A Three-Dimensional Morphometric Study of Craniofacial Shape in Schizophrenia
Abstract
Background: Subtle dysmorphogenesis of the craniofacial region constitutes important corroborating evidence of the neurodevelopmental origins of schizophrenia. Advances in facial visualization now allow for three-dimensional anthropometric evaluations of potentially greater discriminatory power in examining the complex geometric relationships of facial topography. METHOD: Sixty-five anthropometrically derived landmarks were identified from three-dimensional facial images collected from 14 patients with schizophrenia and 11 comparison subjects, imaged with a high-resolution, portable laser scanner. RESULTS: Using the Procrustes morphometric approach for shape analysis, the difference in mean shapes was highly significant, with patients exhibiting superoinferior elongation of the face. CONCLUSIONS: The topography of craniofacial anomalies in schizophrenia is not random and points to midline deformation.
The extent and timing of neurodevelopmental (aberrant) processes in schizophrenia is an important and enduring theme in schizophrenia research (1). Minor physical anomalies, best illustrated in unequivocal neurodevelopmental disorders such as Down’s syndrome, are seemingly inconsequential minor deviances of bodily shape and form. There is an enduring literature indicating that developmental and physical anomalies occur more frequently in patients with schizophrenia (2). Classical and manualized anthropometric approaches, while labor intensive and limited to two-dimensional measurements, have indicated that minor physical anomalies are often concentrated in the craniofacial region in schizophrenia (3, 4).
More technical approaches, incorporating three-dimensional visualization, can be usefully applied to further discriminate these subtle distortions in facial topography. Using three-dimensional laser holography, Hennessy and colleagues found that normal female subjects differed from male subjects in craniofacial shape (5).
Method
Participants from Northcoast Behavioral Healthcare, Northfield, Ohio, gave written informed consent (following Institutional Review Board and Northcoast Behavioral Healthcare’s research committee approval) to have facial pictures obtained from a three-dimensional camera (a VIVID 700 camera, effective image pixel size of 1 mm [Minolta Instrument Systems Division, Ramsey, N.J.]). The study group comprised 14 patients (10 men and four women; mean age=39 years, range=22–53; mean height=70.2 inches) with DSM-IV schizophrenia (N=11) or schizoaffective disorder (N=3) determined by interview and record review who had no significant medical or neurological illnesses. Eleven healthy subjects were recruited from hospital staff (seven men and four women; mean age=42 years, range=19–53; mean height=67.7 inches). Six views were taken: right and left oblique posterior to the ears, right and left oblique anterior to the ears, full frontal, and inferior frontal. The scan was then analyzed internally by the camera and later used to construct a polygonal surface mesh to which color was applied from a simultaneously captured jpeg image. Sixty-five landmarks, localized in our laboratory’s own software interface (developed in the OpenInventor environment of Silicon Graphics, Inc., Mountain View, Calif.), were collected using a protocol derived from classical anthropometrics (6). A single operator collected all landmarks three times, with excellent intraoperator reliability (centroid measurement was about 320 VIVID units, with between-session variation from this centroid for all landmarks of 0.06 to 0.25 units). A pictorial guide and key to these facial landmarks are provided in Figure 1. .
Results
Configurations of 65 landmark locations were analyzed using geometric morphometrics methods as previously published in a neuroimaging study (7). The sample of shapes was averaged by Procrustes method (8), with each specimen represented by a complete set of three-dimensional landmark-by-landmark deviations from the average shape, yielding Procrustes distance (i.e., shape distance) residuals. Significance tests evaluated between-group to within-group squared Procrustes distances by permutation tests. The difference in mean shapes was statistically significant (p=0.003), based upon a permutation test on net Procrustes variance (i.e., 65 three-dimensional landmarks, each weighted inversely by its own variation). The greatest deformation was observed in the sagittal plane as vertical (i.e., superoinferior) elongation of the face in patients relative to comparison subjects. There was also a tendency for patients to have shallower (i.e., shorter anteroposteriorly in the coronal plane) faces, but this finding did not attain statistical significance.
Discussion
The main finding from this preliminary study of morphometric evaluation of craniofacial shape in schizophrenia is of statistically significant and pronounced midline craniofacial elongation in patients with schizophrenia relative to healthy subjects. It has been suggested, in accordance with the normal developmental growth trajectory for frontal brain and facial regions, that facial dysmorphogenesis in schizophrenia may occur in concert with in utero cerebral dysmaturation (9). This study complements other more classical anthropometric approaches to evaluating craniofacial anomalies. The localized shape deformation in the midline is of interest in the context of an emergent literature on the topography of craniofacial anomalies in schizophrenia (3, 4, 9).
It would appear, with increasing sophistication of measurement evolving beyond the Waldrop Scale, that craniofacial anomalies observed in patients with schizophrenia are not random. In the extensive topographic analysis by Lane and colleagues (3), abnormalities were largely confined to the anterior midfacial region. In an Australian study (4), patients exhibited lower facial heights, shortened/widened palates, and wider skull bases. The findings of the present study are consistent with these two detailed, anthropometrically based studies. McGrath and colleagues (4) also noted shape differences in the skulls (more brachycephalic in patients with schizophrenia). We are not able to address skull findings in this study, since the images acquired here are confined to the facial region alone. However, our previous work (using classical anthropometric techniques) failed to reveal skull shape or size differences between patients and comparison subjects (10). We were unable to replicate or address the important issue of gender-specific asymmetries described in another recent morphometrics study of craniofacial dysmorphology (11); our study group size here was modest and not balanced for gender.
The present study demonstrates the utility of laser range-derived landmark morphometrics in evaluating craniofacial anomalies in schizophrenia, indicating that minor physical anomalies, while subtle in appearance, are not of indiscriminate distribution.
Presented in part at the ninth International Congress on Schizophrenia Research, Colorado Springs, March 29–April 2, 2003. Received Feb. 2, 2004; revision received May 19, 2004; accepted May 28, 2004. From the Department of Psychiatry and Health Behavior, Medical College of Georgia; the University Hospitals of Cleveland Research Institute and Department of Neurological Surgery, Cleveland; the Department of Anatomy, Case Western Reserve University, Cleveland; the Institute of Gerontology, University of Michigan, Ann Arbor; the Department of Anatomy, Catholic University of Korea, Seoul; and Northcoast Behavioral Healthcare, Ohio Department of Mental Health, Northfield. Address correspondence and reprint requests to Dr. Buckley, Professor and Chairman, Department of Psychiatry and Health Behavior, Medical College of Georgia, 1515 Pope Ave., Augusta, GA 30912–3800; [email protected] (e-mail). Supported by grants from the Korean Science and Engineering Foundation; the foundation of the Department of Neurological Surgery, Case Western Reserve University/University Hospitals of Cleveland; and the Stanley Foundation.
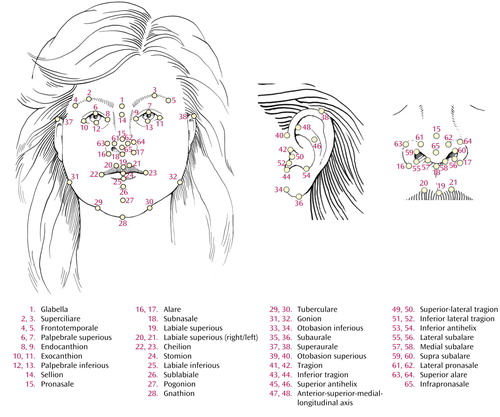
Figure 1. Soft Tissue Facial Landmarks (N=65) Derived From Three-Dimensional Facial Images of 14 Schizophrenia Patients and 11 Healthy Comparison Subjectsa
aFrontal view, ear inset, and nose inset, respectively, are shown. Illustration by Kusawa, 2003.
1. Miyamoto S, LaMantia AS, Duncan GE, Sullivan P, Gilmore JH, Lieberman JA: Recent advances in the neurobiology of schizophrenia. Mol Interv 2003; 3:27–39Crossref, Medline, Google Scholar
2. Nopoulos P, Flaum M, Arndt S, Andreasen NC: Morphometry in schizophrenia revisited: height and body and its relationship in premorbid function. Psychol Med 1998; 28:655–663; correction, 1998; 28:1475Google Scholar
3. Lane A, Kinsella A, Murphy P, Byrne M, Keenan J, Colgan K, Cassidy B, Sheppard N, Horgan R, Waddington JL, Larkin C, O’Callaghan E: The anthropometric assessment of dysmorphic features in schizophrenia as an index of its developmental origins. Psychol Med 1997; 27:1155–1164Crossref, Medline, Google Scholar
4. McGrath J, El-Saadi O, Grim V, Cardy S, Chapple B, Chant D, Lieberman D, Mowry B: Minor physical anomalies and quantitative measures of the head and face in patients with psychosis. Arch Gen Psychiatry 2002; 59:458–464Crossref, Medline, Google Scholar
5. Hennessy RJ, Kinsella A, Waddington JL: 3D laser surface scanning and geometric morphometric analysis of craniofacial shape as an index of cerebro-craniofacial morphogenesis: initial application to sexual dimorphism. Biol Psychiatry 2002; 51:507–514Crossref, Medline, Google Scholar
6. Farkas LG: Anthropometry of the Human Face, 2nd ed. New York, Raven Press, 1994Google Scholar
7. Buckley PF, Dean D, Bookstein FL, Friedman L, Kwon D, Lewin JS, Kamath J, Lys C: Three-dimensional magnetic resonance-based morphometrics and ventricular dysmorphology in schizophrenia. Biol Psychiatry 1999; 45:62–67Crossref, Medline, Google Scholar
8. Rohlf FJ, Slice D: Extensions of the Procrustes method for the optimal super position of landmarks. Syst Zool 1990; 39:40–59Crossref, Google Scholar
9. Waddington JL, Lane A, Larkin C, O’Callaghan E: The neurodevelopmental basis of schizophrenia: clinical clues from cerebro-craniofacial dysmorphogenesis and the roots of a lifetime trajectory of disease. Biol Psychiatry 1999; 46:31–39Crossref, Medline, Google Scholar
10. Buckley PF, Friedman LF, Jesberger JA, Schulz SC, Jaskiw G: Head size and schizophrenia. Schizophr Res 2002; 55:99–104Crossref, Medline, Google Scholar
11. Hennessy RJ, Lane A, Kinsella A, Larkin C, O’Callaghan E, Waddington JL: 3D morphometrics of craniofacial dysmorphology reveals sex-specific asymmetries in schizophrenia. Schizophr Res 2004; 67:261–268Crossref, Medline, Google Scholar



