Different Psychophysiological and Behavioral Responses Elicited by Frustration in Pediatric Bipolar Disorder and Severe Mood Dysregulation
Abstract
Objective: Researchers disagree as to whether irritability is a diagnostic indicator for pediatric mania in bipolar disorder. The authors compared the behavioral and psychophysiological correlates of irritability among children with severe mood dysregulation (i.e., nonepisodic irritability and hyperarousal without episodes of euphoric mood) and narrow-phenotype bipolar disorder (i.e., a history of at least one manic or hypomanic episode with euphoric mood) as well as those with no diagnosis (i.e., healthy comparison children). Method: Subjects with severe mood dysregulation (N=21) or narrow-phenotype bipolar disorder (N=35) and comparison subjects (N=26) completed the affective Posner task, an attentional task that manipulated emotional demands and induced frustration. Mood response, behavior (reaction time and accuracy), and brain activity (event-related potentials) were measured. Results: The severe mood dysregulation and narrow-phenotype bipolar disorder groups both reported significantly more arousal than comparison subjects during frustration, but behavioral and psychophysiological performance differed between the patient groups. In the frustration condition, children with narrow-phenotype bipolar disorder had lower P3 amplitude than children with severe mood dysregulation or comparison subjects, reflecting impairments in executive attention. Regardless of emotional context, children with severe mood dysregulation had lower N1 event-related potential amplitude than comparison subjects or children with narrow-phenotype bipolar disorder, reflecting impairments in the initial stages of attention. Post hoc analyses demonstrated that the N1 deficit in children with severe mood dysregulation is associated with oppositional defiant disorder symptom severity. Conclusions: Results indicate that while irritability is an important feature of severe mood dysregulation and narrow-phenotype bipolar disorder, the pathophysiology of irritability may differ among the groups and is influenced by oppositional defiant disorder severity.
Understanding of pediatric bipolar disorder is hampered by questions about diagnostic criteria and limited pathophysiological research. Despite data suggesting that euphoria is common in pediatric bipolar disorder (1 – 5) and that child and adult mania have similar clinical presentations (3 , 6) , disagreement continues over 1) the importance of irritability as a symptom of mania and 2) whether manic symptoms in pediatric bipolar disorder present episodically. To facilitate research on these questions, we suggested a classification system differentiating children with strictly defined DSM-IV bipolar disorder (i.e., narrow phenotype) from those with nonepisodic irritability and hyperarousal (i.e., broad phenotype) (7 – 11) . Patients with narrow-phenotype bipolar disorder have a lifetime history of at least one episode of mania or hypomania that includes elevated mood or grandiosity. Patients with the broad phenotype, designated as severe mood dysregulation, exhibit nonepisodic irritability accompanied by hyperarousal and hyperreactivity to negative emotional stimuli, without elation or grandiosity ( Figure 1 ).
Most children who meet severe mood dysregulation criteria also meet DSM-IV criteria for attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder, since the severe mood dysregulation category captures many symptoms of these diagnoses. However, when studying pediatric bipolar disorder, there are two advantages to recruiting children with severe mood dysregulation as opposed to ADHD and oppositional defiant disorder. First, because the role of irritability in the diagnosis of pediatric bipolar disorder is controversial (12) , the severe mood dysregulation criteria for irritability are operationalized explicitly (inclusion criteria 2 and 4 in Figure 1 ). Second, the overlap between ADHD symptoms and the symptoms listed under criterion “B” for a manic episode in DSM-IV creates diagnostic questions, and those overlapping symptoms are used to identify hyperarousal in severe mood dysregulation (inclusion criterion 3, Figure 1 ). Thus, children with severe mood dysregulation are the specific focus of debate among bipolar disorder researchers.
Our classification system was designed to facilitate research on the pathophysiology of narrow-phenotype bipolar disorder and severe mood dysregulation. Because low frustration tolerance and irritability are prominent in both syndromes, it is crucial to develop research paradigms that elicit frustration so that its pathophysiology can be studied (13) . The affective Posner task (14 , 15) assesses attention in different emotional contexts, including frustration. Using the affective Posner task, we found that in children with narrow-phenotype bipolar disorder, frustration is associated with attentional dysfunction, perhaps because their attention is drawn to their negative emotional state (16) . Here we compare the pathophysiology of irritability in severe mood dysregulation and narrow-phenotype bipolar disorder, thus bringing physiological data to bear on the debate regarding diagnostic criteria for pediatric bipolar disorder. We use newly acquired data to compare the behavioral and psychophysiological correlates of frustration in children with severe mood dysregulation and children with narrow-phenotype bipolar disorder along with a group of healthy comparison children.
Method
Participants
We enrolled subjects with severe mood dysregulation (N=21), narrow-phenotype bipolar disorder (N=35), and no diagnosis (N=26) in an institutional review board-approved study at the National Institute of Mental Health (NIMH). Parents and children gave written informed consent/assent. Data collection in the narrow-phenotype and comparison subjects was completed before testing the severe mood dysregulation subjects (16) .
The narrow-phenotype bipolar subjects met strict DSM-IV criteria: at least one hypomanic or manic episode (≥ 4 days of hypomania or ≥7 days for mania) with abnormally elevated mood or grandiosity and at least three symptoms listed under criterion “B” for a manic episode (17) . Subjects with bipolar I and bipolar II disorder were included—provided they met narrow-phenotype bipolar disorder criteria—since our goal was to compare narrow-phenotype bipolar disorder and severe mood dysregulation. The severe mood dysregulation subjects were youth with nonepisodic irritability, overreactivity to negative emotional stimuli at least three times weekly, and hyperarousal (at least three of the following: insomnia, intrusiveness, pressured speech, flight of ideas/racing thoughts, distractibility, psychomotor agitation). Symptoms had to begin before age 12 and be present for at least 1 year without remission ≥ 2 months. Symptoms had to cause severe impairment (i.e., hospitalization, marked family discord) in at least one setting (home, school, peers) and mild impairment (i.e., school disciplinary problems, disrupted family activities) in another. Euphoric mood or distinct episodes lasting ≥4 days were exclusionary (7) .
Diagnoses were made by using the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL), administered to parents and children separately. Comorbid diagnoses, also assessed using the K-SADS-PL, were present during euthymia and met DSM-IV criteria for impairment. Diagnosis of severe mood dysregulation was made using a K-SADS supplementary module (kappa ≥ 0.9). Exclusion criteria for patients were IQ<70, pervasive development disorder, unstable medical illness, or substance abuse within 2 months.
Comparison subjects had normal physical and neurological examination results. They and a parent completed the K-SADS to ensure that the subject had no psychiatric history.
Clinicians with interrater reliability administered to patients and their parents the Children’s Depression Rating Scale, the Young Mania Rating Scale, and the Children’s Global Assessment Scale. Patients also completed the Manifest Anxiety Scale for Children (18) .
Posner Task
The Posner paradigm consisted of three tasks, with 100, 50, and 51 trials respectively. Tasks involved the same stimuli and instructions but differed in feedback and contingencies. On all tasks, a fixation cross appeared in the center of the screen, followed by three boxes arranged horizontally. Cues consisted of one box illuminating blue; cues appeared in the central box on 20% of trials, and in the right and left boxes on 40% each. Following cue presentation, a target square appeared inside either the left or right box. Subjects were instructed to press the button corresponding to the target location. Stimuli presentation was controlled by the STIM system (James Long Company, Caroga Lake, NY).
Task 1 was the nonemotional baseline; subjects were told their response accuracy (“Good job!” or “Incorrect!”) without contingencies. On task 2, subjects won or lost 10 cents on each trial, based on performance (“Great Job! Win 10 Cents” or “Wrong! Lose 10 Cents”). Task 3 had the same contingencies as task 2, but rigged feedback was added, making this a frustrating task. On 44% of trials, correct responses resulted in accurate feedback and monetary reward (“You are Quick! Win 10 Cents”). However, on 56% of correct responses, rigged feedback informed the subject that he or she was too slow and lost 10 cents (“Too Slow! Lose 10 Cents”). Incorrect responses always resulted in punishment feedback (“Wrong! Lose 10 Cents”). Task order was fixed to heighten arousal progressively and avoid carry-over arousal from the frustration task preceding other tasks. To examine the impact of reward and punishment on performance, trials were classified as post-reward or post-punishment based on the feedback preceding the trial.
Clinical Data
After each task, subjects rated their response to that task, reward, and punishment using the Self-Assessment Manikin (19) : line-drawings with extremes of happy/unhappy (valence) or calm/aroused (arousal).
EEG signals were recorded with an electrode cap from temporal (T3, T4), frontal (Fz, F3, F4), central (C3, C4, Cz), and parietal (Pz, P3, P4) sites using the international 10/20 system, referenced to right earlobe. As in other developmental event-related potential studies (15) , we used average referencing (for sampling rate and filtering, see Rich et al. [16] ).
Event-related potentials were collected to each target presentation. Peak amplitude within the designated time window was analyzed. Given prior results (16) and a priori hypotheses, we focused on P3 (200–400 msec following target presentation) and N1 and P1 (50–150 msec).
We measured reaction time and response accuracy (i.e., percentage of responses matching target location).
Statistical Analysis
Repeated measures ANOVAs were conducted on P3, N1, and P1 amplitude, with group as the between-group variable and task and electrode site as within-subject factors. Each site (i.e., parietal, temporal, frontal, central) was analyzed separately. For reaction time and accuracy, separate 3×3 ANOVAs were conducted using group and task as factors. Greenhouse-Geisser correction was applied when appropriate, and post hoc comparisons employed the Tukey’s honestly significant difference test.
We conducted post hoc ANCOVAs to explore the impact of ADHD, oppositional defiant disorder, and major depressive disorder on the psychophysiological results. We used K-SADS-PL responses to create a continuous variable assessing symptom severity for each diagnosis; the measures on which our groups differed (Pz P3 amplitude and N1 temporal and central amplitude) were outcome variables. Given their proximity, we combined central and temporal N1 amplitude into one score to reduce the number of analyses and provide a more stable estimate of between-group differences. The presence or absence of bipolar disorder served as a categorical independent variable; ADHD or oppositional defiant disorder symptoms were covaried. Severe mood dysregulation designation could not be included due to multicollinearity among ADHD, oppositional defiant disorder, and severe mood dysregulation. Given the high correlation between ADHD and oppositional defiant disorder symptom scores (r=0.71, p<0.001), two ANCOVAs were conducted, one with ADHD and major depressive disorder symptoms as covariates, and one with oppositional defiant disorder and major depressive disorder as covariates.
Results
Clinical Characteristics
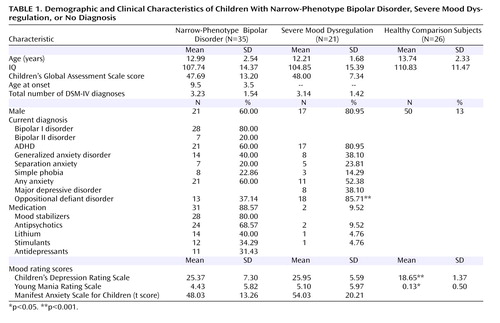
The three subject groups did not differ in terms of age, sex, or IQ. Among children with narrow-phenotype bipolar disorder, 80% (N=28) met criteria for bipolar I disorder. The average age at onset of narrow-phenotype bipolar disorder was 9.5 years (SD=3.5). Comorbid diagnoses were common ( Table 1 ).
ANOVA comparisons of scores on the Children’s Depression Rating Scale (F=10.44, df=2, 56, p<0.001) and Young Mania Rating Scale (F=4.44, df=2, 54, p<0.02) found the comparison subjects to have significantly lower scores than children with narrow-phenotype bipolar disorder (p<0.01 and p<0.04, respectively) and children with severe mood dysregulation (p<0.001 and p<0.02); the patient groups were comparable. Euthymia (i.e., Children’s Depression Rating Scale score ≤40, Young Mania Rating Scale score ≤12) was seen in 31 (88.6%) of the children with narrow-phenotype bipolar disorder and 19 (90.5%) of the children with severe mood dysregulation. For the four noneuthymic narrow-phenotype bipolar subjects, two were hypomanic and two had mixed hypomania. Elevated Young Mania Rating Scale scores in the two noneuthymic children with severe mood dysregulation reflected hyperarousal symptoms. Children’s Global Assessment Scale scores, comparable between patient groups, indicated severe impairment ( Table 1 ). Thirty-one (88.6%) of the narrow-phenotype bipolar subjects were receiving medication, whereas two (9.5%) of those with severe mood dysregulation were medicated ( Table 1 ).
Because the differences in age among the groups approached significance (p<0.08), we repeated all analyses with age as a covariate. The results did not differ, and we report here analyses without age as a covariate.
Affective Data
Repeated-measures ANOVAs of the Self-Assessment Manikin data examined the effects of condition (now, post-reward, post-punishment), type of mood rating (valence, arousal), and task. For arousal post-punishment, the group-by-task interaction was significant (F=2.92, df=4, 92, p<0.05). Post hoc analyses found significant group differences in arousal on task 3 (F=3.44, df=2, 77, p<0.05), with the severe mood dysregulation (5.58 [SD=2.31] and narrow-phenotype bipolar disorder (6.15 [SD=2.54]) groups comparable to each other but significantly more aroused (p<0.05) than comparison subjects (4.44 [SD=2.01]). Thus, the paradigm elicited more frustration in the patient groups than in comparison subjects.
Event-Related Potentials
P3 Amplitude
Across parietal sites there was a significant group-by-task-by-site interaction (F=2.17, df=8, 106, p<0.05) ( Figure 2 ). Post hoc analyses found a significant group-by-task interaction (F=2.71, df=4, 112, p<0.05) at Pz. There were no significant group differences in P3 amplitude on tasks 1 and 2, but there were group differences on task 3 (F=5.25, df=2, 58, p<0.01). Specifically, while the children with severe mood dysregulation had P3 amplitude comparable to the comparison subjects, P3 amplitude of the narrow-phenotype bipolar disorder subjects was significantly lower than that of both severe mood dysregulation (p<0.05) and comparison (p<0.01) subjects. P3 and P4 amplitude did not differ between groups.

a Significantly greater amplitude than seen in subjects with narrow-phenotype bipolar disorder (p<0.01).
b Significantly greater amplitude than seen in subjects with narrow-phenotype bipolar disorder (p<0.05).
N1 and P1 Amplitude
N1 amplitude differed between groups at multiple sites across all tasks ( Figure 3 ). Significant main effects of group were seen for N1 central (F=4.76, df=2, 55, p<0.01), temporal (F=3.70, df=2, 52, p<0.05), and frontal (F=3.26, df=2, 29, p<0.05) locations. Children with severe mood dysregulation had significantly lower N1 amplitude than those with narrow-phenotype bipolar disorder at central (p<0.01) and temporal (p<0.01) sites. The severe mood dysregulation subjects also had significantly lower N1 amplitude than comparison subjects at central (p<0.01), temporal (p<0.01), and frontal (p<0.01) sites. N1 amplitude was comparable between the narrow-phenotype and comparison subjects. Thus, across tasks, children with severe mood dysregulation displayed lower N1 amplitude than did both narrow-phenotype bipolar disorder and comparison subjects, indicating deficient attention orienting independent of task emotionality. For all sites, the task-by-group interactions were nonsignificant.
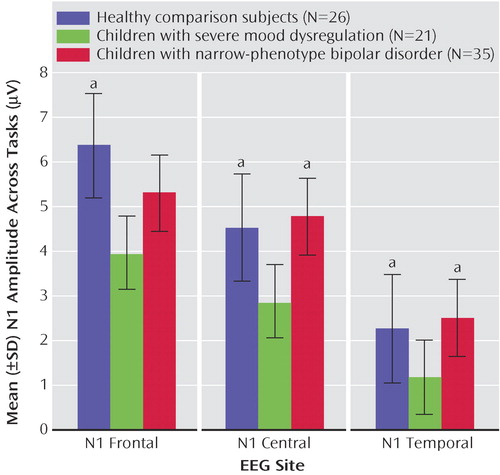
a Significantly greater amplitude than seen in subjects with severe mood dysregulation (p<0.01).
Similarly, for P1 amplitude, we found a significant main effect of group, across tasks, at central sites (F=3.95, df=2, 55, p<0.05). Children with severe mood dysregulation had significantly lower P1 amplitude (1.68 [SD=0.63]) than comparison subjects (4.24 [SD=0.68]; p<0.01) but not differ from those with narrow-phenotype bipolar disorder (2.46 [SD=0.66]), nor did the narrow-phenotype and comparison subjects differ significantly. Task-by-group interaction was nonsignificant, as were between-group differences at other sites
Behavioral Data
Reaction time
The task-by-group interaction for post-punishment reaction time was significant (F=3.37, df=4, 110, p<0.01) ( Figure 4 ). Groups had comparable reaction time on task 1. The task 2 ANOVA was significant (F=8.03, df=2, 61, p<0.001); post hoc analyses found that narrow-phenotype bipolar disorder subjects were slower than the severe mood dysregulation (p<0.02) and comparison (p<0.001) subjects, who were comparable in reaction time. On task 3 (F=21.44, df=2, 79, p<0.0001), narrow-phenotype bipolar subjects were slower than severe mood dysregulation (p<0.02) and comparison (p<0.001) subjects, and the children with severe mood dysregulation were slower than comparison subjects (p<0.01). Thus, the three groups had comparable post-punishment reaction time on nonemotional task 1, but on emotional tasks 2 and 3, the severe mood dysregulation and comparison subjects had significantly faster post-punishment responses than did those with narrow-phenotype bipolar disorder. There were no significant between-group differences on post-reward reaction time.
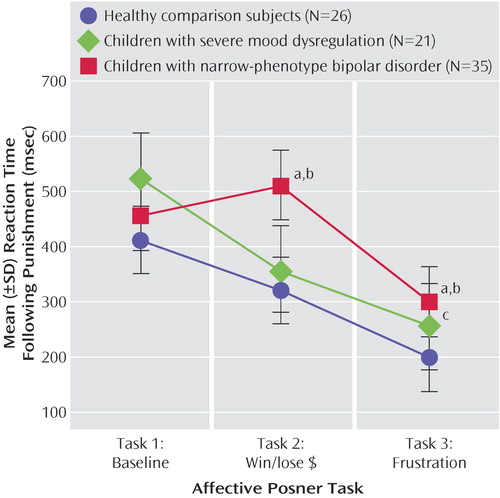
a Significantly slower reaction time than seen in subjects with severe mood dysregulation (p<0.02).
b Significantly slower reaction time than seen in healthy comparison subjects (p<0.001).
c Significantly slower reaction time than seen in healthy comparison subjects (p<0.01).
Accuracy
For the rate of correct responses (children with severe mood dysregulation: 83.22% [SD=1.76], children with narrow-phenotype bipolar disorder: 87.50% [SD=1.27], comparison subjects: 91.79% [SD=1.43]), repeated-measures ANOVA revealed a significant main effect of group (F=7.68, df=2, 54, p<0.001). Post hoc analyses found that across tasks, the comparison subjects had significantly higher accuracy than both the severe mood dysregulation (p<0.0001) and narrow-phenotype (p<0.05) subjects, and subjects with severe mood dysregulation had significantly lower accuracy than did those with narrow-phenotype bipolar disorder (p<0.05). The task-by- group interaction was nonsignificant. Task main effect was significant (F=70.97, df=2, 108, p<0.001), with task 3 accuracy significantly lower than tasks 1 (p<0.001) and 2 (p<0.001).
Comorbid diagnoses
To ascertain the impact of oppositional defiant disorder, ADHD, and major depressive disorder symptoms on our pathophysiological results, we conducted ANCOVA analyses using these symptoms as covariates. The association between narrow-phenotype bipolar disorder and decreased Pz P3 amplitude during frustration remained significant when comorbid symptoms were controlled. Specifically, we found significant group effects with ADHD and major depressive disorder as covariates (F=11.64, df=1, 58, p=0.001) and with oppositional defiant disorder and major depressive disorder as covariates (F=6.36, df=1, 59, p=0.02). The covariates themselves did not predict Pz P3 amplitude in either ANCOVA.
We then conducted ANCOVA analyses using N1 amplitude as our outcome measure. Controlling for ADHD and major depressive disorder, we found a significant group effect of diagnostic category (F=8.92, df=1, 56, p=0.004), with no significant effects of covariates. Thus, controlling for ADHD and major depressive disorder did not alter our N1 results. When we controlled for oppositional defiant disorder, the association with diagnostic category was reduced (F=2.97, df=1, 57, p=0.09), and the effect of oppositional defiant disorder as a covariate was significant (F=5.47, df=1, 57, p=0.02); the effect of major depressive disorder as a covariate was nonsignificant. Thus, when we controlled for oppositional defiant disorder and major depressive disorder, differences in N1 amplitude between our groups became nonsignificant, and the effect of oppositional defiant disorder was significant, demonstrating that oppositional defiant disorder symptoms mediate the association with N1 amplitude independent of diagnosis.
Demographic variables and mood
Bivariate correlations between mood ratings (scores on the Children’s Depression Rating Scale, Young Mania Rating Scale, or Manifest Anxiety Scale for Children) and event-related potential amplitude, reaction time, and accuracy in subjects with severe mood dysregulation were nonsignificant, as was found previously in narrow-phenotype bipolar disorder subjects (16) . Previous analyses in narrow-phenotype subjects also failed to find an association between medication and outcome variables (16) . The small number of medicated subjects with severe mood dysregulation prevented similar analyses. To investigate associations between outcome variables and functional impairment, we performed bivariate correlational analyses between Children’s Global Assessment Scale scores and results. We found significant correlations between score and accuracy in task 1 (r=0.44, p<0.05) and task 2 (r=0.47, p<0.04) in severe mood dysregulation subjects, and between score and task 3 P3 amplitude (r=0.49, p<0.03) in narrow-phenotype subjects. Thus, greater impairment was associated with lower accuracy on tasks 1 and 2 in children with severe mood dysregulation, and with worse deployment of attentional resources during frustration in children with narrow-phenotype bipolar disorder.
Discussion
To investigate the pathophysiology of pediatric bipolar disorder phenotypes, we compared children with severe mood dysregulation (i.e., nonepisodic irritability, marked reactivity, and hyperarousal, but no episodic euphoric mania) to those with narrow-phenotype bipolar disorder (i.e., discrete episodes of mania with elevated mood) (7) . Our results using the affective Posner paradigm (an attention task with emotional manipulations) suggest differences between these phenotypes in the behavioral manifestations and psychophysiological mechanisms of frustration.
Participant reports indicated that the paradigm achieved the desired emotional effect: relative to comparison subjects, both patient groups reported significantly greater arousal on the frustration task. However, the psychophysiological data showed a double dissociation. Specifically, patients with narrow-phenotype bipolar disorder had decreased P3 amplitude when frustrated (suggesting executive attention deficits), but exhibited no N1 amplitude deficit. In contrast, subjects with severe mood dysregulation were unimpaired on P3 amplitude but had decreased N1 amplitude on all three tasks. Thus, the psychophysiological correlates of frustration differed between these two patient groups: comparable perturbations in subjective reports of affect (e.g., increased frustration, relative to comparison subjects) were associated with different physiology.
Attenuated N1/P1 amplitude, previously documented in ADHD (20) , suggests that children with severe mood dysregulation have deficits in initial attention regardless of emotional context, possibly accounting for their low accuracy. Whereas narrow-phenotype bipolar subjects displayed decreased P3 amplitude when frustrated, the children with severe mood dysregulation displayed normal P3 amplitude, suggesting that they can modulate their attention properly in the context of increased emotional demands (21) . P3 deficits, seen here in narrow-phenotype bipolar disorder, have been associated with depression (22) and can co-occur with slow reaction time in anhedonia patients (23) .
Since approximately 90% of patients in both groups were euthymic when tested, the observed deficits may be trait based. Also, the significant correlations between outcome variables and impairment measures in both patient groups indicate that these behavioral and psychophysiological measures may indeed assess processes related to illness severity.
Our primary aim in this study was to move the debate regarding the boundaries of bipolar disorder in children beyond clinical descriptors by supplementing these with measures of brain function. Thus, we compared children with unequivocal bipolar disorder with children whose diagnostic status is controversial; these comparisons were not just on clinical features but also on brain-based measures. Since our narrow-phenotype bipolar disorder criteria are stricter than DSM-IV in that children with irritability only are excluded, an important question is the extent to which our findings are generalizable to other pediatric bipolar disorder samples in the literature. It is notable that the age at onset and level of impairment here are comparable to that in other reported samples (1 , 2 , 4 , 24) . However, direct comparisons to these samples are limited by our lack of data regarding longitudinal course, cycle frequency, or rapid-cycling rates. Further research is needed to ascertain the generalizability of our results to children with bipolar disorder assessed through other techniques.
One important question is the utility of using the term severe mood dysregulation rather than describing our cohort as ADHD and oppositional defiant disorder youth with depression-like irritability. Most important, many children with oppositional defiant disorder or ADHD do not meet severe mood dysregulation criteria because, as noted earlier, the criteria for irritability and impairment secondary to irritability are particularly strict and clearly operationalized. Conversely, not all children with severe mood dysregulation met criteria for both ADHD and oppositional defiant disorder ( Table 1 ). Further, only 38% of the severe mood dysregulation subjects had a history of major depressive disorder. Nonetheless, recruiting children who meet severe mood dysregulation criteria presents challenges in relating findings to severe mood dysregulation as opposed to ADHD or oppositional defiant disorder. Severe mood dysregulation, oppositional defiant disorder, and ADHD are not easily dissociable, and to some extent represent alternative ways to classify a common group of children. Consistent with this possibility, we found that N1 amplitude was related to severity of oppositional defiant disorder symptoms as well as to severe mood dysregulation diagnosis. Regardless, our key observation is that N1 deficits, independent of emotional context, are associated with either oppositional defiant disorder or severe mood dysregulation but not bipolar disorder, whereas P3 deficits, present only during frustration, are associated with bipolar disorder but neither oppositional defiant disorder nor severe mood dysregulation.
The association between N1 amplitude and oppositional defiant disorder symptoms suggests that, whereas the diagnostic controversy in pediatric bipolar disorder has largely focused on the boundary between bipolar disorder and ADHD, the boundary between oppositional defiant disorder and bipolar disorder may be as important. Although usually conceptualized as a disruptive behavior disorder, oppositional defiant disorder has prominent mood components, specifically irritability. Longitudinal studies indicate an association between childhood oppositional defiant disorder and young adult major depressive disorder (25) and between family history of major depressive disorder and childhood oppositional defiant disorder (26) . Thus, whereas several studies compare children with ADHD to those with bipolar disorder (27 , 28) , studies comparing children with oppositional defiant disorder to those with bipolar disorder or severe mood dysregulation would also be helpful. Because of comorbidity in these diagnoses, large patient groups will be required.
A major confound was that the narrow-phenotype bipolar disorder patients were predominantly medicated but the severe mood dysregulation subjects were predominantly unmedicated. Some studies have found that P3 amplitude normalizes (i.e., increases) in adults with schizophrenia treated with antipsychotic medications (29) and in children with ADHD treated with stimulants (30) . Other studies have failed to find such normalization with antipsychotics (31) , hypnotic medications (32) , or multipharmacology (33) . No studies have suggested that medication decreases P3 amplitude. Furthermore, detailed studies of P3 amplitude and medication suggest that, whereas frontal P3 amplitude may be normalized by medications, parietal P3 amplitude, which was deficient in narrow-phenotype bipolar subjects, may be unaffected by medication (34) . Finally, in this study, behavior and psychophysiology were comparable between narrow-phenotype bipolar patients and comparison subjects in nonemotional conditions; differences emerged only in emotional contexts. We are unaware of any reports of medications impacting P3 amplitude differentially based on the emotionality of the context. However, it is possible that our results reflect the interaction of medication effects and task demands. Future studies with unmedicated bipolar disorder patients, and direct comparisons of medicated and unmedicated cohorts, would be informative. Finally, because of the limited number of trials, we were unable to examine habituation or trends in behavior or psychophysiology within each task, which could potentially impact the interpretation of our results.
In conclusion, our results indicate that there may be different psychophysiological mechanisms and behavioral correlates associated with frustration between children with narrow-phenotype bipolar disorder and those with severe mood dysregulation. Whereas the deficits in the narrow-phenotype subjects indicated impaired allocation of attention in the context of frustration, those in the severe mood dysregulation group indicated impairments in the initial stages of attention across emotional and nonemotional tasks. Finally, whereas the deficits in the narrow-phenotype cohort are consistent with those seen in mood disorders, deficits in children with severe mood dysregulation may reflect concurrent oppositional defiant disorder. The current study is the first to provide evidence of behavioral and psychophysiological differences between possible phenotypes of pediatric bipolar disorder.
1. Birmaher B, Axelson D, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Keller M: Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry 2006; 63:175–183Google Scholar
2. Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Bridge J, Keller M: Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry 2006; 63:1139–1148Google Scholar
3. Kowatch RA, Youngstrom EA, Danielyan A, Findling RL: Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disord 2005; 7:483–496Google Scholar
4. Geller B, Tillman R, Craney JL, Bolhofner K: Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry 2004; 61:459–467Google Scholar
5. Geller B, Craney JL, Bolhofner K, Nickelsburg MJ, Williams M, Zimerman B: Two-year prospective follow-up of children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry 2002; 159:927–933Google Scholar
6. Goodwin FK, Jamison KR: Manic-Depressive Illness. Oxford, Oxford University Press, 1990Google Scholar
7. Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS: Defining clinical phenotypes of juvenile mania. Am J Psychiatry 2003; 160:430–437Google Scholar
8. Geller B, Williams M, Zimerman B, Frazier J, Beringer L, Warner KL: Prepubertal and early adolescent bipolarity differentiate from ADHD by manic symptoms, grandiose delusions, ultra-rapid or ultradian cycling. J Affect Disord 1998; 51:81–91Google Scholar
9. Geller B, Zimerman B, Williams M, Delbello MP, Bolhofner K, Craney JL, Frazier J, Beringer L, Nickelsburg MJ: DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. J Child Adolesc Psychopharmacol 2002; 12:11–25Google Scholar
10. Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, Delbello MP, Soutullo CA: Diagnostic characteristics of 93 cases of a prepubertal and early adolescent bipolar disorder phenotype by gender, puberty and comorbid attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 2000; 10:157–164Google Scholar
11. Craney JL, Geller B: A prepubertal and early adolescent bipolar disorder-I phenotype: review of phenomenology and longitudinal course. Bipolar Disord 2003; 5:243–256Google Scholar
12. Leibenluft E, Blair RJ, Charney DS, Pine DS: Irritability in pediatric mania and other childhood psychopathology. Ann N Y Acad Sci 2003; 1008:201–218Google Scholar
13. Leibenluft E, Charney DS, Pine DS: Researching the pathophysiology of pediatric bipolar disorder. Biol Psychiatry 2003; 53:1009–1020Google Scholar
14. Derryberry D, Reed MA: Temperament and attention: orienting toward and away from positive and negative signals. J Pers Soc Psychol 1994; 66:1128–1139Google Scholar
15. Perez-Edgar K, Fox N: A behavioral and electrophysiological study of children’s selective attention under neutral and affective conditions. J Cogn Develop 2005; 6:89–116Google Scholar
16. Rich BA, Schmajuk M, Perez-Edgar KE, Pine DS, Fox NA, Leibenluft E: The impact of reward, punishment, and frustration on attention in pediatric bipolar disorder. Biol Psychiatry 2005; 58:532–539Google Scholar
17. Geller B, Zimerman B, Williams M, DelBello MP, Bolhofner K, Craney JL, Frazier J, Beringer L, Nickelsburg MJ: DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. J Child Adolesc Psychopharmacol 2002; 12:11–25Google Scholar
18. March JS, Parker JD, Sullivan K, Stallings P, Conners CK: The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry 1997; 36:554–565Google Scholar
19. McManis MH, Bradley MM, Berg WK, Cuthbert BN, Lang PJ: Emotional reactions in children: verbal, physiological, and behavioral responses to affective pictures. Psychophysiology 2001; 38:222–231Google Scholar
20. Jonkman LM, Kemner C, Verbaten MN, Van Engeland H, Camfferman G, Buitelaar JK, Koelega HS: Attentional capacity, a probe ERP study: differences between children with attention-deficit hyperactivity disorder and normal control children and effects of methylphenidate. Psychophysiology 2000; 37:334–346Google Scholar
21. Steger J, Imhof K, Steinhausen H, Brandeis D: Brain mapping of bilateral interactions in attention deficit hyperactivity disorder and control boys. Clin Neurophysiol 2000; 111:1141–1156Google Scholar
22. Pause BM, Raack N, Sojka B, Goder R, Aldenhoff JB, Ferstl R: Convergent and divergent effects of odors and emotions in depression. Psychophysiology 2003; 40:209–225Google Scholar
23. Dubal S, Pierson A, Jouvent R: Focused attention in anhedonia: a P3 study. Psychophysiology 2000; 37:711–714Google Scholar
24. Findling RL, Gracious BL, McNamara NK, Youngstrom EA, Demeter CA, Branicky LA, Calabrese JR: Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord 2001; 3:202–210Google Scholar
25. Pine DS, Cohen P, Gurley D, Brook J, Ma Y: The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry 1998; 55:56–64Google Scholar
26. Weissman MM, Wickramaratne P, Nomura Y, Warner V, Verdeli H, Pilowsky DJ, Grillon C, Bruder G: Families at high and low risk for depression: a 3-generation study. Arch Gen Psychiatry 2005; 62:29–36Google Scholar
27. Dickstein DP, Garvey M, Pradella A, Greenstein DK, Sharp WS, Castellanos FX, Pine DS, Leibenluft E. Neurological examination abnormalities in children with bipolar disorder or attention deficit hyperactivity disorder. Biol Psychiatry 2005; 58:517–524Google Scholar
28. Moore CM, Biederman J, Wozniak J, Mick E, Aleardi M, Wardrop M, Dougherty M, Harpold T, Hammerness P, Randall E, Renshaw PF: Differences in brain chemistry in children and adolescents with attention deficit hyperactivity disorder with and without comorbid bipolar disorder: a proton magnetic resonance spectroscopy study. Am J Psychiatry 2006; 163:316–318Google Scholar
29. Wang J, Hirayasu Y, Hokama H, Tanaka S, Kondo T, Zhang M, Xiao Z: Influence of duration of untreated psychosis on auditory P300 in drug-naive and first-episode schizophrenia. Psychiatry Clin Neurosci 2005; 59:209–214Google Scholar
30. Jonkman LM, Kemner C, Verbaten MN, Van Engeland H, Camfferman G, Buitelaar JK, Koelega HS: Attentional capacity, a probe ERP study: differences between children with attention-deficit hyperactivity disorder and normal control children and effects of methylphenidate. Psychophysiology 2000; 37:334–346Google Scholar
31. Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S: Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res 2004; 70:315–329Google Scholar
32. Nakajima T, Takazawa S, Hayashida S, Nakagome K, Sasaki T, Kanno O: Effects of zolpidem and zopiclone on cognitive and attentional function in young healthy volunteers: an event-related potential study. Psychiatry Clin Neurosci 2000; 54:37–40Google Scholar
33. Mathalon DH, Ford JM, Rosenbloom M, Pfefferbaum A: P300 reduction and prolongation with illness duration in schizophrenia. Biol Psychiatry 2000; 47:413–427Google Scholar
34. Gallinat J, Riedel M, Juckel G, Sokullu S, Frodl T, Moukhtieva R, Mavrogiorgou P, Nissle S, Muller N, Danker-Hopfe H, Hegerl U: P300 and symptom improvement in schizophrenia. Psychopharmacology (Berl) 2001; 158:55–65Google Scholar