Structural Brain Magnetic Resonance Imaging of Limbic and Thalamic Volumes in Pediatric Bipolar Disorder
Abstract
Background: Youths with bipolar disorder are ideal for studying illness pathophysiology given their early presentation, lack of extended treatment, and high genetic loading. Adult bipolar disorder MRI studies have focused increasingly on limbic structures and the thalamus because of their role in mood and cognition. On the basis of adult studies, the authors hypothesized a priori that youths with bipolar disorder would have amygdalar, hippocampal, and thalamic volume abnormalities. METHOD: Forty-three youths 6–16 years of age with DSM-IV bipolar disorder (23 male, 20 female) and 20 healthy comparison subjects (12 male, eight female) similar in age and sex underwent structured and clinical interviews, neurological examination, and cognitive testing. Differences in limbic and thalamic brain volumes, on the logarithmic scale, were tested using a two-way (diagnosis and sex) univariate analysis of variance, with total cerebral volume and age controlled. RESULTS: The subjects with bipolar disorder had smaller hippocampal volumes. Further analysis revealed that this effect was driven predominantly by the female bipolar disorder subjects. In addition, both male and female youths with bipolar disorder had significantly smaller cerebral volumes. No significant hemispheric effects were seen. CONCLUSIONS: These findings support the hypothesis that the limbic system, in particular the hippocampus, may be involved in the pathophysiology of pediatric bipolar disorder. While this report may represent the largest MRI study of pediatric bipolar disorder to date, more work is needed to confirm these findings and to determine if they are unique to pediatric bipolar disorder.
Bipolar disorder is one of the most severe neuropsychiatric disorders at any age and is among the most disabling of psychiatric conditions that affect youths (1, 2). Although the chronology of underlying structural brain abnormalities in this population is unknown, such abnormalities may represent disruptions in typical brain growth resulting from an interplay of genetic and environmental factors. MRI studies are critical for advancing our knowledge of brain regions involved in the pathophysiology of pediatric bipolar disorder.
Youths with bipolar disorder are more severely ill and have higher genetic loading than adult-onset cases (3–5). Furthermore, children’s brains are typically free from confounding factors known to affect brain structure and function (e.g., extensive treatment, substance use, history of electroconvulsive therapy). Finally, studying children and adolescents facilitates observation during a time in development when there are hormonal shifts known to have neuromodulatory effects on brain regions such as the temporal lobe (6, 7). By virtue of all of these factors, there is an increased likelihood of uncovering significant brain anatomic abnormalities in this early-onset group.
Although no discrete brain area has been consistently reported abnormal in the adult bipolar disorder MRI (structural) literature, some data lend support to several proposed neuroanatomic models of emotion regulation (8–10). One of these proposed models includes the following brain regions: prefrontal cortex, amygdala-hippocampus complex, hypothalamus, thalamus, insular cortex, ventral striatum, and interconnected structures (8). The amygdala, hippocampus, and thalamus are of particular interest in the study of bipolar disorder because of their functional roles in the brain. For example, the amygdala is integral in emotion-related aspects of behavior, memory, and learning; the thalamus processes sensory information and integrates activity among forebrain regions; and the hippocampus plays a role in learning and memory in providing contextual information (11). Several prior structural MRI studies of adults with bipolar disorder have reported abnormalities in the limbic structures and thalamus (12–18). Abnormalities in these structures might confer a propensity toward dysregulated mood states and vulnerability toward developing a mood disorder.
Prior structural MRI studies in pediatric bipolar disorder have indicated that there are anatomic abnormalities in a number of the structures implicated in the neural systems governing affective and cognitive processes. Botteron and colleagues demonstrated a loss of the normal asymmetry in the frontal lobe (19). A study conducted by Friedman and colleagues (20), which included adolescents with schizophrenia and bipolar disorder in a combined patient group, found that this patient group had reduced intracranial volumes and increased frontal and temporal sulcal sizes relative to healthy subjects. A third report that used the same subjects as in the Friedman et al. study found the patient group had reduced thalamic area relative to healthy subjects (21). A recent study consisting predominantly of adults with bipolar disorder included a subgroup of 14 adolescents (age range=10–22 years). These youths had smaller hippocampal and amygdalar volumes than did 23 healthy adolescents (18). Finally, DelBello and colleagues (22) recently reported that adolescents with bipolar disorder (N=23) had smaller amygdala and enlarged putamen volumes compared with healthy subjects (N=20). In summary, prior MRI studies have suggested that youths with bipolar disorder have abnormalities in a number of the brain areas discussed by Soares and Mann (8) in their neuroanatomic model of emotion regulation: total cerebral volume, frontal lobe, hippocampus, amygdala, putamen, and thalamus. In order to assess anatomic findings in bipolar disorder further, we conducted a structural MRI study to evaluate brain volumes in early-onset bipolar disorder cases. We hypothesized that youths with bipolar disorder would have abnormalities in structures involved in the model of affect regulation discussed by Soares and Mann (8). In particular, the amygdala, hippocampus, and thalamus were chosen a priori on the basis of previous imaging studies of youths and adults with bipolar disorder.
Method
Subjects
The study was approved by institutional review boards at the Massachusetts General Hospital and McLean Hospital. Subjects were recruited through the McLean Hospital outpatient program and professional-patient advocacy groups. Inclusion criteria were DSM-IV diagnosis of bipolar disorder, age 6–16 years, and right-handedness. Male and female subjects of all ethnicities were recruited. Healthy subjects, all right-handed, were recruited through community newspaper advertisements and had no DSM-IV axis I diagnosis according to structured and clinical interviews and no family history of affective disorders or psychotic disorders in first-degree relatives. Exclusion criteria were major sensorimotor handicaps; full-scale IQ <70 or learning disabilities; history of claustrophobia, head trauma, loss of consciousness, autism, schizophrenia, anorexia or bulimia nervosa, electroconvulsive therapy, or alcohol or drug dependence/abuse (in the 2 months preceding the scan or a total history of 12 or more months); active medical or neurologic disease; metal fragments or implants; or current pregnancy or lactation.
Procedure
Seventy subjects (all outpatients) and their parents (or guardians) signed assent and informed consent forms. Three subjects were determined ineligible during interview, and one stopped the study because of lack of interest. Sixty-six scans were obtained; three scans were unreadable due to motion artifact. Therefore, data from 63 subjects scanned as part of an ongoing neuroimaging study are included in this report: 43 youths with DSM-IV bipolar disorder and 20 healthy subjects.
All of the youths underwent a diagnostic semistructured interview (Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version [K-SADS-E] [23]) and a clinical interview by board-certified child psychiatrists (J.A.F., S.C.). In addition, parents were administered an indirect K-SADS-E regarding their children by trained raters. These B.A.-level raters received 4 months of training on the administration of the K-SADS-E under the supervision of senior raters and the senior investigator (J.B.). All raters had established a high degree of interrater reliability: from 175 interviews, the mean kappa was 0.90, and all disorders achieved kappa coefficients >0.82. Final DSM-IV diagnoses were established by the consensus diagnosis of clinical and structured interviews.
Each youth received a physical and neurological examination that included Tanner staging (a I–V scale of pubertal development) (24) and cognitive testing. The age at onset of each illness was determined by parental report of symptoms on the structured interview. Age at illness onset was defined as the time when the youth met full diagnostic criteria (e.g., age at onset of bipolar illness was the age at which the youth first met full diagnostic criteria for mania). Children and adolescents were given several subtests of the Wechsler Intelligence Scale for Children, 3rd ed. (WISC-III) (25) which permitted the estimation of verbal IQ. Handedness was assessed using the Edinburgh Handedness Questionnaire (26).
Measures of current psychopathology were obtained using the Young Mania Rating Scale (27) and Global Assessment of Functioning Scale (GAF) (DSM-IV, p. 32).
Antipsychotic doses (converted to chlorpromazine equivalents) (28, 29), as well as number and type (antipsychotic, antidepressant, stimulant, anticonvulsant, lithium) of psychoactive medications at the time of scan were used as clinical variables.
MRI Protocol
Structural imaging was performed at the McLean Hospital Brain Imaging Center on a 1.5-T Signa scanner (GE Medical Systems, Milwaukee). Acquisitions included a conventional T1-weighted sagittal scout series (20 slices), a proton density/T2-weighted interleaved double-echo axial series (120 slices, slice thickness=3 mm, field of view=24 cm2, TR=3 seconds, TE=30/80 msec, acquisition matrix=256×192, number of excitations=0.5), and a three-dimensional inversion recovery-prepped spoiled gradient recalled echo coronal series, which was used for structural analysis (124 slices, prep=300 msec, TE=1 minute, flip angle=25°, field of view=24 cm2, slice thickness=1.5 mm, acquisition matrix=256×192, number of excitations=2). All scans were reviewed by a clinical neuroradiologist to rule out gross pathology.
Image Analysis
Structural scans were transferred to the NMR Center for Morphometric Analysis-Charlestown Massachusetts General Hospital and coded and catalogued for blind analysis. Imaging analysis was done on Sun Microsystems, Inc. (Mountainview, Calif.) workstations using Cardviews software (30). The datasets were positionally normalized to overcome variations in head position by imposing a standard orientation on each scan using the midpoints of the decussations of the anterior and posterior commissure lines and the midsagittal plane at the level of the posterior commissure as points of reference for rotation and translation. The images were not rescaled to Talairach spatial dimensions in order to preserve individual and interhemispheric differences in the morphometry of structures (31).
The entire image sets were then segmented into gray, white, and CSF tissue classes by three image analysts, under the supervision of one of the authors (N.M.), all of whom had strong backgrounds in neuroanatomy and extensive training in morphometric analysis and who were blind to subject-identifying information. The segmentation method uses a semiautomated intensity contour algorithm for external border definition and signal intensity histogram distributions for delineation of gray-white borders (Figure 1). This technique allows for border definition as the midpoint between the peaks of the bimodal distribution for any given structure and its surrounding tissue (32).
Total cerebral volume
Segmentation of the regions of interest was performed following the anatomic definitions of Filipek and colleagues (33) for the total cerebrum. Total cerebral volume was defined as all gray and white matter in the cerebrum and did not include CSF, cerebellum, or brain stem. The cerebrum was measured across all 124 coronal slices in which it appeared.
Thalamus
Segmentation of the thalamus was performed following the anatomic definitions of Seidman and colleagues (34, 35) by tracing the trajectory of the hypothalamic fissure in the sagittal plane to separate the thalamus proper from the ventral diencephalon. The medial boundary of the structure was the third ventricle, and the lateral boundary was the internal capsule. The superior border was the body of the lateral ventricle, and the inferior border was the hypothalamic fissure.
Hippocampus and amygdala
The method of Filipek et al. (33) defines the amygdala and hippocampus as a continuous gray matter structure in the primary segmentation. These two structures are then separated from each other according to the procedure described by Seidman and colleagues (36) in which the hippocampus is separated from the amygdala at the rostral-coronal plane, where the hippocampus first appears. The segmentation of the amygdala was performed manually in its entirety, comprising approximately 11 subsequent coronal sections.
The coexistence of the amygdala and hippocampus in several coronal sections can make the precise identification of the ventral amygdalar border difficult (37). Therefore, we used the cross-referencing capability of the program Cardviews (38) to draw outlines delimiting the amygdala in axial and sagittal views (Figure 1 B, C); this preliminary procedural step allows a reliable separation of the amygdala from surrounding gray structures, such as the ventral part of the lentiform nucleus, the medial temporal cortex, and the hippocampus, thus eliminating the need to apply conventions to define the anterior amygdalar boundary (12, 22) or the amygdala-hippocampal junction (12). The anterior portion of the amygdala was segmented as it appears beneath the medial temporal cortex (slice K in Figure 2). At this region, the medial temporal cortex and the amygdala can give the impression of being only a thickening of the medial temporal cortex with no amygdala present, as has been reported previously (12, 22). Therefore, the definition of these borders was particularly aided by the tracing of cross-referenced outlines in the axial and sagittal planes (Figure 1 B, C). The choroidal fissure was used as the superior border of the amygdala along with the gray-white matter contrast between the amygdala and surrounding white matter. The gray-white matter contrast between the amygdala and its surrounding temporal white matter (consisting of the centrally located temporal white matter stem), as well as the gray-CSF contrast between the amygdala and the temporal horn of the lateral ventricle, was considered the lateral border of the amygdala. The parahippocampal cortex anteriorly, the brain exterior at the inferior lip of the choroidal fissure, and partially the hippocampus posteriorly were assigned as the medial borders of the amygdala. Finally, the inferior border consisted of the gray-white matter contrast between the amygdala and its surrounding temporal white matter anteriorly and by the alveus (of the hippocampus) and the temporal horn of the lateral ventricle posteriorly.
The volumes for each structure were derived by multiplying the number of voxels assigned to each structure on each slice by the voxel volume (the product of slice thickness and the square of in-plane resolution), followed by summing across all slices in which the structure appeared; the volumes are reported in cm3(31).
For the reliability study, 10 scans from our data set were selected at random and blindly segmented by two raters. Five of the scans were also remeasured in a random order by one of the raters to estimate the intraclass correlation coefficient.
The standard interrater intraclass correlation coefficient for the total cerebrum was 0.93, and the intra- and interrater correlation coefficients, respectively, for the regions of interest were 0.88 and 0.84 for the amygdala, 0.95 and 0.96 for the thalamus, and 0.93 and 0.94 for the hippocampus.
Data Analyses
S-Plus 6.0 (Insightful Corp., Seattle) was used for statistical analysis. All statistical tests were two-sided with alpha set at 0.05.
Differences in demographic and clinical variables were measured using t tests for continuous variables and chi-square tests for categorical variables. In addition, Pearson’s correlations were calculated for clinical variables of the bipolar disorder group and those structures that differed significantly between bipolar disorder youths and healthy subjects. The clinical variables included Young Mania Rating Scale and GAF scores, number of psychoactive medications, chlorpromazine equivalents, and verbal IQ.
Noting that brain structure sizes possess variances that increase with their means, we analyzed our volumetric data on the natural logarithmic scale as a step toward uncoupling this non-Gaussian relationship. We conducted an exploratory multivariate analysis of variance (MANOVA) on the three-dimensional ensemble of log total hippocampal, amygdalar, and thalamic volumes with the effects of log total cerebral volume, age, sex, diagnosis, and the sex-by-diagnosis interaction controlled. In addition, an exploratory MANOVA analysis was performed to assess the effects of age group, mood state, medication type, and presence of ADHD or psychosis on the log hippocampal and log total cerebral volumes.
We then proceeded to analyze the effects of these covariates on log brain structure sizes by three separate univariate linear regression models. Employing our preliminary data summaries and linear regressions, we found no significant laterality effects on any of the volumetric outcomes; hence, we did not include brain hemisphere effects in our model selection procedure. We fit a linear regression model for log total hippocampal volume that contained the same effects as in the preliminary MANOVA. We also analyzed the data using the absolute volumes of the structures and the inferential findings were identical to the log scale analyses.
Results
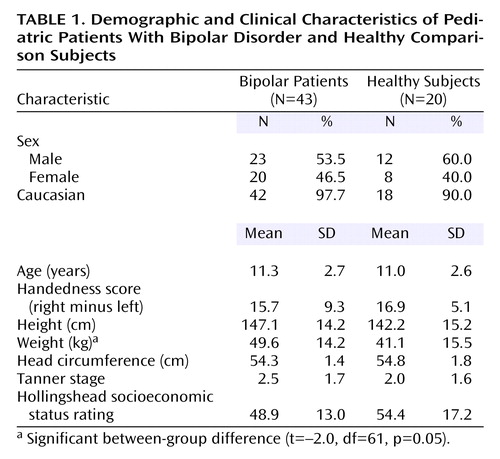
Data from 63 subjects are included: 43 youths with DSM-IV bipolar disorder (mean age=11.3 years, SD=2.7; current episode: mixed=52.3%, manic=15.9%, depressed=11.4%, euthymic=20.5%) and 20 healthy comparison subjects (mean age=11.0 years, SD=2.6). Characteristics of the groups are summarized in Table 1. There were no significant height, head circumference, or Tanner stage differences between groups; there was a significant difference in weight (Table 1).
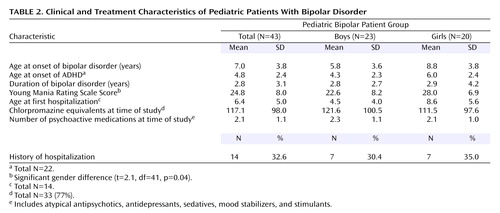
Although the bipolar disorder youths had verbal IQ scores in the normal range, they were significantly lower than those of comparison subjects (mean=100.7 [SD=14.3] versus 116.9 [SD=12.7]; t=4.3, df=61, p<0.001). The bipolar disorder group also scored lower on the GAF (mean=49.5 [SD=6.0] versus 68.8 [SD=1.9]; t=19.0, df=61, p<0.001). The bipolar disorder subjects had a number of comorbid conditions (mean=7.2, SD=3.2). The most common comorbid conditions were oppositional defiant disorder (67% [N=29]) and ADHD (51% [N=22]). Most bipolar disorder youths (86% [N=37]) had experienced at least one episode of major depression (mean age at onset=6.8 years, SD=3.6), and 40% (N=17) had a history of psychosis. One youth had a history of alcohol abuse, which occurred more than 3 months before enrollment. Medications used at the time of MRI included lithium (26% [N=11]), anticonvulsants (42% [N=18]), antidepressants (30% [N=13]), stimulants (21% [N=9]), atypical antipsychotics (76% [N=33]), and other (19% [N=8]), which included anticholinergics and beta-adrenergics. Table 2 presents details of the clinical and treatment characteristics of the bipolar disorder patients.
Clinical neuroradiological interpretations of the scans showed normal variants in three healthy subjects (slightly prominent lateral ventricles [N=1], large cisterna magna [N=1], and pineal cyst [N=1]) and two bipolar disorder subjects (mildly prominent lateral ventricles [N=1] and large cisterna magna [N=1]). One healthy subject had findings that were atypical but of unclear clinical significance (a tiny focus of hypointensity in the subcortical white matter of the superior left frontal lobe). Nine bipolar disorder subjects had findings that were atypical and again of unclear clinical significance (prominent ventricles [N=3], right-greater-than-left asymmetry of the temporal horn [N=2], multiple white matter hyperintensities in the bilateral parietal area [N=1] and in the left hemisphere [N=1], nonspecific punctate T2 foci in the left parietal region [N=1], and bilateral widened perivascular spaces noted in the inferolateral portion of the basal ganglia [N=1]).
Volumetric Measurements
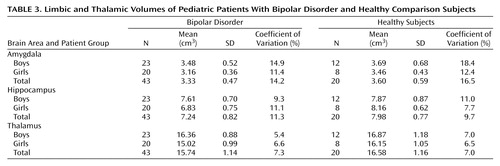
Table 3 contains the limbic and thalamic volume data. The amygdala volumes were measured across a mean of 12.1 slices (SD=1.6, range=9–15) on the right side, and 11.5 slices (SD=1.4, range=8–15) on the left. The hippocampal volumes were measured across a mean of 24.4 slices (SD=1.7, range=21–28) on the right side, and 24.7 slices (SD=2.0, range=20–29) on the left. The thalamic volumes were measured across a mean of 22.7 slices (SD=1.3, range=18–25) on the right side, and 22.9 slices (SD=1.1, range=21–25) on the left.
No significant correlations were seen between any clinical variables and the hippocampus or total cerebral volume for the bipolar disorder group.
When the bipolar disorder group was assessed using the exploratory MANOVA analysis, we found no significant effects of age group, mood state, medication type, or presence of ADHD or psychosis on volume in the hippocampus or cerebrum.
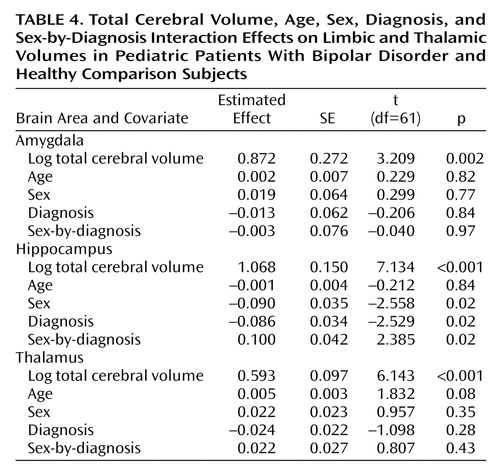
The initial exploratory MANOVA of the three-dimensional brain structure ensemble yielded potentially significant effects for sex (F=2.76, df=3, 55, p=0.05) and the sex-by-diagnosis interaction (F=2.17, df=3, 55, p=0.10).
Hippocampus
The linear regression model for log total hippocampal volume contained the same effects as in the preliminary MANOVA. We found a possible sex-by-diagnosis interaction in raw hippocampal volumes by sex and diagnosis (Table 3). Although not significant, we retained age in the model, since this covariate was fixed by our study design; main effects and the interaction of sex and diagnosis remained significant when age was dropped. We tried controlling for height and weight but found that neither of their effects was significant; the sex-by-diagnosis interaction remained significant. We found significant effects of log cerebral volume, sex, diagnosis, and the sex-by-diagnosis interaction (Table 4). We pursued the source of interaction by fitting separate regression models of the same form to the boys and the girls. This step confirmed our pooled variance assumption when fitting the single model with a sex effect (39). We conclude that the sex-by-diagnosis interaction is being driven by the smaller hippocampus of the girls after log total cerebral volume and age are controlled.
Amygdala and thalamus
The same logarithmic transformations and model selection procedure was applied to the thalamic and amygdalar volumes separately. No significant effects of sex, diagnosis, or their interaction were found.
Discussion
The youths in our study with bipolar disorder had significantly smaller total hippocampal and total cerebral volumes. These findings contribute to the existing literature on bipolar disorder youths, which has shown loss of normal frontal lobe asymmetry (19), reduced intracranial volume (20), increased frontal and temporal sulcal size (20), reduced thalamic area (21), larger putamen volume (12, 22), and smaller amygdala (18, 22) and hippocampal (18) volumes relative to healthy subjects.
These findings in children and adolescents differ somewhat from those in adults, but comparison of MRI results across studies is difficult because of variations in methodology. For example, until recently, it was difficult to even reliably measure structures such as the hippocampus—and particularly the amygdala—on MRI scans (12, 22). It is of note that the quality of the T1-weighted images used in the present study, as well as the outlines traced on the cross-referenced axial and sagittal slices, allow reliable visualization and segmentation of the amygdala, eliminating the need to apply conventions for defining either the anterior amygdalar boundary (12, 22) or the amygdala-hippocampal junction (12). Despite the differences in the methodologies used across studies, it is worth highlighting the anatomic variations that have been reported in adult structural MRI studies relative to the findings reported here and in other pediatric studies of bipolar disorder patients.
Of the structures included in our a priori hypothesis, the thalamus and amygdala have varied findings in adult and child and adolescent studies. Several adult bipolar disorder studies have reported that both structures are increased (12, 13, 15), whereas two others reported smaller volumes: one reported smaller left amygdala (14) and the other smaller bilateral amygdalar volumes (18). In adolescents, one study found reduced thalamic area (although the patient group also included youths with schizophrenia) (21). Blumberg and colleagues (18) and DelBello and colleagues (22) have recently reported decreased amygdalar volumes in adolescents with bipolar disorder. In our study, youths with bipolar disorder had a nonsignificant but possibly meaningful decrement in thalamic volumes compared with healthy subjects; our current study size may be underpowered to detect this effect, and we continue to study this structure as more scans are acquired. However, we found that there were no differences in amygdalar volume between groups.
Interpreting the differences between adult and pediatric findings depends on our understanding of the phenomenology of mood disorders across the lifespan: if early- and adult-onset bipolar disorder are the same illness, then age at onset may differentially affect brain structure. Alternatively, childhood- or adolescent-onset bipolar disorder may be a distinct disorder from adult-onset illness with a different set of neuroanatomic correlates. Cross-sectional measures of anatomic volume also may not adequately reflect the progressive and regressive patterns of growth that affect different brain structures at various stages of development (e.g., the subcortical gray matter structural changes that occur in the temporal lobe during the preteen years [38, 40]).
Our finding of decreased hippocampal volumes is the first report of this finding in children and adolescents with bipolar disorder and further extends the findings of Blumberg and colleagues in which bilateral hippocampal volume reductions were seen in adolescents with bipolar disorder but not in adults with the illness (18). Adult bipolar disorder studies of hippocampal volumes have found either decreased or normal volumes (12, 16, 18, 41). It is possible that the adult studies in which reduced hippocampal volumes were reported may have included at least some adults who had childhood-onset illness. However, this information was not included in those studies. If age at onset, particularly childhood-onset, is related to reduced hippocampal volumes, this could partially explain the mixed results seen in adult studies. Our finding of reduced hippocampal volume, as well as another group’s similar finding among adolescents with the illness (18), may represent a finding unique to early-onset bipolar disorder. This finding in our youths is of interest in light of neuropathologic studies of the hippocampus in bipolar disorder, which have suggested there may be abnormal neurodevelopment and remodeling of synapses in that structure (42–48).
A study of adults that compared monozygotic twins discordant for bipolar disorder found that the right hippocampus was smaller in the sick twin compared with the well twin (17), suggesting that this finding might be a structural correlate for the presence of disease. Given that reduced hippocampal volume was the only significant limbic finding in our group of bipolar disorder youths, and that at least two studies in adults (16, 17) had a similar finding in the right hippocampus, this may also be a finding reflective of disease.
Female bipolar disorder subjects showed a more pronounced decrease in hippocampal volume than did male bipolar youths relative to their comparison subjects. This sex-by-diagnosis interaction could reflect a variety of factors. For example, girls may need to have more significant structural abnormalities in order to reach threshold for disease expression. Alternatively, since the girls with bipolar disorder in our study had significantly higher Young Mania Rating Scale scores than the boys, the smaller hippocampal volumes in girls might be reflective of a greater degree of psychopathology. However, both male and female bipolar disorder youths had reduced hippocampal volumes, and it may be that there is some abnormality in the hippocampus, reflective of disease, that expresses itself more robustly in girls than in boys (49).
In our study we found that total cerebral volume was smaller in bipolar disorder youths (mean=5.4%, SD=6.4%); smaller total cerebral volume has also been reported in four prior studies of bipolar disorder youths (19–21, 50). Most adult bipolar disorder studies, including the recent study published by Blumberg and colleagues (18), have not found differences in total cerebral volume. Therefore, the difference seen in total cerebral volume between early- and adult-onset bipolar disorder cases relative to healthy groups suggests that affected youths may have distinct neurodevelopmental trajectories, possibly from having brains that have developed differently or from early apoptotic pruning of neuronal circuits. A smaller total cerebral volume in early-onset bipolar disorder illness may be reflective of a neurodevelopmental phenomenon. It should be noted that there is evidence of nonuniform scaling across the brain regions studied herein relative to global changes in total cerebral volume (51).
Although abnormal anatomy does not necessarily confer abnormal function, some structural differences may increase the risk of developing dysfunction. Such differences might also be markers of preexisting susceptibility or vulnerability and could aid in identifying different patient phenotypes (52). Bipolar disorder youths often have behavioral and developmental difficulties early in life (2, 52–54), and their symptoms of bipolar disorder typically begin between the ages of 5–11 years (1, 3, 55); in our study the mean age at onset was 7.0 years (SD=3.8). Many of the progressive and regressive events in the brain, particularly in the temporal lobe, occur during this age range, and alterations in normal brain development during this time may result in the symptoms of bipolar disorder.
Adult genetic and neuroimaging studies in both schizophrenia and bipolar disorder lend support for a multifactoral etiology; one possibility is of a “two-hit” hypothesis, a genetic predisposition in combination with environmental influences, resulting in the disorder (35, 36, 56, 57). For example, our finding of smaller hippocampal volumes may reflect developmental or genetic influences or environmental insults (e.g., hypoxia) that have led to reductions in what were previously normally developing volumes. This hypothesis suggests that two hits (genetic and environmental), occurring either independently or in interaction, may result in reduction of hippocampal volumes as well as in the expression of bipolar disorder.
This report describes to our knowledge the largest MRI study of pediatric bipolar disorder to date. However, the number of subjects was still relatively small and represents only a cross-sectional look at youths with bipolar disorder and comparison subjects. Our findings should be considered in light of other limitations, such as the smaller number of comparison subjects relative to the number of youths with bipolar disorder, the lack of rating scales for depressive symptoms, and the difficulty in reliably determining the age at onset of a child or adolescent’s mood instability based on parental recall. Although we did examine the effects of several clinical variables, including age and type of medication as well as chlorpromazine equivalents, and found no significant effects, the power in our study may have been insufficient to fully assess the possible effects of these parameters.
Our findings support the hypothesis that the hippocampus may be involved in the underlying pathophysiology of pediatric bipolar disorder and may represent a unique characteristic of early-onset presentation of the disorder due to a genetic diathesis or derangements in growth processes around the time of illness onset. However, in order to obtain a better sense of the trajectory of abnormalities in this structure in bipolar disorder populations, longitudinal studies are needed. Future studies need large enough sample sizes of both sexes and a sex-matched healthy comparison group to sort out diagnostic, age at onset, developmental, and sexual influences on these structures over time (6, 38, 58). A larger group of subjects is currently being accrued in order to further assess these findings with greater power and to assess the sexual dimorphism of other brain structures.
 |
 |
 |
 |
Received June 24, 2003; revisions received Dec. 29, 2003, and July 9, 2004; accepted July 19, 2004. From the Department of Psychiatry and the Neuroscience Program, Harvard Medical School, Boston; the McLean Hospital Child Psychiatry Outpatient Clinic, Brain Imaging Center, Statistical Neuroimaging Laboratory, and Developmental Neuroscience Laboratory; the Departments of Psychiatry, Pediatric Neurology, Radiology, Neurology, and Pediatrics, the Pediatric Psychopharmacology Unit, and the Center for Morphometric Analysis, Massachusetts General Hospital, Boston; the Department of Psychiatry and Behavioral Sciences, University of California, Davis; the Department of Biostatistics, Harvard School of Public Health, Boston; and the Department of Psychiatry, Massachusetts Mental Health Center, Boston. Address correspondence and reprint requests to Dr. Frazier, Child and Adolescent Neuropsychiatric Research Program, Cambridge Health Alliance/Mystic Center, 1493 Cambridge St., Cambridge, MA 02139; [email protected] (e-mail). Supported by NIMH research grants to Dr. Frazier (K08 MH-01573-01) and Dr. Lange (NS-37483). The authors thank Mary Ahn, M.D., Sandra DeJong, M.D., and Jay Giedd, M.D., for editorial comments and Jill Garroway, Rebecca Melrose, Nathan Stein, Mari Sohma, and Shuna Klaviness for their assistance with the study.
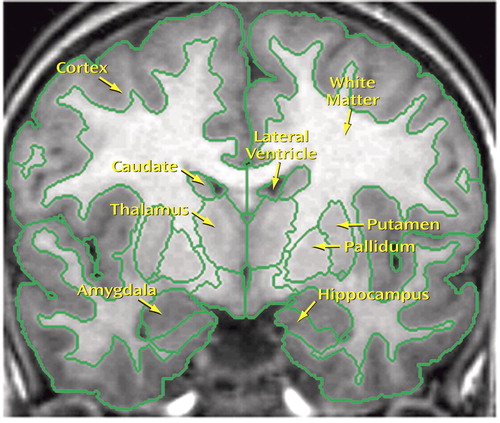
Figure 1. T1-MRI Coronal Section at the Level of the Mamillary Bodiesa
aSegmentation outlines shown in green.
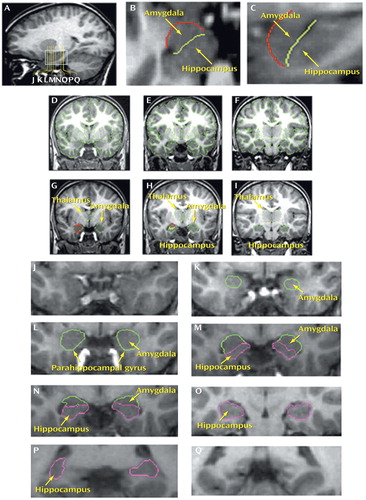
Figure 2. Segmentation of the Amygdala, Hippocampus, and Thalamusa
aThe segmentation method of the amygdala, hippocampus, and thalamus is shown in T1-weighted MR images. In particular, the segmentation method used for the amygdala (developed by N.M.) is shown in detail in images J–P. Image A shows a sagittal slice passing through the hippocampus and amygdala. Yellow vertical lines show the locations of representative coronal sections J–Q. The blue box in A indicates the region containing amygdala (K–N), amygdala-hippocampal transition, anterior hippocampus (L), and the temporal-polar region rostral to the amygdala (J). B shows an axial section (the position of which is indicated by arrow in A) used for the tracing of an outline to separate the most anterior portion of the amygdala from the surrounding medial temporal cortex (red line). C shows an enlarged version of the blue box in A to emphasize the amygdala-hippocampal transition. In both B and C, the light green line represents the border between amygdala and hippocampus. In C, the red line shows the superior, anterior, and inferior limits of the amygdala. These outlines are used as guidelines to complete segmentation on the coronal plane (D–I). Similarly, the thalamus is segmented based on the intensity contrast between this structure and its surrounding white matter; a border delimits its inferior border at the hypothalamic fissure. Structural details from coronal slices J–O are enlarged to show the method of amygdala segmentation. J shows a rostral slice in the temporal lobe where the amygdala is not present. More caudally, the anterior part of the amygdala appears within the rostromedial temporal area beneath the cortex as shown in K. Further posteriorly, the middle portion of the amygdala occupies a position superior and lateral to the anterior parahippocampal gyrus as shown in L. Progressing in the rostrocaudal dimension, the hippocampus appears as shown in M and N, in which the amygdala is also present. In a more posterior location (O), there is only hippocampus as amygdala is no longer present. In P, the posterior most segment of the hippocampus is shown flanked laterally by the atrium of the lateral ventricle. Finally, Q is the coronal posterior to the hippocampus. To emphasize the amygdala and hippocampal outlines, the segmentation outlines of the other brain structures were omitted from images J–Q.
1. Wozniak J, Biederman J, Kiely K, Ablon JS, Faraone SV, Mundy E, Mennin D: Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995; 34:867–876Crossref, Medline, Google Scholar
2. Geller B, Luby J: Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 1997; 36:1168–1176Crossref, Medline, Google Scholar
3. Geller B, Craney JL, Bolhofner K, DelBello MP, Williams M, Zimerman B: One-year recovery and relapse rates of children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry 2001; 158:303–305Link, Google Scholar
4. Lewinsohn PM, Klein DN, Seeley JR: Bipolar disorders in a community sample of older adolescents: prevalence, phenomenology, comorbidity, and course. J Am Acad Child Adolesc Psychiatry 1995; 34:454–463Crossref, Medline, Google Scholar
5. Johnson L, Andersson-Lundman G, Åberg-Wistedt A, Mathé AA: Age of onset in affective disorder: its correlation with hereditary and psychosocial factors. J Affect Disord 2000; 59:139–148Crossref, Medline, Google Scholar
6. Goldstein JM, Seidman LJ, O’Brien LM, Horton NJ, Kennedy DN, Makris N, Caviness VS Jr, Faraone SV, Tsuang MT: Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch Gen Psychiatry 2002; 59:154–164Crossref, Medline, Google Scholar
7. Reiter EO, Fuldauer VG, Root AW: Secretion of the adrenal androgen, dehydroepiandrosterone sulfate, during normal infancy, childhood, and adolescence, in sick infants, and in children with endocrinologic abnormalities. J Pediatr 1977; 90:766–770Crossref, Medline, Google Scholar
8. Soares JC, Mann JJ: The anatomy of mood disorders: review of structural neuroimaging studies. Biol Psychiatry 1997; 41:86–106Crossref, Medline, Google Scholar
9. Phillips ML, Drevets WC, Rauch SL, Lane R: The neurobiology of emotion perception, I: the neural basis of normal emotion perception. Biol Psychiatry 2003; 54:504–514Crossref, Medline, Google Scholar
10. Phillips ML, Drevets WC, Rauch SL, Lane RD: The neurobiology of emotion perception, II: implications for major psychiatric disorders. Biol Psychiatry 2003; 54:515–528Crossref, Medline, Google Scholar
11. Packard MG, Cahill L: Affective modulation of multiple memory systems. Curr Opin Neurobiol 2001; 11:752–756Crossref, Medline, Google Scholar
12. Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, Wilkins J, Gerner R, Mintz J: An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry 2000; 48:147–162Crossref, Medline, Google Scholar
13. Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER: Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry 1999; 56:254–260Crossref, Medline, Google Scholar
14. Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, Federman EB, Chase GA, Petty RG, Tien AY: Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry 1997; 41:1–14Crossref, Medline, Google Scholar
15. Dupont RM, Jernigan TL, Heindel W, Butters N, Shafer K, Wilson T, Hesselink J, Gillin JC: Magnetic resonance imaging and mood disorders: localization of white matter and other subcortical abnormalities. Arch Gen Psychiatry 1995; 52:747–755Crossref, Medline, Google Scholar
16. Swayze VW II, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC: Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry 1992; 31:221–240Crossref, Medline, Google Scholar
17. Noga JT, Vladar K, Torrey EF: A volumetric magnetic resonance imaging study of monozygotic twins discordant for bipolar disorder. Psychiatry Res 2001; 106:25–34Crossref, Medline, Google Scholar
18. Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, Charney DS, Krystal JH, Peterson BS: Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry 2003; 60:1201–1208Crossref, Medline, Google Scholar
19. Botteron KN, Vannier MW, Geller B, Todd RD, Lee BC: Preliminary study of magnetic resonance imaging characteristics in 8- to 16-year-olds with mania. J Am Acad Child Adolesc Psychiatry 1995; 34:742–749Crossref, Medline, Google Scholar
20. Friedman L, Findling RL, Kenny JT, Swales TP, Stuve TA, Jesberger JA, Lewin JS, Schulz SC: An MRI study of adolescent patients with either schizophrenia or bipolar disorder as compared to healthy control subjects. Biol Psychiatry 1999; 46:78–88Crossref, Medline, Google Scholar
21. Dasari M, Friedman L, Jesberger J, Stuve TA, Findling RL, Swales TP, Schulz SC: A magnetic resonance imaging study of thalamic area in adolescent patients with either schizophrenia or bipolar disorder as compared to healthy controls. Psychiatry Res 1999; 91:155–162Crossref, Medline, Google Scholar
22. DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM: Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord 2004; 6:43–52Crossref, Medline, Google Scholar
23. Orvaschel H, Puig-Antich J: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (K-SADS-E), 4th revision. Fort Lauderdale, Fla, Nova University, Center for Psychological Studies, 1987Google Scholar
24. Tanner JM: Growth at Adolescence. Oxford, UK, Blackwell Scientific Publications, 1962Google Scholar
25. Wechsler D: Manual for the Wechsler Intelligence Scale for Children. San Antonio, Tex, Psychological Corp (Harcourt), 1991Google Scholar
26. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97–113Crossref, Medline, Google Scholar
27. Young RC, Biggs JT, Ziegler VE, Meyer DA: A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
28. Woods SW: Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003; 64:663–667Crossref, Medline, Google Scholar
29. Stoll AL: The Psychopharmacology Reference Card, 2001Google Scholar
30. Caviness VS Jr, Meyer J, Makris N, Kennedy DN: MRI-based topographic parcellation of human neocortex: an anatomically specified method with estimate reliability. J Cogn Neurosci 1996; 8:566–587Crossref, Medline, Google Scholar
31. Kennedy DN, Filipek PA, Caviness VS Jr: Autonomic segmentation and volumetric calculations in nuclear magnetic resonance imaging. IEEE Trans Med Imaging 1989; 8:1–7Crossref, Medline, Google Scholar
32. Worth AJ, Makris N, Caviness VS Jr, Kennedy DN: Neuroanatomical segmentation in MRI: technological objectives. Intern J Pattern Recognit Artif Intell 1997; 11:1161–1187Crossref, Google Scholar
33. Filipek PA, Richelme C, Kennedy DN, Caviness VS Jr: The young adult human brain: an MRI-based morphometric study. Cereb Cortex 1994; 4:344–360Crossref, Medline, Google Scholar
34. Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Matsuda G, Hoge EA, Kennedy D, Makris N, Caviness VS Jr, Tsuang MT: Reduced subcortical brain volumes in nonpsychotic siblings of schizophrenic patients: a pilot magnetic resonance imaging study. Am J Med Genet 1997; 74:507–514Crossref, Medline, Google Scholar
35. Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kreman WS, Toomey R, Tourville J, Kennedy D, Makris N, Caviness VS, Tsuang MT: Thalamic and amygdala-hippocampal volume reductions in first-degree relatives of patients with schizophrenia: an MRI-based morphometric analysis. Biol Psychiatry 1999; 46:941–954Crossref, Medline, Google Scholar
36. Seidman LJ, Faraone SV, Goldstein JM, Kreman WS, Horton NH, Makris N, Toomey R, Kennedy D, Caviness VS, Tsuang MT: Left hippocampal volume as a vulnerability indicator for schizophrenia. Arch Gen Psychiatry 2002; 59:839–849Crossref, Medline, Google Scholar
37. Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL: Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res 1997; 75:31–48Crossref, Medline, Google Scholar
38. Caviness VS Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA: The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex 1996; 6:726–736Crossref, Medline, Google Scholar
39. Lange N, Giedd JN, Castellanos FX, Vaituzis AC, Rapoport JL: Variability of human brain structure size: ages 4–20 years. Psychiatry Res 1997; 74:1–12Crossref, Medline, Google Scholar
40. Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H: Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry 2001; 40:1012–1020Crossref, Medline, Google Scholar
41. Hauser P, Matochik J, Altshuler LL, Denicoff KD, Conrad A, Li X, Post RM: MRI-based measurements of temporal lobe and ventricular structures in patients with bipolar I and bipolar II disorders. J Affect Disord 2000; 60:25–32Crossref, Medline, Google Scholar
42. Benes FM, Kwok EW, Vincent SL, Todtenkopf MS: A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry 1998; 44:88–97Crossref, Medline, Google Scholar
43. Fatemi SH, Earle JA, Stary JM, Lee S, Sedgewick J: Altered levels of the synaptosomal associated protein SNAP-25 in hippocampus of subjects with mood disorders and schizophrenia. Neuroreport 2001; 12:3257–3262Crossref, Medline, Google Scholar
44. Fatemi SH, Earle JA, McMenomy T: Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry 2000; 5:654–663Crossref, Medline, Google Scholar
45. Eastwood SL, Harrison PJ: Hippocampal synaptic pathology in schizophrenia, bipolar disorder and major depression: a study of complexin mRNAs. Mol Psychiatry 2000; 5:425–432Crossref, Medline, Google Scholar
46. Dowlatshahi D, MacQueen G, Wang JF, Chen B, Young LT: Increased hippocampal supragranular Timm staining in subjects with bipolar disorder. Neuroreport 2000; 11:3775–3778Crossref, Medline, Google Scholar
47. Law AJ, Deakin JF: Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport 2001; 12:2971–2974Crossref, Medline, Google Scholar
48. Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann JJ, Latov N, Hays AP, Dwork AJ: Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry 2000; 57:349–356Crossref, Medline, Google Scholar
49. Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy D, Caviness VS, Faraone S, Tsuang MT: Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex 2001; 11:490–497Crossref, Medline, Google Scholar
50. DelBello MP, Zimerman ME, Getz GE, Strakowski S: Structural MRI analysis of adolescents with bipolar disorder. Biol Psychiatry 2001; 49:20S-21SCrossref, Medline, Google Scholar
51. Kennedy DN, Lange N, Makris N, Bates J, Meyer J, Caviness VS Jr: Gyri of the human neocortex: an MRI-based analysis of volume and variance. Cereb Cortex 1998; 8:372–384Crossref, Medline, Google Scholar
52. Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK: Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet 2003; 361:281–288Crossref, Medline, Google Scholar
53. Sigurdsson E, Fombonne E, Sayal K, Checkley S: Neurodevelopmental antecedents of early-onset bipolar affective disorder. Br Med J 1999; 174:121–127Google Scholar
54. Hellgren L, Gillberg IC, Bagenholm A, Gillberg C: Children with deficits in attention, motor control and perception (DAMP) almost grown up: psychiatric and personality disorders at age 16 years. J Child Psychol Psychiatry 1993; 35:1255–1271Crossref, Google Scholar
55. Frazier JA, Biederman J, Tohen M, Feldman PD, Jacobs TG, Toma V, Rater MA, Tarazi RA, Kim GS, Garfield SB, Sohma M, Gonzalez-Heydrich J, Risser RC, Nowling ZM: A prospective open-label treatment trial of olanzapine monotherapy in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol 2001; 11:239–250Crossref, Medline, Google Scholar
56. Tsuang M: Schizophrenia: genes and environment. Biol Psychiatry 2000; 47:210–220Crossref, Medline, Google Scholar
57. Todd RD, Reich W, Petti TA, Joshi P, DePaulo JR Jr, Nurnberger J Jr, Reich T: Psychiatric diagnoses in the child and adolescent members of extended families identified through adult bipolar affective disorder probands. J Am Acad Child Adolesc Psychiatry 1996; 35:664–671Crossref, Medline, Google Scholar
58. Goldstein JM, Kennedy DN, Caviness VS Jr: Brain development, XI: sexual dimorphism (image, neuro). Am J Psychiatry 1999; 156:352Link, Google Scholar



