Decline in Treatment of Pediatric Depression After FDA Advisory on Risk of Suicidality With SSRIs
Abstract
Objective: In October 2003, the U.S. Food and Drug Administration (FDA) issued a public health advisory about the risk of suicidality in pediatric patients taking selective serotonin reuptake inhibitors (SSRIs) for depression. This study used data from a large national pediatric cohort to examine patterns of diagnosis of depression, prescription of antidepressants, prescription of pharmacological alternatives to antidepressants, and use of psychosocial care before and after the FDA advisory was issued. Method: A large pediatric cohort with newly diagnosed episodes of depression was created from a national integrated claims database of managed care plans from October 1998 to September 2005 (N=65,349). Time-series models were used to compare diagnosing and prescribing trends during the 2 years after the FDA advisory and the expected trends based on data from the 5-year period preceding the advisory. Results: From 1999 to 2004, pediatric diagnoses of depression increased from 3 to 5 per 1,000. After the FDA advisory was issued, the national rate decreased to 1999 levels, a significant deviation from the historical trend. Pediatricians and nonpediatrician primary care physicians accounted for the largest reductions in new diagnoses. Among patients with depression, the proportion receiving no antidepressant increased to three times the rate predicted by the preadvisory trend, and SSRI prescription fills were 58% lower than predicted by the trend. There was no evidence of a significant increase in use of treatment alternatives (psychotherapy, atypical antipsychotics, and anxiolytics). Conclusions: The FDA advisory was associated with significant reductions in aggregate rates of diagnosis and treatment of pediatric depression.
Depression is one of the most burdensome diseases worldwide (1) . For youths, it endangers health, development, and economic attainment across the lifespan (2 – 5) . Practice guidelines for pediatric patients recommend selective serotonin reuptake inhibitor (SSRI) antidepressants as first-line treatment in the acute stage of depression (6) . Fluoxetine is the only drug in this class approved by the U.S. Food and Drug Administration (FDA) specifically for pediatric use (7) . However, the FDA changed regulatory policy for all antidepressants, culminating in 2005 in a black box warning about suicidality risk for pediatric patients with depression being treated with SSRIs.
The FDA’s risk communication began in October 2003 with a public health advisory calling health care professionals’ attention to the possibility of an increased risk of suicidality in pediatric patients with depression who were being treated with antidepressant medications. This policy action created a natural experiment because it heightened concern among the public and clinicians about the potential risks of using antidepressants. APA and the American Academy of Child and Adolescent Psychiatry urged the FDA to track prescribing patterns of antidepressants in the pediatric population (8) , and experts have expressed concern for the unknown effects on community treatment given the immense public health burden of pediatric depression (9 – 11) . However, the effect of this policy change on treatment of pediatric depression in the community has not been studied.
The FDA’s antidepressant policy action followed recent developments. FDA announcements regarding unpublished efficacy studies suggested that negative results had not been made public, and SSRIs for pediatric treatment came under increased scrutiny (12) . In June 2003, the FDA issued an alert citing uncontrolled studies showing a link between paroxetine treatment and suicide attempts among pediatric patients with depression. The FDA’s British counterpart, the Committee on Safety of Medicines, banned the use of paroxetine with this population for the same reasons (13) . As noted, the first FDA public health advisory was announced in October 2003. In March 2004, the FDA issued a strong warning calling on manufacturers of 10 specific antidepressants, mostly the newer agents, to add to their labeling a warning that all patients (adult and pediatric) being treated with these drugs should be monitored for suicidality. In September 2004, the FDA held a publicized advisory committee meeting that culminated in a second public health advisory in October 2004 requiring the black box warning for all antidepressants regarding the risk of suicidality in pediatric patients. In February 2005, the FDA provided specific language for the warning and required a patient medication guide.
By September 2004, media sources were reporting reduced SSRI prescriptions; one study showed that all SSRI prescriptions for one pharmacy benefit manager declined by 20% in the 3 months following the March 2004 warning (8 , 14 , 15) . The decline in pediatric SSRI prescriptions could be interpreted as positive (e.g., SSRIs were overmarketed and overused, and the decline was a correction), negative (e.g., it was a halt to decades of progress toward cost-effective treatment of depression in primary care), or both. Although research continues on the link between depression and suicidality (10 , 16 – 21) , no studies have yet illuminated the implications of the FDA’s action for clinical practice.
The purpose of this study was to evaluate the impact of the FDA warnings on market-level patterns of care, using a large national community-based pediatric cohort of patients with new episodes of depression. This study draws on data with unique strengths: 1) an integrated file with comprehensive accounting for health care visits and prescriptions; 2) large samples of pediatric patients that permit the creation of an analytic cohort of significant size; and 3) a time span covering 5 years before and 2 years after the FDA policy action (the 2003 advisory), allowing robust estimates of preadvisory and postadvisory trends in patterns of care. The results of this study illustrate aggregate effects on community medical practice for depression among U.S. pediatric managed care enrollees.
Method
Data
The data for this study, from the PharMetrics Patient-Centric Database, span the period of January 1997 to December 2005. The data universe includes medical, specialty, facility, and pharmacy paid claims from more than 85 managed care plans nationally, representing more than 47 million covered lives. The distributions of age, gender, and region in these national data are not statistically different from those in the 2000 U.S. Census data.
Enrollment and claims data were extracted from the database for enrollees of all ages who met either of two criteria: they had a diagnosis of major depressive disorder or a related psychiatric disorder (ICD-9-CM codes 296.xx–300.xx or 311.xx) on a medical claim assigned by a clinician, or they had a paid claim for a filled prescription of any antidepressant drug (generic product identifier [GPI] code 58.xx). This process resulted in a base population of some 4.1 million patients. Because the data were unidentified and anonymous, an expedited review was obtained, and the study was approved by the Colorado Multiple Institutional Review Board.
The analytical file was built by creating a cohort of new cases of pediatric depression and then aggregating the data for time-series regressions. First, claims data were used to create a cohort of new episodes of depression. A new episode was defined using the following specifications of the National Committee for Quality Assurance’s Healthplan and Employer Data and Information Set (HEDIS): an ICD-9 code of 296.2, 296.3, 300.4, or 311 (i.e., major depressive disorder, single episode; major depressive disorder, recurrent episode; neurotic depression; and depression not otherwise specified, respectively); a period of 120 days before diagnosis during which no other depression-related diagnoses appeared in the claims history, and a period of 90 days before diagnosis during which no other antidepressant medication claims appeared in the history (22 , 23) . Continuous enrollment for 120 days before and after diagnosis was required. The HEDIS outpatient depression indicators were derived from expert consensus on treatment research and clinical care and have been used in published depression treatment and outcomes research (22 – 25) .
The time horizon for the study was anchored by the first clinical trial of the safety and efficacy of an SSRI for youths (26) . To account for seasonal trends, annual data were anchored on the same month. The resulting time horizon, which accounted for episode creation, follow-up, and seasonality, spanned October 1998 to September 2005. From the total cohort of 541,187 unique new episodes of depression, cases of patients 5 to 18 years of age at diagnosis were selected, yielding a pediatric cohort of 65,349 unique patients with a diagnosis of depression.
The second step for the analytic file was to create time series based on aggregated measures of the cohort. As new episodes of depression accrued within the cohort over time, relevant measures were aggregated into successive monthly values. Thus, each observation is an aggregate measure of the new episodes of depression that were diagnosed nationally in that month. These data constituted the analytic file for time series of variables with aggregated national values spanning 60 months before and 24 months after the 2003 FDA advisory was issued.
Measures
FDA Advisory
As noted, the October 2003 FDA advisory was selected as the policy action of interest in our analysis. This choice was determined empirically by when the FDA action was reflected in aggregated series, and it is consistent with other reports of market-level changes (14) . Sensitivity analyses were conducted on the timing of the differential impact of a series of FDA warnings (e.g., examining the second FDA advisory in October 2004), and the results supported the choice of the first FDA advisory as the point of interest.
For each month in the 7-year period, process-of-care measures were calculated based on the pediatric cohort. National aggregate measures were calculated for each month for the five measures described below. The measures that were specified as requiring longer follow-up periods (180 days, or 6 months) were excluded from the monthly cohorts for the last 5 months of 2005.
Provider Types Who Diagnosed Pediatric Depression
We computed the percentage of cases of depression that were diagnosed by each of the following types of providers: pediatrician, nonpediatrician primary care physician (primary care, internal medicine, or obstetrics-gynecology), psychiatrist, other mental health provider (psychologist, social worker, or therapist), other specialty (not already listed), or unknown specialty.
Antidepressant Drug Prescribing for Pediatric Depression
We computed the percentage of episodes of depression for which the following types of prescriptions were filled within 30 days of the diagnosis date: SSRI, tricyclic antidepressant, other antidepressant, multiple antidepressants (of any class, concurrently or consecutively); we also included the category “no antidepressants prescribed.” This measure required that cohorts have at least 30 days of follow-up.
Types of Providers Prescribing Antidepressants for Pediatric Depression
We computed the percentage of antidepressant prescriptions filled within 30 days of the diagnosis date that were written by the following types of provider: pediatrician, nonpediatrician primary care physician, psychiatrist, other mental health provider, other specialty (non-mental health), or unknown specialty. This measure required that cohorts have at least 30 days of follow-up.
Use of Psychotherapy After Pediatric Diagnosis of Depression
We computed the percentage of episodes of depression for which any patient visit was coded as psychotherapy within 180 days of the diagnosis date. This measure required a cohort with at least 180 days of follow-up.
Use of Alternatives to Antidepressants After Diagnosis of Depression
We computed the percentage of episodes of depression for which a prescription for an atypical antipsychotic drug (GPI 590700, 591520, 591530, 591540, 591570, 592500, or 594000) or an anxiolytic drug (GPI 601000–609980) was filled within 30 days of diagnosis. This measure required that cohorts have at least 30 days of follow-up.
Statistical Analysis
The first analysis was conducted to examine rates of diagnosis of depression in the general managed care population. Total numbers of unique diagnoses of depression were determined from the physician visit file. Population sizes were provided by PharMetrics as single annual counts by age and gender bands for 7 calendar years (1999–2005). Because of the small number of data time points and the binomial distribution of the numerator and denominator data, a linear regression line was fit on a logit scale. The regression line was fit to years 1999–2004 and was used to estimate a predicted diagnosis rate for 2005. This predicted rate was then compared with the observed rate for 2005 using a t test.
The second set of analyses focused on the process-of-care measures among monthly cohorts of subjects diagnosed with depression. Segmented time-series regression analysis was used. This method is the most common for evaluating effects of an “interruption” that occurs at a specific point in a time series (27 – 29) —in this case, the October 2003 FDA advisory. The time series spanned 79–84 months, depending on follow-up restrictions, with 60 months before and up to 24 months after the advisory was issued, which is well above the rule-of-thumb recommendations of 12 observations each before and after the interruption (28) .
Monthly measures were first plotted in order to examine patterns over the 7-year period. The timing of the interruption was selected by inspection of these plots and was varied to establish robust findings. Segmented time-series regression models were used to measure the effect of the October 2003 FDA advisory on each process-of-care measure; the linear regression models included variables to test for a change in level and rate (slope) after the FDA advisory compared with preadvisory estimations. Given the large sample sizes, normality was assumed and linear regression was deemed appropriate. The following model was fit for each measure (y t ):
y t = β 0 + β 1 (time) t + β 2 (interruption) t + β 3 (time after interruption) t + e t
where time is a continuous variable indicating the number of months between October 1998 and month t; interruption is an indicator variable equal to 0 for months up to the interruption month (October 2003) and equal to 1 for the interruption month and subsequent months; time after interruption is a variable equal to 0 for months up to the interruption month, and counts from 1 to 24 starting with the FDA advisory month through all subsequent months; β 0 is the mean baseline estimate of the outcome variable; β 1 is the estimate of the preinterruption slope; β 2 is the estimate of the level change immediately after the interruption; and β 3 is the estimate of the change in the slope after the interruption, compared to the preinterruption slope. β 1 and β 3 were summed to produce an estimate of the postinterruption slope. The error term e t consists of a normally distributed random error at month t; time-dependent data are often correlated, and thus the error terms may not be independent (28) . Autocorrelation was investigated using correlograms (residuals versus time) and the Durbin-Watson test statistic produced by Stata (30) . First-order autocorrelation was detected for each process-of-care measure and was adjusted for by estimating the autocorrelation parameter and including it in each regression model (31 , 32) . Models were implemented using autoregressive-moving average (ARMA) interrupted time series in Stata (30) .
Results
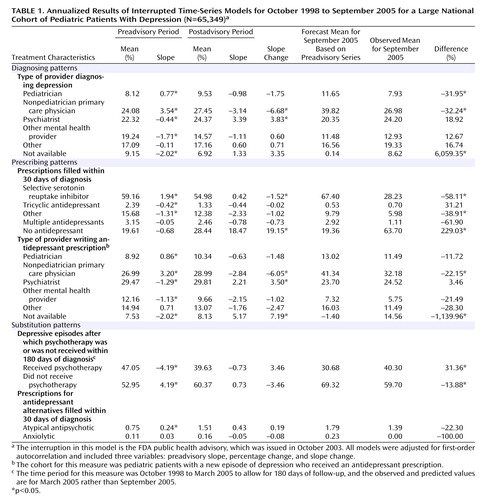
Figure 1 presents the annual rates of depression in the pediatric general medical and specialty managed care enrollee population by gender from 1999 to 2005. From 1999 to 2004, the rate of diagnosed new episodes of pediatric depression increased steadily, and in 2005 the rate decreased sharply. For both male and female patients, the observed 2005 rate was significantly lower than the rate predicted from the regression line (p<0.0001), indicating that the observed rate in 2005 was significantly lower than would have been expected on the basis of the historical trend. For male pediatric patients, the observed rate of diagnosed new episodes in 2005 was 2.3 per 1,000 enrollees, while the trend predicted a rate of 3.8 (65% higher than observed). For females, the observed rate in 2005 was 3.5 per 1,000 enrollees, whereas the trend predicted a rate of 6.0 (71% higher than observed). This finding also indicated that diagnosis rates among enrollees were not explained by changes in the base population.
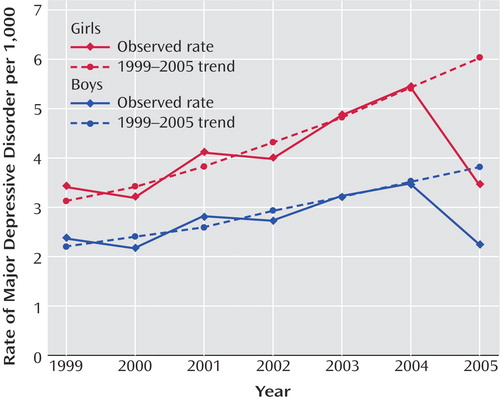
a For boys, the observed rate for 2005 (2.3 per 1,000) was significantly lower than the rate predicted by the 1999–2004 trend (3.8 per 1,000) (p<0.0001). For girls, the observed rate for 2005 (3.5 per 1,000) was significantly lower than the rate predicted by the 1999–2004 trend (6.0 per 1,000) (p<0.0001).
In time-series regressions, measures were monthly aggregates of new episodes of pediatric depression comprising the cohort that accrued in each month, so each monthly observation is a national measure characterized by new cases of depression in that period ( Table 1 ). Although statistical models were estimated using monthly aggregates, annualized results are reported here for ease of interpretation. The first two columns report the preadvisory mean level and trend (slope), followed by the postadvisory mean level and slope. The slope change from pre- to postadvisory indicates the policy change. There were no statistically and clinically significant changes in mean levels, as is evident in the graphical representations of selected series. The baseline preadvisory linear trend was used to forecast to September 2005, and a t test was used to compare the observed rate for September 2005 with this forecast estimate. The last column presents the percentage of the projected value accounted for by the observed value.
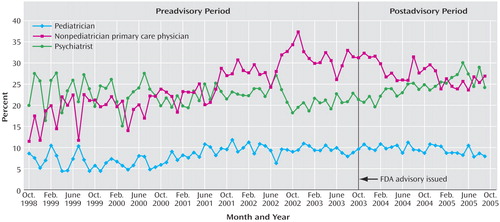
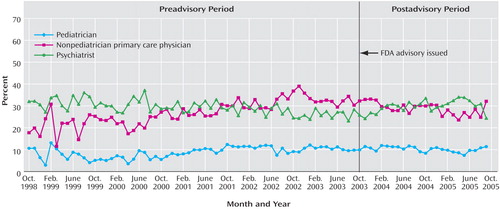
Diagnosing patterns were measured by the percentage of new episodes of depression diagnosed by each provider type during the month. These were mutually exclusive categories that summed to 100%, and they were measured for each monthly national cohort that was new in a given month. Before the 2003 FDA advisory was issued, the percentage of episodes of depression diagnosed by pediatricians and nonpediatrician primary care physicians increased steadily and together accounted for the majority of diagnoses. After the advisory was issued, there was no abrupt level shift, but the rate of diagnosing new episodes of depression by nonpediatrician primary care physicians significantly decreased; the rate did not significantly change for pediatricians. By September 2005, the shares of diagnoses of depression for nonpediatrician primary care physicians and pediatricians were each 32% lower than would have been predicted on the basis of the history from 1999. The percentage of episodes diagnosed by a psychiatrist significantly increased to an annual rate of 3.39%, indicating some shifting of diagnosis patterns away from primary care to psychiatry; psychiatrists accounted for about one-fourth of diagnoses of depression in each period, and in September 2005, the psychiatrists’ share of diagnoses was 19% higher than would have been predicted by history. Figure 2 displays these time series.
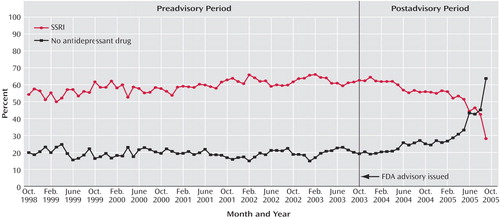
The second part of Table 1 summarizes antidepressant prescription fills within 30 days after the initial diagnosis of depression, and Figure 3 shows the trends for SSRIs and no antidepressant graphically. Before the advisory, the percentage of episodes of depression in which the patient received a prescription of an SSRI averaged 59% and increased significantly over time, averaging an annual rate of 1.94%, and after the advisory, the percentage of SSRI prescriptions decreased significantly to an annual rate of 0.42%. The historical trend predicted that in 67% of episodes of depression the patient would receive an SSRI in September 2005, whereas actual SSRI fills averaged 28% (58% less than history predicted). Before the advisory, there was no significant trend in the percentage of depressive episodes in which the patient received no antidepressant prescription; after the advisory, however, the trend significantly changed, with the percentage increasing sharply to an annual rate of 18.47%. The predicted level for “no antidepressant” was 19%, whereas the observed level was 64% in September 2005.
Nonpediatrician primary care physicians wrote more than one-quarter of filled antidepressant prescriptions and wrote them at an increasing rate (3.20%) before the advisory was issued; after the advisory, the trend reversed significantly (–2.84%). Before the advisory, there was a small but significant downward trend (–1.29%) in the percentage of episodes in which an antidepressant was prescribed by a psychiatrist, and this trend reversed after the advisory, to an annual rate of 3.50%. Among these major prescriber types, only nonpediatrician primary care physicians filled prescriptions significantly less (–22%) than would have been expected on the basis of the historical trend (p<0.05). Time series are shown in Figure 4 .
Lastly, Table 1 presents models of possible antidepressant substitutes. Before the advisory was issued, the trend of patients having at least one visit for psychotherapy within 180 days of diagnosis of depression was significantly decreasing (–4.19% annual rate); after the advisory, this trend did not significantly change. The observed rate of psychotherapy received (40%), however, was significantly higher than history would have predicted in September 2005, reflecting a flattening of the negative slope. Before the advisory, the percentage of episodes of depression for which the patient filled a prescription for an atypical antipsychotic or an anxiolytic within 30 days of diagnosis was low. Although the trends did not statistically change, the observed September 2005 levels were lower than predicted (remaining less than 2% for atypical antipsychotics and less than 1% for anxiolytics).
Discussion
Time-series analyses of treatment for pediatric depression in the community showed statistically and clinically significant effects associated with the 2003 FDA public health advisory relative to previous trends. Before the FDA advisory was issued, the diagnosis rate of pediatric depression was increasing, as was the rate of SSRI prescriptions for depression. After the FDA advisory, declines in treatment indicated a reversal of the previous trend: the overall rate of diagnosis declined, and among patients diagnosed, the proportion treated with antidepressants declined. Whereas there was substantial growth in diagnosis and antidepressant treatment of pediatric depression in primary care settings before the FDA advisory, there were substantial reversals afterward for both pediatricians and nonpediatrician primary care physicians. Treatment by psychiatrists increased after the FDA advisory, but not enough to compensate for the decline observed among primary care physicians.
These changes may be driven by both providers and consumers. While providers are diagnosing depression less frequently, antidepressant prescriptions are also being filled less frequently. It is possible that part of the reduced rate of diagnosis of depression stems from a new reluctance on the part of families, in the wake of the advisory, to seek treatment or to disclose depressive symptoms. Similarly, providers may be writing prescriptions for antidepressants that families do not fill.
Those who think America’s youth overdiagnosed and overmedicated might find these results to be good news. However, the observed rates of diagnoses of depression, 3–5 per 1,000 (that is, 0.3%–0.5%), were lower than the published incidences of child (0.4%–2.5%) and adolescent (0.4%–8.3%) depression (33 – 35) . It may be that the previous upward trend reflected improved recognition of depression by the public and practitioners.
A more sobering perspective on these data gives cause for concern. Pharmacoepidemiological studies examining the relationship between trends in sales or prescription fills of SSRIs have consistently shown a relationship between increases in SSRI prescription rates and declines in adolescent suicide rates (9 , 35 – 37) . On the basis of those studies, one might expect that the adolescent suicide rate would begin increasing in the wake of the FDA advisory after a decade of steady decline.
Some of this study’s limitations are related to the nature of claims records, including incorrect reporting or underreporting (e.g., undercounting of free medication samples), limited clinical detail in the ICD-9 system, and incorrect demographic information (38) . Physicians and patients may underreport depression because of the stigma associated with mental disorders (39 , 40) , and there may be variability in physicians’ ability to diagnose depression (41) . Stigma and other factors may motivate families to seek treatment outside their insurance plan, and this care would not be reflected in the claims data we used. In addition, the FDA took a series of actions over time, so the postinterruption period measures may be transitional; data over a longer period are needed to detect the durability of observed trends.
Strengths of this study include the patterns of community care observed in the data, as well as implications for future policies on drug risks. The FDA’s drug safety policy has moved toward broader and more proactive communication of new safety warnings for marketed drugs (42) . The FDA has emphasized its “aggressive development of electronic health information” to directly alert patients and providers about the risks of prescription medications. The agency has issued draft recommendations for a “Drug Watch” system to disseminate emerging drug safety information as safety signals emerge and before causality is determined (43) . The results of our study suggest a need for public health interventions to ease unintended but predictable changes in treatment patterns that may increase “the risk of doing nothing” (10) .
1. Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, Angermeyer MC, Bernert S, de Girolamo G, Morosini P, Polidori G, Kikkawa T, Kawakami N, Ono Y, Takeshima T, Uda H, Karam EG, Fayyad JA, Karam AN, Mneimneh ZN, Medina-Mora ME, Borges G, Lara C, de Graaf R, Ormel J, Gureje O, Shen Y, Huang Y, Zhang M, Alonso J, Haro JM, Vilagut G, Bromet EJ, Gluzman S, Webb C, Kessler RC, Merikangas KR, Anthony JC, Von Korff MR, Wang PS, Brugha TS, Aguilar-Gaxiola S, Lee S, Heeringa S, Pennell BE, Zaslavsky AM, Ustun TB, Chatterji S; WHO World Mental Health Survey Consortium: Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA 2004; 291:2581–2590Google Scholar
2. Voelker R: Researchers probe depression in children. JAMA 2003; 289:3078–3079Google Scholar
3. Weissman M, Wolk S, Wickramaratne P, Goldstein R, Adams P, Greenwald S, Ryan N, Dahl R, Steinberg D: Children with prepubertal-onset major depressive disorder and anxiety grown up. Arch Gen Psychiatry 1999; 56:794–801Google Scholar
4. Weissman M, Wolk S, Goldstein R, Moreau D, Adams P, Greenwald S, Klier C, Ryan N, Dahl R, Wickramaratne P: Depressed adolescents grown up. JAMA 1999; 281:1707–1713Google Scholar
5. Berndt ER, Koran LM, Finkelstein SN, Gelenberg AJ, Kornstein SG, Miller IM, Thase ME, Trapp GA, Keller MB: Lost human capital from early-onset chronic depression. Am J Psychiatry 2000; 157:940–947Google Scholar
6. American Academy of Child and Adolescent Psychiatry: Practice parameters for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 1997; 36:138–157Google Scholar
7. Emslie G, Heiligenstein J, Wagner K, Hoog S, Ernest D, Brown E, Nilsson M, Jacobson J: Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry 2002; 41:1205–1215Google Scholar
8. Daly R: Drop in youth antidepressant use prompts call for FDA monitoring. Psychiatr News 2005; 40:18Google Scholar
9. Gibbons RD, Hur K, Bhaumik DK, Mann JJ: The relationship between antidepressant prescription rates and rate of early adolescent suicide. Am J Psychiatry 2006; 163:1898–1904Google Scholar
10. Brent DA: Antidepressants and pediatric depression: the risk of doing nothing. N Engl J Med 2004; 351:1598–1601Google Scholar
11. Goodman W, Murphy T, Lazoritz M: Risk of suicidality during antidepressant treatment of children and adolescents. Prim Psychiatry 2006; 13:43–50Google Scholar
12. Harris G: FDA links drugs to being suicidal. New York Times, Sept 14, 2004Google Scholar
13. Abbott A: British panel bans use of antidepressant to treat children. Nature 2003; 423:792Google Scholar
14. Rosack J: New data show declines in antidepressant prescribing. Psychiatr News 2005; 40:1Google Scholar
15. Reuters: Youth antidepressant use down due to risks—Medco. Reuters, Sept 21, 2004Google Scholar
16. Olfson M, Marcus SC, Shaffer D: Antidepressant drug therapy and suicide in severely depressed children and adults: a case-control study. Arch Gen Psychiatry 2006; 63:865–872Google Scholar
17. Rappaport N, Bostic JQ, Prince JB, Jellinek M: Treating pediatric depression in primary care: coping with the patients’ blue mood and the FDA’s black box. J Pediatr 2006; 148:567–568Google Scholar
18. Varley CK: Treating depression in children and adolescents: what options now? CNS Drugs 2006; 20:1–13Google Scholar
19. Bridge JA, Salary CB, Birmaher B, Asare AG, Brent DA: The risks and benefits of antidepressant treatment for youth depression. Ann Med 2005; 37:404–412Google Scholar
20. Richmond TK, Rosen DS: The treatment of adolescent depression in the era of the black box warning. Curr Opin Pediatr 2005; 17:466–472Google Scholar
21. Licinio J, Wong ML: Depression, antidepressants, and suicidality: a critical appraisal. Nat Rev Drug Discov 2005; 4:165–171Google Scholar
22. Scholle SH: NCQA behavioral health measurement efforts. J Manag Care Pharm 2005; 11(3 suppl):S9–S11Google Scholar
23. National Committee for Quality Assurance: HEDIS Volume 2: Technical Specifications. Washington, DC, National Committee for Quality Assurance, 2004Google Scholar
24. Rost K, Dickinson LM, Fortney J, Westfall J, Hermann RC: Clinical improvement associated with conformance to HEDIS-based depression care. Ment Health Serv Res 2005; 7:103–112Google Scholar
25. National Committee for Quality Assurance: The State of Health Care Quality 2005. Washington, DC, National Committee for Quality Assurance, 2005Google Scholar
26. Emslie G, Rush A, Weinberg W, Kowatch R, Hughes C, Carmody T, Rintelmann J: A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry 1997; 54:1031–1037Google Scholar
27. Cook TD, Campbell DT: Quasi-Experimentation: Design and Analysis Issues for Field Settings. Chicago, Rand McNally, 1979Google Scholar
28. Wagner A, Sourmerai S, Zhang F, Ross-Degnan D: Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002; 27:299–309Google Scholar
29. Catalano R, Libby A, Snowden L, Cuellar AE: The effect of capitated financing on mental health services for children and youth: the Colorado experience. Am J Public Health 2000; 90:1861–1865Google Scholar
30. Stata: Intercooled Stata for Windows. College Station, Tex, Stata Corp, 2006Google Scholar
31. Durbin J, Watson G: Testing for serial correlation in least squares regression. Biometrika 1951; 37:409–428Google Scholar
32. Ostrom C: Time series analysis, in Sage Publications University Papers Series on Quantitative Applications in the Social Sciences, vol 07-009. Thousand Oaks, Calif, Sage Publications, 1990Google Scholar
33. Birmaher B, Brent DA, Benson RS: Practice parameters for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry 1998; 37:63S–83SGoogle Scholar
34. Birmaher B, Ryan N, Williamson D, Brent D, Kaufman J, Dahl RE, Perel J, Nelson B: Childhood and adolescent depression: a review of the past 10 years, part I. J Am Acad Child Adolesc Psychiatry 1996; 35:1427–1439Google Scholar
35. Ludwig J, Marcotte D: Anti-depressants, suicide, and drug regulation. J Policy Anal Manage 2005; 24:249–272Google Scholar
36. Gibbons R, Hur K, Bhaumik D, Mann J: The relationship between antidepressant medication use and rate of suicide. Arch Gen Psychiatry 2005; 62:165–172Google Scholar
37. Olfson M, Shaffer D, Marcus SC, Greenberg T: Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry 2003; 60:978–982Google Scholar
38. Spettell C, Wall T, Allison J, Calhoun J, Kobylinski R, Fargason R, Kiefe C: Identifying physician-recognized depression from administrative data: consequences for quality measurement. Health Serv Res 2003; 38:1081–1102Google Scholar
39. Rost K, Smith R, Matthews D, Guise B: The deliberate misdiagnosis of major depression in primary care. Arch Fam Med 1994; 3:333–337Google Scholar
40. Hirschfeld R, Keller M, Panico S, Arons B, Barlow D, Davidoff F, Endicott J, Froom J, Goldstein M, Gorman J, Marek R, Maurer T, Meyer R, Phillips K, Ross J, Schwenk T, Sharfstein S, Thase M, Wyatt R: The National Depressive and Manic-Depressive Association consensus statement on the undertreatment of depression. JAMA 1997; 277:333–340Google Scholar
41. Lemelin J, Hotz S, Swensen R, Elmslie T: Depression in primary care: why do we miss the diagnosis? Can Fam Physician 1994; 40:104–108Google Scholar
42. Food and Drug Administration: FDA’s New Drug Safety Initiative. May 5, 2005. http://www.fda.gov/cder/drugsafety.htmGoogle Scholar
43. Food and Drug Administration: Draft Guidance for Industry on the Food and Drug Administration’s “Drug Watch” for Emerging Drug Safety Information. Washington, DC, Food and Drug Administration, May 10, 2005Google Scholar