Amygdala Hypoactivity to Fearful Faces in Boys With Conduct Problems and Callous-Unemotional Traits
Abstract
Objective: Although early-onset conduct problems predict both psychiatric and health problems in adult life, little research has been done to index neural correlates of conduct problems. Emerging research suggests that a subgroup of children with conduct problems and elevated levels of callous-unemotional traits may be genetically vulnerable to manifesting disturbances in neural reactivity to emotional stimuli indexing distress. Using functional MRI, the authors evaluated differences in neural response to emotional stimuli between boys with conduct problems and elevated levels of callous-unemotional traits and comparison boys. Method: Seventeen boys with conduct problems and elevated levels of callous-unemotional traits and 13 comparison boys of equivalent age (mean=11 years) and IQ (mean=100) viewed blocked presentations of fearful and neutral faces. For each face, participants distinguished the sex of the face via manual response. Results: Relative to the comparison group, boys with conduct problems and elevated levels of callous-unemotional traits manifested lesser right amygdala activity to fearful faces. Conclusions: This finding is in line with data from studies of adults with antisocial behavior and callous-unemotional traits (i.e., psychopaths), as well as from a recent study of adolescents with callous-unemotional traits, and suggests that the neural substrates of emotional impairment associated with callous-unemotional antisocial behavior are already present in childhood.
Early-onset conduct problems predict both psychiatric and physical health problems in adult life (1 , 2) . Conduct problems that manifest early in life are also thought to reflect biological vulnerability to antisocial behavior (3) . Recent studies have highlighted the finding that within this early-onset group, callous-unemotional traits index a particularly serious form of conduct disturbance. Callous-unemotional traits include such characteristics as lack of guilt and empathy, which are also considered primary in clinical descriptions of adult psychopathy (4) . Recent data from studies of twins suggest that conduct problems in callous-unemotional children are under strong genetic influence (5 , 6) .
Both adult psychopaths and children with conduct problems and callous-unemotional traits have difficulty processing visual and auditory displays of fear and sadness (7 – 9) . Both groups also experience difficulties in aversive learning paradigms (8) . This profile is also seen in neuropsychological patients with damage to the amygdala, one of the brain’s key affect-processing structures (10) . Consequently, it has been proposed that the difficulties in affective processing seen in adult psychopaths and children with conduct problems and callous-unemotional traits are accompanied by amygdala impairment (8) . Early amygdala dysfunction may have a negative impact on the development of empathy (8) .
Functional brain imaging data from studies of healthy adults and children support the amygdala’s role in processing emotional facial expressions and other affective stimuli (11 – 13) . To date, there have been a handful of functional brain imaging studies of adult psychopathy and one study of adolescents with conduct problems and callous-unemotional traits. All implicate reduced amygdala reactivity in individuals with psychopathy or elevated levels of psychopathic personality traits as compared with both healthy and institutionalized comparison subjects, although the laterality of the reported difference varies among studies, probably reflecting the paradigm used (14 – 18) . Amygdala hyporeactivity has been suggested to be associated with the emotional dysfunction observed in psychopathy (8) .
Studies of healthy adults suggest a role for the rostral anterior cingulate cortex in extinguishing amygdala reactivity during emotional arousal (19 , 20) . The amygdala hypoactivity observed in adults with psychopathy could thus reflect down-regulation of amygdala activity by the anterior cingulate cortex. Aside from the finding of amygdala hypoactivity in psychopathy, the rostral anterior cingulate cortex has been implicated in more than one brain imaging study of psychopathy (14 , 15) . In each case, adults with psychopathy showed less rostral anterior cingulate cortex reactivity than comparison subjects. This finding suggests that the reduced amygdala reactivity in adults with psychopathy might not, in this case, be due to increased emotional regulation by the anterior cingulate cortex.
Despite a prominent model of psychopathy proposing that it is a developmental disorder of early amygdala dysfunction, to date, only one neuroimaging study of adolescents with conduct problems and callous-unemotional traits has been published (18) . The study reported amygdala hyporeactivity to fearful faces in adolescents with conduct problems and callous-unemotional traits relative to comparison subjects and adolescents with attention deficit hyperactivity disorder. We also know of one functional MRI (fMRI) study of emotion processing in adolescents with conduct disorder, which demonstrated reduced left amygdala and right dorsal anterior cingulate cortex activation to nonfacial emotional stimuli in adolescents with conduct disorder relative to healthy comparison subjects after co-occurring anxiety and depression symptoms were controlled for (21) . Structural MRI data from the same group indicated reduced left amygdala volume in adolescents with conduct disorder (22) .
Given that lack of anxiety is thought to be a core characteristic of psychopathy (23) , it is possible to infer from the findings of the previous fMRI study of adolescents with conduct disorder (21) a hypothesis that children with callous-unemotional traits and conduct problems may show reduced amygdala reactivity. This hypothesis was supported by the recent study of adolescents by Marsh et al. (18) . Behavioral genetic findings of increased genetic vulnerability to antisocial behavior in children with conduct problems and callous-unemotional traits (5 , 6) , as well as previous behavioral studies (7 – 9) , further underscore the importance of studying the brain reactivity to emotional stimuli in this group.
The task used in this study was designed to investigate amygdala reactivity to fearful faces. In previous studies, this and similar tasks employing fearful facial stimuli have been shown to reliably elicit activation of the amygdala in healthy adults (12) and children (11 , 13) . Our primary goal was to examine differences in amygdala reactivity to fearful faces between boys with conduct problems and elevated levels of callous-unemotional traits and an age- and IQ-matched comparison group. We hypothesized that boys with conduct problems and elevated levels of callous-unemotional traits would show decreased amygdala reactivity to fearful faces relative to the comparison subjects. We further hypothesized that this decreased amygdala reactivity would not be associated with stronger anterior cingulate cortex reactivity in this group, although, in line with adult data, it is possible that children with conduct problems and callous-unemotional traits may show reduced anterior cingulate cortex reactivity.
Method
Participants
This study reports data from 30 boys 10–12 years of age (mean age=11.6 years). Participants were recruited from the 9-year longitudinal Twins Early Development Study (24) as part of an ongoing neuroimaging project to study the heritability of affect circuitry in children at high versus low risk for callous-unemotional antisocial behavior. Children were selected as potential participants on the basis of behavioral ratings collected at 9 years of age. Families were recruited by letter. Parents completed screening questionnaires indicating MRI contra-indicators and provided consent to be contacted about the study. The study and recruitment procedure were approved by the Institute of Psychiatry and Maudsley Research Ethics Committee. After children and their parents received a complete description of the study, both provided written informed consent. All participants had normal or corrected-to-normal vision, and all but one were right-handed.
Combined parent and teacher ratings (the highest rating given by parent or teacher for each question was recorded) (25) on the conduct problems subscale of the Strengths and Difficulties Questionnaire (26) and the Antisocial Process Screening Device (27) were used to assign children to one of two groups for our analyses: those with conduct problems and elevated levels of callous-unemotional traits (N=17) and age- and IQ-matched (full-scale IQ) comparison subjects (N=13). The grouping procedure is described in Figure 1 , and the groups’ basic characteristics are summarized in Table 1 .
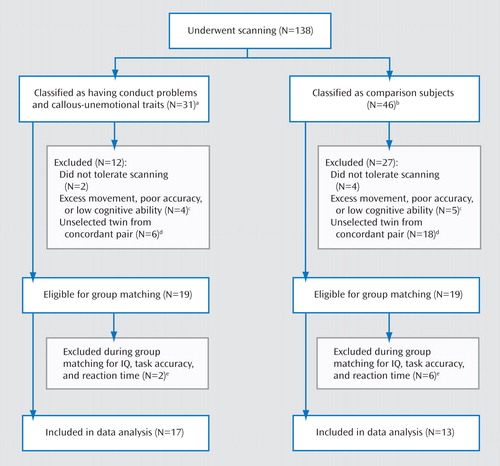
a Scored in the top 10% on both conduct problems and callous-unemotional traits. These criteria were based on earlier behavior genetic studies from our group demonstrating a strong heritability for antisocial behavior and callous-unemotional traits using these cutoffs (5, 6).
b Scored within one standard deviation of the population mean for the Twins Early Development Study on the conduct problems subscale of the Strengths and Difficulties Questionnaire and on the callous-unemotional scale of the Antisocial Process Screening Device.
c Movement ≤4 degrees or ≤4 mm in any plane.
d One twin from each concordant pair was selected to avoid bias due to nonindependence of data. If a twin pair was concordant for conduct problems and callous-unemotional traits, the eligible twin with the higher score on callous-unemotional traits was selected. In the comparison group, the twin with the lower score on callous-unemotional traits was selected; if a twin pair had the same score, the twin with the lower score on conduct problems was selected.
e Boys with extreme high or low scores on the matching variables were excluded from the analysis.
Fearful Faces fMRI Task
A categorical blocked design was used. Participants viewed blocks of fearful or neutral faces and, to ensure that they were attending to the stimuli, were required to indicate the sex of the target using a button-box response. Each block consisted of faces derived from a standard set of pictures of facial affect (28) . Fearful faces were displayed at 100% emotional intensity, while neutral faces were morphed to a 25% “happy” intensity in order to avoid appearing threatening ( Figure 2 ) (29) . Each face was presented sequentially for 3000 msec, remaining on-screen for the full 3000 msec even if a response was made before the end of the period. Interstimulus intervals were 750 msec. Responses to five neutral, five fearful, and two rest blocks (showing just a fixation cross and displayed at the beginning and end of the scanning run) were acquired. The epoch length for each block (comprising eight face presentations, plus instructions) was 32 seconds, including a 2-second fixation cross presentation at the beginning of each block. Participant performance was measured as accuracy on the sex discrimination task (percent correct responses). Before entering the scanner room, all participants underwent a brief ability screen using the short version of the Wechsler Abbreviated Scales of Intelligence (30) and were trained on the imaging paradigm.

fMRI Measurement, Processing, and Functional Analyses
Functional image volumes were acquired using blood-oxygen-level-dependent (BOLD) contrasts in a 3-T scanner (GE Signa Excite), using the body coil for radiofrequency transmission and the manufacturer’s eight-channel head coil for signal reception. Image volumes were collected using T 2 *-weighted gradient echo-planar imaging sequence with 28 slices (slice thickness=3.5 mm, gap=0.3 mm) designed to cover the whole brain. Other parameters were repetition time=2 seconds, echo time=25 msec, field of view=220×220 mm, matrix size=64×64. To reduce T 1 losses at this relatively short repetition time, an excitation flip angle of 70 degrees was used, and at the start of each scan series (prior to the presentation of stimuli), four “dummy acquisitions” with no data collection were played out to ensure that T 1 effects had reached a steady state. The orientation of the oblique axial slices was parallel to the anterior commissure-posterior commissure line. Slices were acquired in interleaved order.
Functional imaging data were preprocessed and analyzed using a statistical parametric mapping software package (SPM2, Wellcome Department of Cognitive Neurology, London) implemented in Matlab, release 12 (MathWorks, Natick, Mass.). For each participant, images were manually readjusted to the anterior commissure-posterior commissure line before being realigned to the first volume in the time series to correct for head motion during the scan. A mean functional image was constructed for each participant and then used to derive parameters for spatial normalization into the standard stereotaxic space implemented in SPM2 (Montreal Neurological Institute [MNI] template) (31 , 32) . Both affine and nonlinear components were used in the spatial normalization. The spatial normalization parameters for each mean image were applied to the corresponding realigned images from the session and resampled into isotropic 3-mm voxels. Normalized images were smoothed with a Gaussian filter with full width at half maximum of 6 mm. A high-pass filter (cutoff period=128 seconds) was incorporated to remove noise associated with low-frequency confounds (e.g., scanner drift).
Within-Group Analyses
For each participant, a contrast image was created in which voxel values represented the difference in the amplitude of the fitted hemodynamic response elicited during fearful face processing relative to that elicited during neutral face processing and another for neutral relative to fearful face processing. None of the participants exhibited absence of signal in the regions of interest. The neutral blocks were designed to act as baseline stimuli of equal visual complexity. These contrasts were then entered into one-sample t tests for each group to determine whether there were any brain regions in which the mean difference between the stimulus types departed significantly from zero. Region-of-interest analyses were restricted to the amygdala and the anterior cingulate cortex using anatomical masks defined by the Pick-Atlas software program (Functional MRI Laboratory, Wake Forest University School of Medicine, http://www.fmri.wfubmc.edu). Activations exceeding a cluster size of five voxels and an uncorrected p value of <0.05 were reported in the a priori specified amygdala region of interest, and activations exceeding a cluster size of five voxels and an uncorrected p value of <0.005 were set for the a priori specified anterior cingulate cortex region of interest. These significance levels were selected to be in line with those used in Marsh and colleagues’ recent study of children with conduct problems and callous-unemotional traits (18) . All brain coordinates are provided in Talairach space after adjustment (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach) for differences between MNI and Talairach coordinates. Additionally, activation differences outside the amygdala and anterior cingulate were assessed across the whole brain (p<0.001, uncorrected for multiple comparisons across the whole brain).
Between-Group Analyses
The individual contrast images were entered into one-way ANOVAs to test the null hypothesis that there would be no difference in amygdala or anterior cingulate activity between boys with conduct problems and callous-unemotional traits and the comparison group. The significance of activation was assessed for the a priori defined regions of interest described above (cluster size greater than five voxels and p<0.05, uncorrected, for the amygdala region of interest; and cluster size greater than five voxels and p<0.005, uncorrected, for the anterior cingulate cortex region of interest) and across the whole brain (p<0.001, uncorrected), according to the criteria applied in Marsh et al. (18) .
Results
Behavioral Responses
The mean accuracy levels on the sex identification task for fearful and neutral faces for the comparison subjects were 94.62% (SD=6.00) and 94.62% (SD=3.00), respectively. For the boys with conduct problems and callous-unemotional traits, the mean accuracy levels were 93.53% (SD=6.00) and 94.12% (SD=3.00), respectively. The main effects for group or emotion category were not statistically significant, and we did not observe a group-by-expression category interaction. The mean reaction times for fearful and neutral faces for the comparison subjects were 1068.83 msec (SD=167.80) and 1058.90 msec (SD=190.97), respectively. For the boys with conduct problems and callous-unemotional traits, the mean reaction times were 1036.80 msec (SD=251.27) and 1007.72 msec (SD=227.31). The main effects for group and for emotion category were not statistically significant, nor was the group-by-expression category interaction.
Bold fMRI Responses
Within-group analyses of fear versus neutral contrast
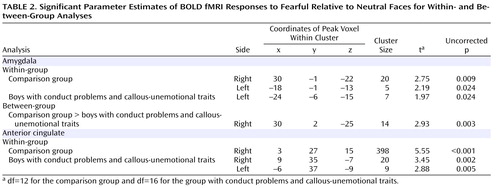
The comparison group showed significant bilateral amygdala activation (right: t=2.75, df=12, p=0.009; left: t=2.19, df=12, p=0.024) for the contrast comparing fearful versus neutral faces ( Table 2 ). That is, comparison boys exhibited a relatively greater activation in both the left and right amygdala during fearful face processing than during neutral baseline. In the anterior cingulate, greater response to fearful versus neutral faces was observed in the right anterior cingulate cortex (t=5.55, df=12, p<0.001).
For the boys with conduct problems and callous-unemotional traits, a significant activation was observed in the left amygdala (t=1.97, df=16, p=0.024) for the contrast of fearful versus neutral faces ( Table 2 ). Bilateral anterior cingulate activation was also observed (right: t=3.45, df=16, p=0.002; left: t=2.88, df=16, p=0.005).
Within-group whole brain analysis at p<0.001 (uncorrected) showed no other areas of significant activation for either group during fearful relative to neutral face processing.
Between-group comparisons of fear versus neutral contrast
Differential activation between the groups was examined for the contrast of fearful faces versus neutral faces in the amygdala and anterior cingulate regions of interest. The comparison group showed significantly greater right-sided amygdala activation to fearful relative to neutral faces (t=2.93, df=28, p=0.003; Figure 3 ). There was no difference between the groups in the anterior cingulate cortex region of interest at the prescribed uncorrected p level of <0.005. Whole-brain analysis (p<0.001, uncorrected) did not reveal any additional brain areas in which differential activation was observed between the groups. Furthermore, whole brain and region-of-interest analyses revealed no significant areas of greater activation for the group with conduct problems and callous-unemotional traits relative to the comparison group.
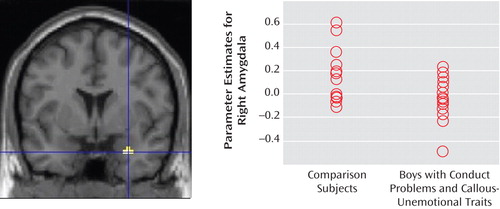
a Coordinates (x, y, z) are 30, 2, –25; cluster size=14 voxels. Significant difference between groups (t=2.93, p=0.003).
In accordance with previous data (4) , the groups differed in hyperactivity symptoms (F=5.41, p=0.03) in addition to conduct problems and callous-unemotional traits. We therefore tested for group differences in brain activation to fearful relative to neutral faces using an analysis of covariance with hyperactivity symptoms as a covariate. The significant group difference in the right amygdala was slightly reduced in magnitude but remained statistically significant at the significance threshold of p<0.05 (F=2.23, df=28, p=0.018). The neutral versus fearful faces contrast did not yield any statistically significant activations for the amygdala or anterior cingulate regions of interest in either the within-group or the between-group analyses.
Discussion
The results of this study are consistent with previous child and adult data suggesting that the amygdala typically activates more strongly to fearful faces than to neutral faces (12 , 13) . These data confirm previous child brain imaging findings (11 , 13) and indicate that amygdala involvement in the processing of emotional facial stimuli has already developed by middle childhood. In our comparison group, both the right and left amygdala were activated significantly more to fearful faces than to neutral expressions. Boys with conduct problems and callous-unemotional traits showed only left-sided amygdala activation to fearful faces as compared with neutral expressions. When the two groups were compared, the boys with conduct problems and callous-unemotional traits thus showed relatively decreased amygdala reactivity to fearful facial stimuli in the right amygdala. Our data suggest that the emotional impairment evident in children with conduct problems and callous-unemotional traits is accompanied by reduced right-sided amygdala reactivity to others’ fear. Both groups showed statistically significant reactivity in the anterior cingulate cortex region of interest, but there were no statistically significant group differences. Crucially, the reduced amygdala activation in boys with conduct problems and callous-unemotional traits was not associated with stronger anterior cingulate cortex reactivity.
Frick and Marsee have highlighted the importance of charting psychopathic personality markers (callous and unemotional traits) in childhood (4) . The Antisocial Process Screening Device we used to assess callous-unemotional traits in this study was designed to extend assessment of psychopathic traits to children, with the view that the callous and unemotional traits mark one important risk factor for lifelong persistent antisocial behavior. Callous and unemotional traits include such characteristics as lack of guilt and empathy, which are also considered primary in clinical descriptions of adult psychopathy (4) . Children with callous-unemotional traits show a specific behavioral and neurocognitive profile that is similar to that seen in adult psychopaths (8) . At a behavioral level, conduct problems with elevated levels of callous-unemotional traits are associated with a poorer long-term outcome and greater severity of antisocial behavior as compared with children with conduct problems but without callous-unemotional traits (4) . At the neurocognitive level, children with conduct problems and callous-unemotional traits appear to suffer from emotional dysfunction characterized by difficulty in processing fear and sadness, as well as deficits in reinforcement learning (8 , 18) . Data from our own group indicate that antisocial behavior in children with elevated levels of callous-unemotional traits is strongly heritable, suggesting that children who display these traits may be particularly vulnerable genetically to antisocial behavior (5 , 6) . This finding raises the possibility that psychopathy may be a developmental disorder with particular personality and neurocognitive markers that can be delineated successfully in children (8) .
Our findings are in line with fMRI studies investigating emotion processing in adult psychopaths, adolescents with conduct disorder, and adolescents with conduct problems coupled with callous-unemotional traits (14 – 18 , 21) . Reduced amygdala activation in individuals with psychopathy has been demonstrated with various affective stimuli (14 – 18) , with reduced right amygdala activation previously reported for a face processing paradigm (16 , 18) . Our study offers a replication of Marsh and colleagues’ (18) finding of amygdala hyporeactivity in response to fearful facial expressions in adolescents with both conduct problems and callous-unemotional traits, and it extends the work of Sterzer et al. (21) , who did not assess these traits in their sample of adolescents with conduct disorder. Our focus on younger children adds to findings by Marsh et al. (18) and others (14 – 17) by providing evidence suggesting that amygdala hypoactivity associated with callous-unemotional conduct problems is already present in some pre-adolescent and early adolescent children and lends support to the notion that psychopathy may be a developmental disorder related to amygdala dysfunction (8) .
Our findings are in line with earlier behavioral data demonstrating difficulties in recognition of fearful expressions in children with conduct problems and callous-unemotional traits (7) . Of course it would be unethical to label children as “psychopaths,” but it is worth noting that vulnerability to psychopathy appears to be present from childhood and manifests in both detectable trait differences and associated neurocognitive differences.
It has been proposed that psychopathy may be genetic in origin, and there are preliminary data to support this notion (5 , 6 , 8) . Genetic vulnerability may set the tone for an individual’s neural reactivity to emotional stimuli, which is likely to be further moderated by environmental factors (33) . Current imaging genetic studies have focused on reactive, nonpsychopathic antisocial behavior (20 , 34 , 35) , but our own group and others are planning to extend these investigations to psychopathic/callous-unemotional antisocial behavior.
There are several limitations to this study. First, we cannot exclude the possibility that with larger sample sizes, between-group differences might have emerged in additional brain areas. Second, the p values for our regions of interest were uncorrected for multiple comparisons. To increase our confidence in the findings, we reported data on only two regions of interest with empirical precedence (the amygdala and the anterior cingulate) and where cluster size exceeded five voxels. Third, the format of our task did not allow for the rest condition to be used as an additional baseline condition. Instead, neutral faces were used as the baseline condition. However, a 2×2 analysis of group by emotion may have provided more power to detect group differences. Fourth, we were not able to measure the gaze patterns of participants while they studied the stimuli in the scanner. Thus, although our behavioral data reveal no differences in accuracy or reaction time between the groups (and thus suggest that both groups were efficient in processing the stimuli), we do not know on which part of the face the participants fixated. Previous studies indicate that individuals with amygdala damage do not fixate on the most informative part of a fearful face, the eyes (36) . Decreased gaze fixation to crucial aspects of emotional stimuli accounts for a moderate proportion of variance in amygdala activation in healthy adult females (37) . Furthermore, overt fear recognition can be improved in children with callous-unemotional traits when they are asked to fixate on eyes in a face (38) . It remains to be seen whether we could elicit stronger amygdala activation in children with conduct problems and callous-unemotional traits if they were to engage effortfully with processing the eyes in facial stimuli. Finally, our study did not include a group of children with conduct problems and no callous-unemotional traits. We hope to include such a group in future studies.

1. Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE: MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry 2006; 11:903–913Google Scholar
2. Odgers CL, Caspi A, Broadbent JM, Dickson N, Hancox RJ, Harrington H, Poulton R, Sears MR, Thomson WM, Moffitt TE: Prediction of differential adult health burden by conduct problem subtypes in males. Arch Gen Psychiatry 2007; 64:476–484Google Scholar
3. Moffitt TE: Life-course-persistent and adolesence-limited antisocial behavior, in Causes of Conduct Disorder and Juvenile Delinquency. Edited by Lahey BB, Moffitt TE, Caspi A. New York, Guilford, 2003Google Scholar
4. Frick PJ, Marsee MA: Psychopathy and developmental pathways to antisocial behavior in youth, in Handbook of Psychopathy. Edited by Patrick CJ. New York, Guilford, 2006Google Scholar
5. Viding E, Jones AP, Frick P, Moffitt TE, Plomin R: Heritability of antisocial behaviour at 9: do callous-unemotional traits matter? Dev Sci 2008; 11:17–22Google Scholar
6. Viding E, Blair RJR, Moffitt TE, Plomin R: Evidence for substantial genetic risk for psychopathy in 7-year-olds. J Child Psychol Psychiatry 2005; 46:592–597Google Scholar
7. Blair RJ, Colledge E, Murray L, Mitchell DG: A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. J Abnorm Child Psychol 2001; 29:491–498Google Scholar
8. Blair RJR, Peschardt KS, Budhani S, Mitchell DGV, Pine DS: The development of psychopathy. J Child Psychol Psychiatry 2006; 47:262–275Google Scholar
9. Blair R, Mitchell DG, Richell RA, Kelly S, Leonard A, Newman C, Scott SK: Turning a deaf ear to fear: impaired recognition of vocal affect in psychopathic individuals. J Abnorm Psychol 2002; 111:682–686Google Scholar
10. Adolphs R: Is the human amygdala specialized for processing social information? Ann NY Acad Sci 2003; 985:326–340Google Scholar
11. Herba C, Phillips ML: Annotation: development of facial expression recognition from childhood to adolescence: behavioural and neurological perspectives. J Child Psychol Psychiatry 2004; 45:1–14Google Scholar
12. Hariri A, Tessitore A, Mattay V, Fera F, Weinberger D: The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage 2002; 17:317–323Google Scholar
13. Lobaugh N, Gibson E, Taylor M: Children recruit distinct neural systems for implicit emotional face processing. Neuroreport 2006; 17:215–219Google Scholar
14. Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, Liddle PF: Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry 2001; 50:677–684Google Scholar
15. Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H: Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch Gen Psychiatry 2005; 62:799–805Google Scholar
16. Gordon HL, Baird AA, End A: Functional differences among those high and low on a trait measure of psychopathy. Biol Psychiatry 2004; 56:516–521Google Scholar
17. Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, Birbaumer N: Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neurosci Lett 2002; 328:233–236Google Scholar
18. Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJ: Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry 2008; 165:712–720Google Scholar
19. Paus T: Primate anterior cingulate cortex: where motor control, drive, and cognition interface. Nat Rev Neurosci 2001; 2:417–424Google Scholar
20. Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger D: Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA 2006; 103:6269–6274Google Scholar
21. Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poutska F: Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry 2005; 57:7–15Google Scholar
22. Sterzer P, Stadler C, Poustka F, Kleinschmidt A: A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage 2007; 37:335–342Google Scholar
23. Cleckley H: The Mask of Sanity, 5th ed. St Louis, Mosby, 1976Google Scholar
24. Trouton A, Spinath FM, Plomin R: Twins Early Development Study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition, and behavior problems in childhood. Twin Res 2002; 5:444–448Google Scholar
25. Piacentini J, Cohen P, Cohen C: Combining discrepant diagnostic information from multiple sources: are complex algorithms better than simple ones? J Abnorm Child Psychol 1992; 20:51–63Google Scholar
26. Goodman R: The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry 1997; 38:581–586Google Scholar
27. Frick PJ, Hare RD: Antisocial Process Screening Device. Toronto, Multi Health Systems, 2001Google Scholar
28. Ekman P, Freisen W: Pictures of Facial Affect. Palo Alto, Calif, Consulting Psychologist Press, Inc, 1976Google Scholar
29. Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, Williams SC, Bullmore ET, Brammer M, Gray JA: Neural responses to facial and vocal expressions of fear and disgust. Proc Biol Sci 1998; 265:1809–1817Google Scholar
30. Wechsler D: Wechsler Abbreviated Scales of Intelligence. San Antonio, Tex, Psychological Corporation, 1999Google Scholar
31. Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL: The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage 2002; 17:184–200Google Scholar
32. Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL: Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage 2002; 19:16–28Google Scholar
33. Viding E, Jones AP: Cognition to genes via the brain in the study of conduct disorder. Q J Exp Psychol (Colchester) 2008; 61:171–181Google Scholar
34. Eisenberger NI, Way BM, Taylor SE, Welch WT, Lieberman MD: Understanding genetic risk for aggression: clues from the brain’s response to social exclusion. Biol Psychiatry 2007; 35:1601–1612Google Scholar
35. Buckholtz J, Callicott J, Kolachana B, Hariri A, Goldberg T, Genderson M, Egan M, Mattay V, Weinberger D, Meyer-Lindenberg A: Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Mol Psychiatry 2008; 13:313–324Google Scholar
36. Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR: A mechanism for impaired fear recognition after amygdala damage. Nature 2005; 433:68–72Google Scholar
37. van Reekum CM, Johnstone T, Urry HL, Thurow ME, Schaefer HS, Alexander AL, Davidson RJ: Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage 2007; 36:1041–1055Google Scholar
38. Dadds MR, Perry Y, Hawes DJ, Merz S, Riddell AC, Haines DJ, Solak E, Abeygunawardane AI: Attention to the eyes and fear-recognition deficits in child psychopathy. Br J Psychiatry 2006; 189:280–281Google Scholar