and some patients exhibit behavioral responses without concurrent improvements in cognition.
Cholinergic agents affect many aspects of cognition, which suggests that the primary effect may be on an attentional or executive system with a secondary, pan-intellectual modulating influence on memory, language, and visuospatial skills (87). Conversely, anticholinergics have disproportionately adverse effects on attention, immediate memory, and executive processes (88–90). Improvement in attention may underlie the reductions in apathy that are commonly associated with acetylcholinesterase inhibitors, possibly explaining why apathy is among the most responsive of neuropsychiatric symptoms to cholinergic therapy and why it correlates highly with cognitive improvement.
Regulatory Issues
FDA guidelines specify that a claim of efficacy in dementia can arise only 1) if a drug beneficially affects a core symptom or sign of dementia (i.e., has cognitive effects) or 2) if the effect of the drug is expressed only or is differentially expressed in patients with dementia who exhibit the symptom, sign, or behavior (91). It is likely that cholinergic agents will benefit only patients who have disturbances of cholinergic function, and, thus, a specific indication for the treatment of neuropsychiatric symptoms in Alzheimer’s disease may be allowable under the second FDA criterion. These considerations are important since cholinergic agents sometimes have emotional benefits for patients who do not improve cognitively; cholinergic agents may reduce neuropsychiatric symptoms late in the course of the disease, when cognitive enhancement may be limited; subpopulations of patients with behaviors that are specifically responsive to cholinergic agents may be identified; and agents may differ substantially in their efficacy regarding the treatment of neuopsychiatric symptoms. Thus, acetylcholinesterase inhibitors may have a psychotropic role that is independent of their cognitive effects and deserves regulatory recognition.
Clinical Importance
Reducing behavioral disturbances in patients with Alzheimer’s disease is an important treatment goal. Neuropsychiatric disturbances are distressing to the patient who experiences fear, sadness, or anxiety; they are a source of marked distress for the caregiver (92); and they may precipitate institutionalization (93). Thus, the treatment of behavioral disturbances in Alzheimer’s disease may ameliorate a patient’s emotional distress and have secondary beneficial effects on the caregiver and on the opportunity for the patient to remain at home. Cholinergic agents may play an important role in these treatment goals.
Determining the magnitude of the psychotropic effect of acetylcholinesterase inhibitors is critical to establishing the clinical importance of these agents as treatments for neuropsychiatric symptoms. The psychotropic effects of individual cholinergic agents must be determined by prospective, randomized, controlled trials in which specific behavioral criteria are used for the selection of participants. The magnitude of the behavioral response can be anticipated by reviewing the change of symptoms in patients who were included from existing trials who were symptomatic at baseline. For example, of the patients treated with metrifonate, more than 50% had at least a 30% reduction in depression, anxiety, apathy, and total Neuropsychiatric Inventory scores (68). A 30% reduction in such symptoms is clinically relevant and comparable in magnitude to the changes observed with conventional psychotropic agents.
Role of Cholinergic Therapy in Other Conditions
Cholinergic treatments were developed for use in patients with Alzheimer’s disease but may be applicable to patients with other disorders with cholinergic abnormalities. As already noted, preliminary evidence suggests that patients with dementia with Lewy bodies (64, 67) or Parkinson’s disease with dementia (77) may exhibit a behavioral response to treatment with acetylcholinesterase inhibitors. Cortical cholinergic deficits have been identified in a variety of other neurological disorders, including some cases of Pick’s disease (94), olivopontocerebellar atrophy (95), progressive supranuclear palsy (96), the parkinsonism dementia complex of Guam (97), alcoholism with Wernicke’s encephalopathy (98), Creutzfeldt-Jakob disease (99), subacute sclerosing panencephalitis (99), dementia pugilistica (100, 101), and traumatic brain injury (102). Patients with vascular dementia may have lesions that interrupt projections from the nucleus basalis and produce a cortical cholinergic deficit, or they may have mixed Alzheimer’s disease plus cerebrovascular disease, which render them potentially responsive to acetylcholinesterase inhibitors (103). In addition, there are age-related changes in the nucleus basalis; cell numbers decline from approximately 450,000 in children to approximately 150,000 in elderly control subjects (104). Neuropsychiatric disturbances in these conditions might be reduced with the use of acetylcholinesterase inhibitors.
Open-label studies suggest that acetylcholinesterase inhibitors are of potential benefit in bipolar disorder (105), and cholinergic dysfunction may be present in some patients with schizophrenia(106). These findings raise the possibility that these disorders could be treated with cholinergic agents. Cholinesterase inhibitors also might benefit other disorders that putatively affect cholinergic systems, including attention deficit hyperactivity disorder, autism, and sleep disorders.
Behavioral Effects of Cholinergic Agonists
The hypothesis that cholinergic enhancement has psychotropic effects is strengthened by the observation that cholinergic agonists, as well as acetylcholinesterase inhibitors, have been reported to relieve neuropsychiatric symptoms. Xanomeline, an M1- and M4-selective muscarinic receptor agonist, was studied in a 6-month randomized, double-blind, placebo-controlled, parallel-group, multiple-dose trial (107). The agent exhibited substantial behavioral effects and significant dose-dependent reductions in vocal outbursts, suspiciousness, delusions, agitation, hallucinations, wandering, fearfulness, compulsiveness, tearfulness, mood swings, and threatening behaviors. The emergence of neuropsychiatric symptoms in patients in whom these were absent at baseline was suppressed. SB-202026, an M1 partial agonist, produced an improvement in behavior, compared to the deterioration in behavior in the placebo group, when assessed with the noncognitive portion of the Alzheimer’s Disease Assessment Scale (108).
The BPRS was used to assess behavioral responses to intravenous administration of the cholinergic agonist arecoline to patients with Alzheimer’s disease (109). The anergia subscale of the BPRS showed a biphasic response, with decreases following low-dose infusions and increases following high-dose infusions. Similarly, the score on the thought disorder subscale increased with high-dose infusions.
Penn and colleagues (110) noted a trend toward decreased abnormal behavior scores during intraventricular administration of the cholinergic agonist bethanechol, whereas my colleagues and I (111) observed that two of five patients treated in the course of a dose-finding study exhibited distress, restlessness, agitation, and depression. Oxotremorine, a long-acting, direct cholinergic agonist, was administered to seven patients with Alzheimer’s disease and was noted to precipitate depressive reactions in five (112). Intravenous nicotine has been used to assess the effect of nicotinic receptor stimulation in Alzheimer’s disease, and Newhouse and colleagues (113) found that at the highest doses, patients exhibited significant elevations in scores for anxiety and depression.
Thus, whereas cholinergic agonists have produced improvement in neuropsychiatric symptoms in some studies of patients with Alzheimer’s disease, the information available is limited, and the responses reported are less consistent than those observed with acetylcholinesterase inhibitors.
Summary
Acetylcholinesterase inhibitors have psychotropic as well as cognition-enhancing effects. The amelioration of apathy and reduction of visual hallucinations are the most reproducible effects on neuropsychiatric symptoms in Alzheimer’s disease, but other neuropsychiatric symptoms have responded in some studies. There may be variations among acetylcholinesterase inhibitors in their psychotropic properties. The beneficial effects of acetylcholinesterase inhibitors on emotion and behavior are most likely mediated through cholinergic influences on limbic and paralimbic brain structures. Patients who exhibit substantial cognitive improvement usually have a concomitant behavioral response, but behavioral and cognitive responses may be dissociated. Clinical trials should be designed to clarify the neuropsychiatric effects of acetylcholinesterase inhibitors in Alzheimer’s disease and to explore the potential use of these agents in other conditions.
Received Jan. 19, 1999; revision received July 16, 1999; accepted July 21, 1999. From the Department of Neurology and Department of Psychiatry and Biobehavioral Sciences, UCLA School of Medicine. Address reprint requests to Dr. Cummings, UCLA Department of Neurology, 710 Westwood Plaza, Los Angeles, CA 90095-1769; [email protected] (e-mail).Supported by Alzheimer’s Disease Center grant AG-16570 and Alzheimer’s Disease Cooperative study grant AG-10483 from the National Institute on Aging, an Alzheimer’s Disease Research Center of California grant, the Sidell-Kagan Foundation, General Clinical Research Center grant RR-00865 to the University of California at Los Angeles from the NIH National Center for Research Resources, and Alzheimer’s Disease Cooperative Study grant AG-10483 from the National Institute on Aging. Images provided by the UCLA Laboratory of Neuroimaging, supported by grant RR-13642 from the NIH National Center for Research Resources. Dr. Cummings has served as a consultant or performed research in conjunction with Novartis, Parke-Davis, Pfizer, Inc./Eisai, Bayer Corporation, Eli Lilly and Company, Janssen Pharmaceutica, Inc., and SmithKline Beecham Consumer Healthcare regarding agents relevant to this article.
 |
 |
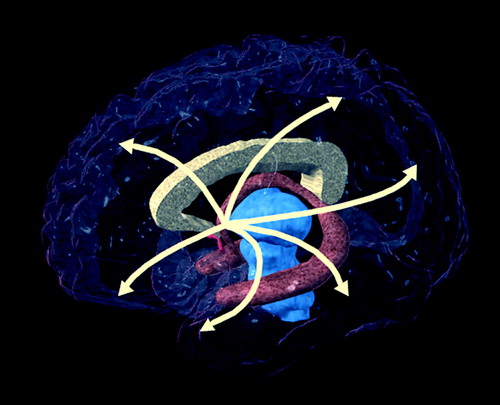
FIGURE 1. Cholinergic Projections From the Nucleus Basalis to the Cerebral Cortex and Related Structuresa
aImage provided by the UCLA Laboratory of Neuroimaging; courtesy of Andrew Lee, John Bachelor, and Arthur Toga.
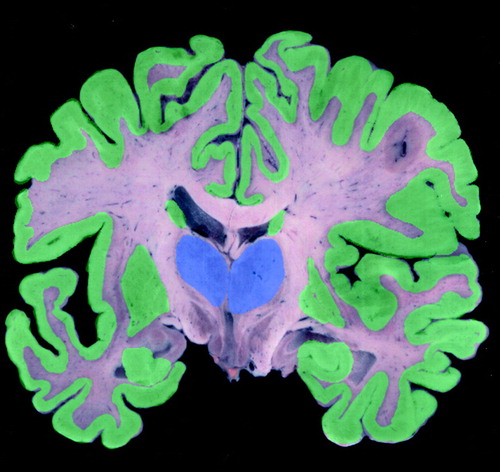
FIGURE 3. Regional Concentrations of Nicotinic Cholinergic Receptorsa
aHigh=green, medium=pink, low=blue. Image provided by the UCLA Laboratory of Neuroimaging; courtesy of Andrew Lee, John Bachelor, and Arthur Toga.
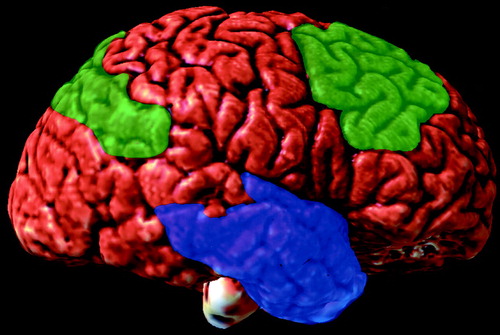
FIGURE 4. Regional Reductions of Cholinergic Markers in Alzheimer’s Disease in the Lateral (Left) Brain Regiona
aMarked=blue, medium=green. Image provided by the UCLA Laboratory of Neuroimaging; courtesy of Andrew Lee, John Bachelor, and Arthur Toga.
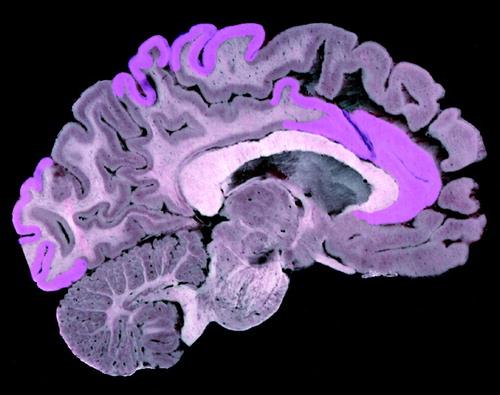
FIGURE 5. Regional Reductions of Cholinergic Markers in Alzheimer’s Disease in the Medial (Right) Brain Regiona
aModest cholinergic reductions in areas shown in purple. Image provided by the UCLA Laboratory of Neuroimaging; courtesy of Andrew Lee, John Bachelor, and Arthur Toga.
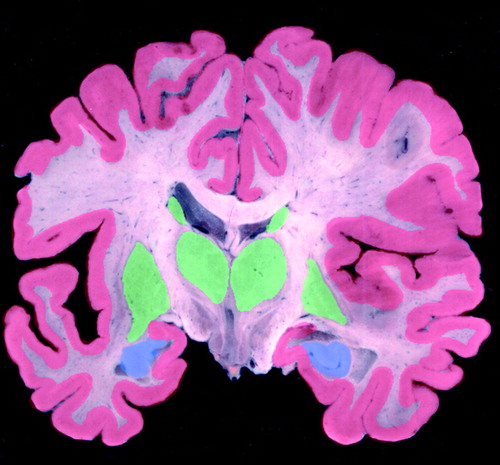
FIGURE 2. Regional Concentrations of M1 Muscarinic Cholinergic Receptorsa
aHigh=green, low=blue. Image provided by the UCLA Laboratory of Neuroimaging; courtesy of Andrew Lee, John Bachelor, and Arthur Toga.
1. Davis KL, Thal LJ, Gamzu ER, Davis CS, Woolson RF, Gracon SI, Drachman DA, Schneider LS, Whitehouse PJ, Hoover TM, Morris JC, Kawas CH, Knopman DS, Earl NL, Kumar V, Doody RS (the Tacrine Collaborative Study Group): A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer’s disease. N Engl J Med 1992; 327:1253–1259Google Scholar
2. Farlow M, Gracon SI, Hershey LA, Lewis KW, Sadowsky CH, Dolan-Ureno J (Tacrine Study Group): A controlled trial of tacrine in Alzheimer’s disease. JAMA 1992; 268:2523–2529Google Scholar
3. Knapp MJ, Knopman DS, Solomon PR, Pendlebury WW, Davis CS, Gracon SI (Tacrine Study Group): A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer’s disease. JAMA 1994; 271:985–994Crossref, Medline, Google Scholar
4. Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT (Donepezil Study Group): A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology 1998; 50:136–145Crossref, Medline, Google Scholar
5. Rogers SL, Doody RS, Mohs RC, Friedhoff LT (Donepezil Study Group): Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Arch Intern Med 1998; 158:1021–1031Google Scholar
6. Rogers SL, Friedhoff LT (Donepezil Study Group): The efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a US multicentre, randomized, double-blind, placebo-controlled trial. Dementia 1996; 7:293–303Medline, Google Scholar
7. Cummings JL, Cyrus PA, Bieber F, Mas J, Orazem J, Gulanski B (Metrifonate Study Group): Metrifonate treatment of the cognitive deficits of Alzheimer’s disease. Neurology 1998; 50:1214–1221Google Scholar
8. Morris JC, Cyrus PA, Orazem J, Mas J, Bieber F, Ruzicka BB, Gulanski B: Metrifonate benefits cognitive, behavioral, and global function in patients with Alzheimer’s disease. Neurology 1998; 50:1222–1230Google Scholar
9. Corey-Bloom J, Anand R, Veach J (ENA 713 B352 Study Group): A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriatr Psychopharmacol 1998; 1:55–65Google Scholar
10. Sramek JJ, Anand R, Wardle TS, Irwin P, Hartman RD, Cutler NR: Safety/tolerability trial of SDZ ENA 713 in patients with probable Alzheimer’s disease. Life Sci 1996; 58:1201–1207Google Scholar
11. Rosler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, Dal-Bianco P, Stahelin H, Hartmann R, Gharabawi M: B303 Exelon study group: efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. Br Med J 1999; 318:633–640Crossref, Medline, Google Scholar
12. Thal LJ, Ferguson JM, Mintzer J, Raskin A, Targum SD: A 24-week randomized trial of controlled-release physostigmine in patients with Alzheimer’s disease. Neurology 1999; 52:1146–1152Google Scholar
13. Imbimbo BP, Lucca U, Lucchelli F, Alberoni M, Thal LJ (Eptastigmine Study Group): A 25-week placebo-controlled study of eptastigmine in patients with Alzheimer’s disease. Alzheimer Dis Assoc Disord 1998; 12:313–322Crossref, Medline, Google Scholar
14. Tariot PN, Schneider L: Contemporary treatment approaches to Alzheimer’s disease. Consultant Pharmacist 1996; 11(suppl E):16–24Google Scholar
15. Rainer M: Galantamine in Alzheimer’s disease: a new alternative to tacrine? CNS Drugs 1997; 2:89–97Google Scholar
16. Antuono PG (Mentane Study Group): Effectiveness and safety of velnacrine for the treatment of Alzheimer’s disease: a double-blind, placebo-controlled study. Arch Intern Med 1995; 155:1766–1772Google Scholar
17. Rosen WG, Mohs RC, Davis KL: A new rating scale for Alzheimer’s disease. Am J Psychiatry 1984; 141:1356–1364Google Scholar
18. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
19. Knopman DS, Knapp MJ, Gracon SI, Davis CS: The Clinician Interview-Based Impression (CIBI): a clinician’s global change rating scale in Alzheimer’s disease. Neurology 1994; 44:2315–2321Google Scholar
20. Cummings JL, Kaufer D: Neuropsychiatric aspects of Alzheimer’s disease: the cholinergic hypothesis revisited. Neurology 1996; 47:876–883Crossref, Medline, Google Scholar
21. Cummings JL, Back C: The cholinergic hypothesis of neuropsychiatric symptoms in Alzheimer’s disease. Am J Geriatr Psychiatry 1998; 6(suppl 1):S64–S78Google Scholar
22. Schegg KM, Harrington LS, Nielsen S, Zwieg RM, Peacock JH: Soluble and membrane-bound forms of brain acetylcholinesterase in Alzheimer’s disease. Neurobiol Aging 1992; 13:697–704Crossref, Medline, Google Scholar
23. Younkin SG, Goodridge B, Katz J, Lockett G, Nafziger D, Usiak MF, Younkin LH: Molecular forms of acetylcholinesterase in Alzheimer’s disease. Fed Proc 1986; 45:2982–2988Google Scholar
24. Mesulam M-M: Structure and function of cholinergic pathways in the cerebral cortex, limbic system, basal ganglia, and thalamus of the human brain, in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom FE, Kupfer DJ. New York, Raven Press, 1995, pp 135–146Google Scholar
25. Xiang Z, Huguenard JR, Prince DA: Cholinergic switching within neocortical inhibitory networks. Science 1998; 281:985–988Crossref, Medline, Google Scholar
26. Guela C: Abnormalities of neural circuitry in Alzheimer’s disease: hippocampus and cortical cholinergic innervation. Neurology 1998; 51(suppl 1):S18–S29Google Scholar
27. Mesulam M-M, Mufson EJ: Neural inputs into the nucleus basalis of the substantia innominata (Ch4) in the rhesus monkey. Brain 1984; 107:253–274Crossref, Medline, Google Scholar
28. Richelson E: Cholinergic transduction, in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom FE, Kupfer DJ. New York, Raven Press, 1995, pp 125–134Google Scholar
29. Arneric SP, Sullivan JP, Williams M: Neuronal nicotinic acetylcholine receptors: novel targets for central nervous system therapeutics. Ibid, pp 995–1010Google Scholar
30. Nicholls DG: Proteins, Transmitters and Synapses. London, Blackwell Science, 1994Google Scholar
31. Flynn DD, Ferrari-DiLeo G, Mash DC, Levery AL: Differential regulation of modular subtypes of muscarinic receptors in Alzheimer’s disease. J Neurochem 1995; 64:1888–1891Google Scholar
32. Schliebs R, Robner S: Distribution of muscarinic acetylcholine receptors in the CNS, in CNS Neurotransmitters and Neuromodulators: Acetylcholine. Edited by Stone TW. Boca Raton, Fla, CRC Press, 1995, pp 67–83Google Scholar
33. Court JA, Perry EK: Distribution of nicotinic receptors in the CNS. Ibid, pp 85–104Google Scholar
34. Taylor P: Development of acetylcholinesterase inhibitors in the therapy of Alzheimer’s disease. Neurology 1998; 51(suppl 1):S30–S35Google Scholar
35. Zhou H-X, Wlodek ST, McCammon JA: Conformation gating as a mechanism for enzyme specificity. Proc Natl Acad Sci USA 1998; 95:9280–9283Google Scholar
36. Fishman EB, Siek GC, MacCallum RD, Bird ED, Volicer L, Marquis JK: Distribution of the molecular forms of acetylcholinesterase in human brain: alterations in dementia of the Alzheimer type. Ann Neurol 1986; 19:246–252Crossref, Medline, Google Scholar
37. Cummings JL, Vinters HV, Cole GM, Khachaturian ZS: Alzheimer’s disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology 1998; 51(suppl 1):S2–S17Google Scholar
38. Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR: Alzheimer’s disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol 1981; 10:122–126Crossref, Medline, Google Scholar
39. Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT: Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 1982; 215:1237–1239Google Scholar
40. Rasool CG, Svendsen CN, Selkoe DJ: Neurofibrillary degeneration of cholinergic and noncholinergic neurons of the basal forebrain in Alzheimer’s disease. Ann Neurol 1986; 20:482–488Crossref, Medline, Google Scholar
41. Davies P: Studies on the neurochemistry of central cholinergic systems in Alzheimer’s disease, in Alzheimer’s Disease: Senile Dementia and Related Disorders. Edited by Katzman R, Terry RD, Bick KL. New York, Raven Press, 1978, pp 453–459Google Scholar
42. Guela C, Mesulam M-M: Cholinergic systems and related neuropathological predilection patterns in Alzheimer disease, in Alzheimer Disease. Edited by Terry RD, Katzman R, Bick KL. New York, Raven Press, 1994, pp 263–291Google Scholar
43. Rodriguez-Puertas R, Pascual J, Vilaro T, Pazos A: Autoradiographic distribution of M1, M2, M3, and M4 muscarinic receptor subtypes in Alzheimer’s disease. Synapse 1997; 26:341–350Crossref, Medline, Google Scholar
44. Perry EK, Perry RH, Smith CJ, Purohit D, Bonham J, Dick DJ, Candy JM, Edwardson JA, Fairbairn A: Cholinergic receptors in cognitive disorders. Can J Neurol Sci 1986; 13:521–527Crossref, Medline, Google Scholar
45. Pontecorvo MJ, Parys W: Clinical development of galantamine: evaluation of a compound with possible acetylcholinesterase inhibiting and nicotinic modulating activity (abstract). Neurobiol Aging 1998; 19(suppl 7):57Google Scholar
46. Imbimbo BP, Martelli P, Troetel WM, Lucchelli F, Lucca U, Thal LJ (Eptastigmine Study Group): Efficacy and safety of eptastigmine for the treatment of patients with Alzheimer’s disease. Neurology 1999; 52:700–708Crossref, Medline, Google Scholar
47. Harrell LE, Calloway R, Morere D, Falgout J: The effect of long-term physostigmine administration in Alzheimer’s disease. Neurology 1990; 40:1350–1354Google Scholar
48. Shader RI, Harmatz JS, Salzman C: A new scale for clinical assessment in geriatric populations: Sandoz Clinical Assessment—Geriatric (SCAG). J Am Geriatr Soc 1974; 22:107–113Crossref, Medline, Google Scholar
49. Schwartz AS, Kohlstaedt EV: Physostigmine effects in Alzheimer’s disease: relationship to dementia severity. Life Sci 1986; 38:1021–1028Google Scholar
50. Wirkowski E, Prohovnik I, Young WL: Observations on the physostigmine syndrome in patients with Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 1991; 3:73–75Crossref, Medline, Google Scholar
51. Molchan SE, Vitiello B, Minichiello M, Sunderland T: Reciprocal changes in psychosis and mood after physostigmine in a patient with Alzheimer’s disease (letter). Arch Gen Psychiatry 1991; 48:1113Crossref, Medline, Google Scholar
52. Cummings JL, Gorman DG, Shapira J: Physostigmine ameliorates the delusions of Alzheimer’s disease. Biol Psychiatry 1993; 33:536–541Crossref, Medline, Google Scholar
53. Gorman DG, Read S, Cummings JL: Cholinergic therapy of behavioral disturbances in Alzheimer’s disease. Neuropsychiatry Neuropsychol Behav Neurol 1993; 6:229–234Google Scholar
54. Reisberg B, Borenstein J, Salob SP, Ferris SH, Franssen E, Georgotas A: Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment. J Clin Psychiatry 1987; 48(suppl 5):9–15Google Scholar
55. Qizilbash N, Whitehead A, Higgins J, Wilcock G, Schneider L, Farlow M (Dementia Trialists’ Collaboration): Cholinesterase inhibition for Alzheimer disease: a meta-analysis of the tacrine trials. JAMA 1998; 280:1777–1782Google Scholar
56. Raskind MA, Sadowsky CH, Sigmund WR, Beitler PJ, Auster SB: Effect of tacrine on language, praxis, and noncognitive behavioral problems in Alzheimer’s disease. Arch Neurol 1997; 54:836–840Crossref, Medline, Google Scholar
57. Kaufer DI, Cummings JL, Christine D: Effect of tacrine on behavioral symptoms in Alzheimer’s disease: an open-label study. J Geriatr Psychiatry Neurol 1996; 9:1–6Crossref, Medline, Google Scholar
58. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J: The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Google Scholar
59. Kaufer D, Cummings JL, Christine D: Differential neuropsychiatric symptom responses to tacrine in Alzheimer’s disease: relationship to dementia severity. J Neuropsychiatry Clin Neurosci 1998; 10:55–63Crossref, Medline, Google Scholar
60. Raskin A, Crook T: Relative’s Assessment of Global Symptomatology (RAGS). Psychopharmacol Bull 1988; 24:759–763Medline, Google Scholar
61. Kaufer D, Catt K, Pollack B, Lopez O, DeKosky S: Donepezil in Alzheimer’s disease: relative cognitive and neuropsychiatric responses and impact on caregiver distress (abstract). Neurology 1998; 50:A89Google Scholar
62. Mega M, Masterman DM, O’Connor SM, Barclay TR, Burzynski MJ, Cummings JL: The spectrum of behavioral responses in cholinesterase inhibitor therapy in Alzheimer’s disease. Arch Neurol (in press)Google Scholar
63. Small G, Donohue J, Brooks R: An economic evaluation of donepezil in the treatment of Alzheimer’s disease. Clin Ther 1998; 20:838–850Crossref, Medline, Google Scholar
64. Shea C, MacKnight C, Rockwood K: Aspects of dementia: donepezil for treatment of dementia with Lewy bodies: a case series of nine patients. Int Psychogeriatr 1998; 10:229–238Crossref, Medline, Google Scholar
65. Perry EK, Kerwin J, Perry RH, Irving D, Blessed G, Fairbairn A: Cerebral cholinergic activity is related to the incidence of visual hallucinations in senile dementia of Lewy body type. Dementia 1990; 1:2–4Google Scholar
66. Aarsland D, Bronnick K, Karlsen K: Donepezil for dementia with Lewy bodies: a case study. Int J Geriatr Psychiatry 1999; 14:69–72Crossref, Medline, Google Scholar
67. Kaufer DI, Catt KE, Lopez OL, DeKosky ST: Dementia with Lewy bodies: response of delirium-like features to donepezil. Neurology 1998; 51:1512Crossref, Medline, Google Scholar
68. Cummings JL, Cyrus PA, Ruzicka BB, Gulanski B: The efficacy of metrifonate in improving the behavioral disturbances of Alzheimer’s disease patients (abstract). Neurology 1999; 524:4Google Scholar
69. Raskind M, Cyrus PA, Ruzicka BB, Gulanski B: Metrifonate Study Group: the effects of metrifonate on the cognitive, behavioral, and functional performance of Alzheimer’s disease patients. J Clin Psychiatry 1999; 60:318–325Crossref, Medline, Google Scholar
70. Becker R, Colliver J, Elble R, Feldman E, Giacobini E, Kumar V, Markwell S, Moriearty P, Parks R, Shillcutt S, Unni L, Vicari S, Womack C, Zec R: Effects of metrifonate, a long-acting cholinesterase inhibitor, in Alzheimer disease: report of an open trial. Drug Dev Res 1990; 19:425–434Crossref, Google Scholar
71. Becker RE, Colliver JA, Markwell SJ, Moriearty PL, Unni LK, Vicari S: Double-blind, placebo-controlled study of metrifonate, an acetylcholinesterase inhibitor, for Alzheimer’s disease. Alzheimer Dis Assoc Disorder 1996; 10:124–131Crossref, Medline, Google Scholar
72. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
73. Spagnoli A, Lucca U, Menasce G: Long-term acetyl-L-carnitine treatment in Alzheimer’s disease. Neurology 1991; 41:1726–1732Google Scholar
74. Jan G, McKeith IG: Special workshop in dementia with Lewy bodies (abstract). Neurobiol Aging 1998; 19:45Google Scholar
75. Sunderland T, Tariot PN, Cohen RM, Weingartner H, Mueller EA III, Murphy DL: Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls: a dose-response study. Arch Gen Psychiatry 1987; 44:418–426Crossref, Medline, Google Scholar
76. Gershon S, Olariu J: JB 329—a new psychotomimetic, its antagonism by tetrahydroaminoacridine and its comparison with LSD, mescaline and serynl. J Neuropsychiatry 1960; 1:283–292Medline, Google Scholar
77. Hutchinson M, Fazzini E: Cholinesterase inhibition in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1996; 61:324–325Crossref, Medline, Google Scholar
78. Zweig RM, Schegg K, Peacock JH, Melarkey D: A case of Alzheimer’s disease and hippocampal sclerosis with normal cholinergic activity in basal forebrain, neocortex, and hippocampus. Neurology 1989; 39:288–290Crossref, Medline, Google Scholar
79. Whitehouse PJ, Hedreen JC, White CL III, Price DL: Basal forebrain neurons in the dementia of Parkinson disease. Ann Neurol 1983; 13:243–248Crossref, Medline, Google Scholar
80. Davis K, Mohs R, Marin D, Purohit D, Perl D, Lantz M, Austin G, Haroutunian V: Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA 1999; 281:1401–1406Google Scholar
81. Poirier J, Delisle M-C, Quirion R, Aubert I, Farlow M, Lahiri D, Hui S, Bertrand P, Nalbantoglu J, Gilfix BM, Gauthier S: Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer’s disease. Proc Natl Acad Sci USA 1995; 92:12260–12264Google Scholar
82. Allen SJ, MacGowan SH, Tyler S, Wilcock GK, Robertson AGS, Holden PH, Smith SKF, Dawbarn D: Reduced cholinergic function in normal and Alzheimer’s disease brain is associated with apolipoprotein E4 genotype. Neurosci Lett 1997; 239:33–36Crossref, Medline, Google Scholar
83. Salehi A, Gonzalez Martinez V, Swaab DF: A sex difference and no effect of ApoE type on the amount of cytoskeletal alterations in the nucleus basalis of Meynert in Alzheimer’s disease. Neurobiol Aging 1998; 19:505–510Crossref, Medline, Google Scholar
84. Farlow MR, Lahiri DK, Poirier J, Davignon J, Schneider L, Hui SL: Treatment outcome of tacrine therapy depends on apolipoprotein genotype and gender of the subjects with Alzheimer’s disease. Neurology 1998; 50:669–677Crossref, Medline, Google Scholar
85. Schneider LS, Farlow MR, Henderson VW, Pogoda JM: Effects of estrogen replacement therapy on response to tacrine in patients with Alzheimer’s disease. Neurology 1996; 46:1580–1584Google Scholar
86. Decker MW, McGaugh JL: The role of interactions between the cholinergic system and other neuromodulatory systems in learning and memory. Synapse 1991; 7:151–168Crossref, Medline, Google Scholar
87. Lawrence AD, Sahakian BJ: Alzheimer disease, attention, and the cholinergic system. Alzheimer Dis Assoc Disord 1995; 9(suppl 2):43–49Google Scholar
88. Cooper JA, Sagar HJ, Doherty SM, Jordan N, Tidswell P, Sullivan EV: Different effects of dopaminergic and anticholinergic therapies on cognitive and motor function in Parkinson’s disease. Brain 1992; 115:1701–1725Google Scholar
89. Dubois B, Pillon B, Lhermitte F, Agid Y: Cholinergic deficiency and frontal dysfunction in Parkinson’s disease. Ann Neurol 1990; 28:117–121Crossref, Medline, Google Scholar
90. Van Spaendonck KP, Berger HJ, Horstink MW, Buytenhuijs EL, Cools AR: Impaired cognitive shifting in parkinsonian patients on anticholinergic therapy. Neuropsychologia 1993; 31:407–411Crossref, Medline, Google Scholar
91. Leber P: Guidelines for the Clinical Evaluation of Antidementia Drugs. Washington, DC, Food and Drug Administration, 1990Google Scholar
92. Kaufer DI, Cummings JL, Christine D, Bray T, Castellon S, Masterman D, MacMillan A, Ketchel P, DeKosky ST: Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc 1998; 46:210–215Crossref, Medline, Google Scholar
93. Steele C, Rovner B, Chase GA, Folstein M: Psychiatric symptoms and nursing home placement of patients with Alzheimer’s disease. Am J Psychiatry 1990; 147:1049–1051Google Scholar
94. Uhl GR, Hilt DC, Hedreen JC, Whitehouse PJ, Price DL: Pick’s disease (lobar sclerosis): depletion of neurons in the nucleus basalis of Meynert. Neurology 1983; 33:1470–1473Google Scholar
95. Tagliavini F, Pilleri G: Neuronal loss in the basal nucleus of Meynert in a patient with olivopontocerebellar atrophy. Acta Neuropathol 1985; 66:127–133Crossref, Medline, Google Scholar
96. Tagliavini F, Pilleri G, Gemignani F, Lechi A: Neuronal loss in the basal nucleus of Meynert in progressive supranuclear palsy. Acta Neuropathol 1983; 61:157–160Crossref, Medline, Google Scholar
97. Nakano I, Hirano A: Neuron loss in the nucleus basalis of Meynert in parkinsonism-dementia complex of Guam. Ann Neurol 1983; 13:87–91Crossref, Medline, Google Scholar
98. Cullen KM, Halliday GM: Mechanisms of cell death in cholinergic basal forebrain neurons in chronic alcoholics. Metab Brain Dis 1995; 10:81–91Crossref, Medline, Google Scholar
99. Rogers JD, Brogan D, Mirra SS: The nucleus basalis of Meynert in neurological disease: a quantitative morphological study. Ann Neurol 1985; 17:163–170Crossref, Medline, Google Scholar
100. Tagliavini F, Pilleri G: The basal nucleus of Meynert in cerebral aging and degenerative dementias, in Brain Pathology, 1. Edited by Pilleri G, Tagliavini F. Berne, Switzerland, Brain Anatomy Institute, 1984, pp 181–218Google Scholar
101. Cummings JL, Benson DF: The role of the nucleus basalis of Meynert in dementia: review and reconsideration. Alzheimer Dis Assoc Disord 1987; 1:128–145Crossref, Medline, Google Scholar
102. Murdoch I, Perry EK, Court JA, Graham DI, Dewar D: Cortical cholinergic dysfunction after human head injury. J Neurotrauma 1998; 15:295–305Crossref, Medline, Google Scholar
103. Mendez M, Younesi F, Perryman K: Use of donepezil for vascular dementia: preliminary clinical experience. J Neuropsychiatry Clin Neurosci 1999; 11:268–270Crossref, Medline, Google Scholar
104. McGeer PL, McGeer EG, Suzuki J, Dolman CE, Nagai T: Aging, Alzheimer’s disease, and the cholinergic system of the basal forebrain. Neurology 1984; 34:741–745Crossref, Medline, Google Scholar
105. Burt T, Sachs G, Demopulos C: Donepezil in treatment-resistant bipolar disorder. Biol Psychiatry 1999; 45:959–964Crossref, Medline, Google Scholar
106. White K, Cummings JL: Schizophrenia and Alzheimer’s disease: clinical and pathophysiologic analogies. Compr Psychiatry 1996; 37:188–195Crossref, Medline, Google Scholar
107. Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, Hurley DJ, Potter WZ, Paul SM: Effects of xanomeline, a selective receptor agonist, on cognitive function and behavioral symptoms in Alzheimer’s disease. Arch Neurol 1997; 4:465–473Crossref, Google Scholar
108. Kumar R, Orgogozo J: Efficacy and safety of SB 202026 as a symptomatic treatment for Alzheimer’s disease, in Alzheimer’s Disease: Biology, Diagnosis, and Therapeutics. Edited by Iqbal K, Winbald B, Nishimura T, Takeda M, Wisniewski HM. New York, John Wiley & Sons, 1997, pp 677–685Google Scholar
109. Tariot PN, Cohen RM, Welkowitz JA, Sunderland T, Newhouse PA, Murphy DL, Weingarten H: Multiple-dose arecoline infusions in Alzheimer’s disease. Arch Gen Psychiatry 1988; 45:901–905Crossref, Medline, Google Scholar
110. Penn RD, Martin EM, Wilson RS, Fox JH, Savoy SM: Intraventricular bethanechol infusion for Alzheimer’s disease: results of double-blind and escalating-dose trials. Neurology 1988; 38:219–222Crossref, Medline, Google Scholar
111. Read SL, Frazee J, Shapira J, Smith C, Cummings JL, Tomiyasu U: Intracerebroventricular bethanechol for Alzheimer’s disease: variable dose-related responses. Arch Neurol 1990; 47:1025–1030Google Scholar
112. Davis KL, Hollander E, Davidson M, Davis BM, Mohs RC, Horvath TB: Induction of depression with oxotremorine in patients with Alzheimer’s disease. Am J Psychiatry 1987; 144:468–471Link, Google Scholar
113. Newhouse PA, Sunderland T, Tariot PN, Blumhardt CL, Weingarten H, Mellow A, Murphy DL: Intravenous nicotine in Alzheimer’s disease: a pilot study. Psychopharmacology (Berl) 1988; 95:171–175Crossref, Medline, Google Scholar