Performance on a Virtual Reality Spatial Memory Navigation Task in Depressed Patients
Abstract
Objective: Findings on spatial memory in depression have been inconsistent. A navigation task based on virtual reality may provide a more sensitive and consistent measure of the hippocampal-related spatial memory deficits associated with depression. Method: Performance on a novel virtual reality navigation task and a traditional measure of spatial memory was assessed in 30 depressed patients (unipolar and bipolar) and 19 normal comparison subjects. Results: Depressed patients performed significantly worse than comparison subjects on the virtual reality task, as assessed by the number of locations found in the virtual town. Between-group differences were not detected on the traditional measure. The navigation task showed high test-retest reliability. Conclusions: Depressed patients performed worse than healthy subjects on a novel spatial memory task. Virtual reality navigation may provide a consistent, sensitive measure of cognitive deficits in patients with affective disorders, representing a mechanism to study a putative endophenotype for hippocampal function.
Of great value for endophenotype studies in psychiatric disorders will be an understanding of the neuroanatomical and functional relationships between neuropsychological deficits and brain structure abnormalities (1) . Neuropsychological testing has long established the presence of memory deficits in patients with unipolar depression and, more recently, in those suffering from bipolar depression (see reference 2 for a review). Owing to the role of the hippocampus (3) , spatial memory may be of particular interest because of reports of hippocampal volume reduction in patients with mood disorders (4 – 7) . Unfortunately, despite the myriad of memory deficits reported, studies of spatial memory have produced mixed results (2 , 8 – 10) . These variations are due, in part, to various methodological issues, including ascertainment limitations, the method for matching patients and comparison groups, studying patients during different phases of illness, medication confounds, and the multifaceted nature of spatial ability and its measurement. Traditionally, tasks assessing spatial memory require individuals to remember the position of items in an array. These tasks, however, focus on only one small aspect of spatial cognition and do not provide scenarios that resemble the majority of the demands of real-life situations. Because of their multifaceted nature, navigation tasks based on virtual reality may provide a more consistent, sensitive measure of spatial ability and are more likely to require hippocampal involvement, thereby increasing their sensitivity to the impact of depression on this cognitive domain.
As part of larger protocols, we tested the hypothesis that depression is associated with specific cognitive deficits resulting from hippocampal dysfunction. We administered to depressed patients and healthy subjects a novel virtual reality navigation task in which performance is impaired by hippocampal damage (11 , 12) and reflects hippocampal activation (13 , 14) .
Method
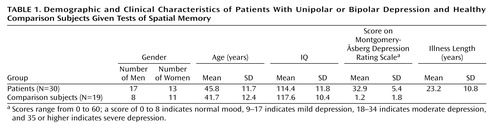
Participants were recruited from inpatient and outpatient psychiatric units at the National Institute of Mental Health (NIMH) as part of randomized clinical medication trials for unipolar or bipolar depression. The subjects included men and women, ages 21 to 65 years, with a diagnosis of current major depressive disorder (N=10) or bipolar I or II disorder in the depressive phase (N=20) without psychotic features, as assessed by the Structured Clinical Interview for DSM-IV (SCID). All subjects had been free of comorbid substance abuse or dependence for 3 months (eight patients had a history of substance abuse) and had no acute medical illnesses. Nineteen normal comparison subjects participated in the study. Subjects were excluded from the comparison group on the basis of the presence of current or past psychiatric or neurological illness, any medical condition that could affect cognitive performance, and a family history of psychiatric illness; none had a history of substance abuse. The subjects with unipolar depression had been medication free for at least 2 weeks. Those with bipolar depression had had therapeutic levels of lithium or valproic acid for at least 4 weeks at the time of testing. They had been free of other medications for at least 4 weeks. No other concomitant medications were permitted. Given a likely relationship between familiarity with video games and the outcome measure, individuals reporting high expertise in video games were excluded. The Montgomery-Åsberg Depression Rating Scale (15) was used to assess the severity of depression.
The study was approved by the NIMH institutional review board. After a complete description of the study to the subjects, written informed consent was obtained.
The present study employed a virtual reality town ( Figure 1 ) developed by N. Burgess and previously used by Maguire et al. (13) and Pine et al. (14) . On day 1, the subjects were oriented to the town for 20 minutes. They were then given 30 minutes to navigate the town through a computer-generated sequence of destinations. After this period, the subjects had to find at least three predetermined destinations within the town during two 3-minute practice runs. If the subjects were able to do so, the training period terminated. If not, they were given an additional 30 minutes to explore the town to ensure adequate familiarity with the various locations. Three days later, the subjects explored the town for 20 minutes in order to refamiliarize themselves with the task. Two to 4 hours later, they were tested on their memory of the town (as assessed by the number of locations found) in a manner identical to that used in the practice sessions but beginning at different locations. The software recorded the locations visited. Preliminary data from a group of healthy subjects revealed high test-retest reliability on this task (r=0.85, N=15, p<0.001). Additionally, a subset of patients and comparison subjects also completed the Spatial Working Memory task, a component of the Cambridge Automated Neuropsychological Testing Battery (Cambridge Cognition, Cambridge, U.K.), which likely taps both frontal and hippocampal functions.

a A, aerial view of the entire town. B–D, first-person views of locations within the town. Subjects navigate through the town by using a first-person, three-dimensional (nonaerial) view.
Independent-sample t tests were used to evaluate differences between the patients and comparison subjects on the outcome measures (i.e., number of locations found on the navigation task and performance on the Spatial Working Memory task) and demographic characteristics. Pearson r correlations were used to examine the relationships between independent variables and the outcome measures. All statistics were evaluated at p≤0.05, two-tailed.
Results
The patients fell within the moderately to severely depressed range on the Montgomery-Åsberg Depression Rating Scale (score=18–45). There were no significant differences in age or IQ between the patients and comparison subjects ( Table 1 ). The patients found significantly fewer locations (mean=2.4, SD=1.7) than the normal subjects (mean=3.8, SD=2.0) on the spatial navigation task (t=–2.70, df=47, p=0.01). There was no significant difference between men and women (t=–0.01, df=47, p=0.99). Among patients only, the number of locations found was correlated with the score on the Montgomery-Åsberg Depression Rating Scale (r=–0.33, N=30, p=0.02) such that the more severely depressed patients performed worse. For the subset of subjects (24 patients and 11 comparison subjects) who completed the Spatial Working Memory task, the number of locations found was correlated with overall task performance (r=–0.45, p=0.007). It was interesting, however, that there was no difference in scores on the Spatial Working Memory task between the patients (mean=33.3, SD=4.8) and comparison subjects (mean=33.4, SD=6.4) (t=–0.06, df=33, p=0.95), while there remained a performance difference on the virtual reality spatial navigation task within this subset (t=–2.36, df=33, p=0.03) whereby the patients found significantly fewer locations (mean=2.2, SD=1.5) than the normal comparison subjects (mean=4.0, SD=2.3). There were no differences between patients with and without a history of substance abuse on the outcome measures (number of locations found on the navigation task and performance on the Spatial Working Memory task). In post hoc comparisons of the three diagnostic groups (unipolar depressed, bipolar depressed, and healthy), there was no difference between the unipolar and bipolar depressed patients on navigation task performance. Finally, there was no difference on the navigation task between the bipolar depressed patients taking lithium and those taking valproic acid.
Discussion
The depressed patients performed significantly worse than the healthy comparison subjects on a novel virtual reality measure of spatial memory, which uses a navigation format based on virtual reality (13) . This difference was manifested through a lower number of locations found in the task. An important finding was that, while performance on the navigation task was correlated with performance on the Spatial Working Memory task, the patients and comparison subjects did not differ in performance on the Spatial Working Memory test. Differences detected on the virtual reality task suggest that it may be a sensitive measure of hippocampal-related spatial memory deficits and may more clearly elucidate the relationship between depression and this cognitive domain. Thus, despite the fact that the present task is more involved than existing measures, such as the Spatial Working Memory task, it has the advantage of detecting performance deficits in depressed subjects. Prior research has demonstrated a robust correlation between engagement of the hippocampus and the dependent measure used in this study (14) . Because of the role of the hippocampus in this task (11 – 14) and studies suggesting hippocampal volume reduction in depression (4 – 7) , it is reasonable to speculate that the patient deficit being measured is dependent on altered hippocampal function, which was not detected on the Spatial Working Memory test. However, while this task has previously been shown to involve the hippocampus and require normal function of the hippocampus, we cannot be certain that the differences on this task that we observed in the depressed patients are due to altered function of the hippocampus (as opposed to other brain areas that could be involved). The incorporation of additional control measures that target various structures within the medial temporal lobe will be necessary to more clearly delineate the neuroanatomical structures involved in the task performance. Nevertheless, our results suggest that spatial memory performance on a virtual reality navigation task may represent a quantifiable endophenotypic measure to assess possible hippocampal deficits in patients with depression (1 , 16) . This hypothesis can be further confirmed by applying spatial memory tests with a virtual reality navigation format to euthymic patients, unaffected first-degree relatives of depressed patients, and medication-free bipolar patients, in order to assess the cognitive effects of mood stabilizers, and by correlating performance with structural and functional neuroimaging measures of relevant structures, such as the hippocampus.
1. Gottesman II, Gould TD: The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 2003; 160:636–645Google Scholar
2. Quraishi S, Frangou S: Neuropsychology of bipolar disorder: a review. J Affect Disord 2002; 72:209–226Google Scholar
3. O’Keefe J, Nadel L: The Hippocampus as a Cognitive Map. Oxford, UK, Oxford University Press, 1978Google Scholar
4. Sheline YI, Gado MH, Kraemer HC: Untreated depression and hippocampal volume loss. Am J Psychiatry 2003; 160:1516–1518Google Scholar
5. MacMaster FP, Kusumakar V: Hippocampal volume in early onset depression. BMC Med 2004; 2:2Google Scholar
6. Videbech P, Ravnkilde B: Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry 2004; 161:1957–1966Google Scholar
7. Hickie I, Naismith S, Ward PB, Turner K, Scott E, Mitchell P, Wilhelm K, Parker G: Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry 2005; 186:197–202Google Scholar
8. Clark L, Iversen SD, Goodwin GM: Sustained attention deficit in bipolar disorder. Br J Psychiatry 2002; 180:313–319Google Scholar
9. Sweeney JA, Kmiec JA, Kupfer DJ: Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry 2000; 48:674–684Google Scholar
10. Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES: Emotional bias and inhibitory control processes in mania and depression. Psychol Med 1999; 29:1307–1321Google Scholar
11. Spiers HJ, Burgess N, Maguire EA, Baxendale SA, Hartley T, Thompson PJ, O’Keefe J: Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain 2001; 124(pt 12):2476–2489Google Scholar
12. Spiers HJ, Burgess N, Hartley T, Vargha-Khadem F, O’Keefe J: Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus 2001; 11:715–725Google Scholar
13. Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O’Keefe J: Knowing where and getting there: a human navigation network. Science 1998; 280:921–924Google Scholar
14. Pine DS, Grun J, Maguire EA, Burgess N, Zarahn E, Koda V, Fyer A, Szeszko PR, Bilder RM: Neurodevelopmental aspects of spatial navigation: a virtual reality fMRI study. Neuroimage 2002; 15:396–406Google Scholar
15. Montgomery SA, Åsberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Google Scholar
16. Hasler G, Drevets WC, Manji HK, Charney DS: Discovering endophenotypes for major depression. Neuropsychopharmacology 2004; 29:1765–1781Google Scholar