Osteoporosis in Patients With Schizophrenia
Abstract
OBJECTIVE: Osteoporosis is regularly mentioned as a possible consequence of treatment with prolactin-increasing antipsychotic medications, but little is known about the prevalence and the degree of loss of bone mineral density in patients suffering from schizophrenia. The authors’ goals were to investigate the association between schizophrenia and a decrease in bone mineral density and to get more insight into potential underlying pathophysiological mechanisms. METHOD: In a cross-sectional study, the authors used dual x-ray absorptiometry to determine bone mineral density of 75 inpatients and outpatients suffering from schizophrenia. All patients had been treated with antipsychotics for at least 1 year, and only patients between the ages of 19 and 50 were studied to exclude patients with age-related idiopathic osteoporosis. RESULTS: In men but not women with schizophrenia, bone mineral density was significantly lower than normal in the lumbar region. A comparison of loss of bone mineral density in male and female patients showed significant differences between the sexes. Bone mineral density showed a negative correlation with negative symptoms and Positive and Negative Syndrome Scale total score and a positive correlation with 25-hydroxy-vitamin D3 levels and body mass index in male patients. In female patients, a positive correlation between body mass index and bone mineral density was found. Exposure to prolactin-increasing antipsychotics was not related to bone mineral density. CONCLUSIONS: The male patients with schizophrenia in this study suffered from low bone density. This finding as well as other reports lend support to directing more attention to bone metabolism in patients with schizophrenia, although there is no universally accepted screening policy to identify individuals at high risk for osteoporosis.
Despite the fact that treatment with prolactin-increasing antipsychotics is regularly mentioned as a risk factor for osteoporosis, little is known about the prevalence and the degree of loss of bone mineral density in patients suffering from schizophrenia (1, 2). Bone, both of cortical and trabecular structure, undergoes continuous changes throughout life, called bone remodeling (3). This regulatory process of remodeling can be influenced in many ways, and one of the pathological consequences is osteoporosis, defined as an absolute decrease in total bone mass. Osteoporosis is caused either by a greater frequency or duration of activity of osteoclasts, by a decrease in activity or duration of the action of osteoblasts, or a combination of both (4). Osteoporosis is a disorder that increases the susceptibility to fractures (5).
The most common primary forms of bone loss are postmenopausal osteoporosis and age-related osteoporosis (6). A main cause of secondary osteoporosis is drug-induced bone loss.
In patients suffering from schizophrenia, antipsychotic-induced elevations of prolactin levels, caused by a blockade of D2 receptors in the hypothalamic-pituitary axis, may be associated with hypogonadism in both men and women. In women, a chronic elevation of prolactin levels induces inhibition of the hypothalamic secretion of luteinizing hormone-releasing hormone. This, in turn, lowers levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which regulate gonadal steroid production and release (7). The resulting estrogen deficiency may reduce bone density in women (8). Testosterone deficiency has been shown to be associated with osteopenia (9), and a decrease of estrogen can influence the activity of interleukins, which are known to influence bone dynamic homeostasis (9). High levels of interleukin 2 have been found in neuroleptic-free patients with schizophrenia (10). Other risk factors that might contribute to the development of osteoporosis are alcohol and drug abuse and heavy smoking, often observed in patients with schizophrenia (11–13). Immobility, dietary deficiency, and a reduced exposure to sunshine resulting in vitamin D deficiency (9) have also been thought to contribute to the risk of osteoporosis.
A cumulative effect of several disease- and medication-related processes renders a higher risk of osteoporosis plausible in patients with schizophrenia. Therefore, we conducted a cross-sectional study to evaluate the association between schizophrenia and a decrease in bone mineral density and to get more insight into potential underlying pathophysiological mechanisms.
Method
In a cross-sectional study, we investigated 75 inpatients and outpatients suffering from schizophrenia diagnosed according to ICD-10 (14). All patients had been treated with antipsychotics for at least 1 year. To exclude patients with age-related idiopathic osteoporosis, we studied patients who were between 19 and 50 years of age. Furthermore, we excluded patients suffering from alcohol abuse, nutritional impairment, or comorbid somatic diseases, as well as patients treated with comedications known to have an influence on bone mineral density (e.g., glucocorticoids, heparin, anticonvulsant agents, oral contraceptives) and bedridden patients or patients confined to a wheelchair. After a thorough description of the study to the patients, written informed consent was obtained.
We determined bone mineral density by dual x-ray absorptiometry with a QDR*4500-Hologic densitometer (Hologic, Inc., Bedford, Mass.) in the lumbar spine (L1–L4) and the proximal right femur (femoral neck, trochanter, intertrochanteric region, Ward’s area). According to World Health Organization guidelines (15), osteopenia was defined as bone mineral density more than one standard deviation below the young adult mean but less than 2.5 standard deviations below this value (T score <–1 and >–2.5), and osteoporosis was defined as bone mineral density 2.5 standard deviations or more below the young adult mean (T score ≤–2.5 ).
All patients had blood drawn for the analysis of hormones, vitamins, and other factors known to play an important role in the regulatory process of bone remodeling: estrogen, testosterone, dehydroepiandrosterone, LH, FSH, prolactin, 25-hydroxy-vitamin D3, parathyroid hormone, osteocalcin, crosslaps, cortisol, thyrotropin, creatinine, calcium, phosphorus, serum glutamic-pyruvic transaminase, serum glutamic-oxaloacetic transaminase, γ-glutamyl transpeptidase, alkaline phosphatase, and blood count.
We also used the Positive and Negative Syndrome Scale (16) to calculate the influence of psychopathological symptoms on bone mineral density. A semistructured clinical interview was conducted to evaluate smoking behavior (packs per year). Information about the duration of treatment with antipsychotics was obtained from patients’ hospital records. Flupentixol, fluphenazine, haloperidol, amisulpride, sulpiride, and risperidone were defined as prolactin-increasing antipsychotics. Clozapine, olanzapine, quetiapine, sertindole, and zotepine were defined as non-prolactin-increasing antipsychotics.
Body composition was determined by impedance analysis with a multifrequency bioelectric impedance 2000-M analyzer (Data Input, Hofheim, Germany).
The study conformed to the Declaration of Helsinki and was approved by the Ethics Committee of the Medical Faculty of Innsbruck University.
We investigated the following main hypotheses: 1) Patients suffering from schizophrenia have a decrease in bone mineral density compared with an age- and sex-matched normal population. 2) Reductions of bone mineral density are comparable in men and women. 3) There is a relationship between the duration of treatment with prolactin-increasing antipsychotics and bone mineral density.
Statistical Analysis
Bone mineral density of individual patients was compared with a normative curve and computed as a z score (difference from the mean, divided by the standard deviation) to enable comparisons of values across age and sex. Comparison of the patients’ z scores with normative values (z score=0) (hypothesis 1) as well as comparisons between male and female patients (hypothesis 2) were performed by using both multivariate (MANOVA) and univariate (ANOVA) analysis of variance. The same procedure was applied to compare patients treated with prolactin-increasing antipsychotics and those treated with other antipsychotics (hypothesis 3). To investigate the potential effect of prolactin-increasing antipsychotics on bone mineral density, patients were divided into two subgroups according to their total duration of treatment with one or several prolactin-increasing antipsychotics: less than 6 months (including 0) versus at least 6 months. This analysis was supplemented by a correlation analysis (Spearman rank correlation) to take the duration of treatment into account. In addition to the three hypotheses stated above, the associations of clinical variables (Positive and Negative Syndrome Scale score, body mass index) and biochemical measures with bone mineral density were evaluated in an exploratory fashion by using Spearman rank correlation coefficients.
Results
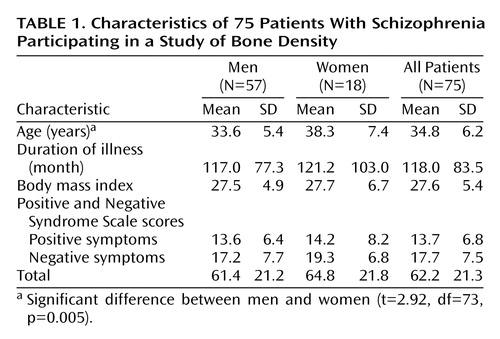
Demographic characteristics of the study group are shown in Table 1. Fifty-two (69.3%) of the patients were smokers. On the day of investigation, 45 (78.9%) of the 57 men and 13 (72.2%) of the 18 women were being treated with non-prolactin-increasing antipsychotics, and 10 (17.5%) of the men and four (22.2%) of the women were being treated with prolactin-increasing antipsychotics. Treatment for two men and one woman included a prolactin-increasing as well as a non-prolactin-increasing antipsychotic at the same time.
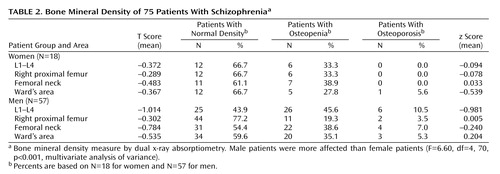
Table 2 shows the bone mineral density of the male and female patients: 33.3% of the women suffered from osteopenia in the lumbar region and in the right proximal femur. In the lumbar region, osteopenia was found in 45.6% and osteoporosis in 10.5% of the men. In the right proximal femur, osteopenia was found in 19.3% and osteoporosis in 3.5% of the men. MANOVA revealed that, overall, bone mineral density (z scores) differed significantly from normal values, i.e., from z=0, in the male patients (F=34.31, df=4, 53, p<0.001). Subsequent univariate ANOVAs showed that bone mineral density in men, but not women, was significantly lower than age- and sex-matched normal data (z scores) in the lumbar region (p<0.001). This phenomenon was not found in the femur of either male or female patients.
A comparison of loss of bone mineral density (z scores) in male and female patients showed significant differences between the sexes (Table 2). Subsequent ANOVAs revealed that male patients were more severely affected than female patients in the lumbar region (p<0.05) (Table 2). Again, these differences were not found in the femur.
MANOVAs found no significant difference in bone mineral density between the men or women who had received prolactin-increasing antipsychotics for less than 6 months or for 6 months or longer (for men, F=0.99, df=4, 48, p=0.42; for women, F=0.60, df=4, 13, p=0.67). In particular, reduced bone mineral density values in the lumbar region were found both in the men treated with prolactin-increasing antipsychotics for less than 6 months (z=–0.77, SD=1.26, N=15) and for at least 6 months (z=–1.04, SD=1.12, N=38); there were no significant differences between the groups (t=0.76, df=51, p=0.45). This finding did not depend on the particular choice of the cutoff value for treatment duration. Calculations of the relationship between duration of treatment with prolactin-increasing antipsychotics and bone mineral density by correlation analysis yielded similar results. Although for one of the individual regions, L2, the correlation approached significance in male patients (rs=–0.25, N=57, p<0.08) (Figure 1), this finding was apparently mainly due to one extreme duration-of-treatment outlier (14.5 years). After exclusion of this patient from the analysis the statistical trend disappeared. One male patient suffered from osteoporosis and three from osteopenia despite the fact that they had never been treated with prolactin-increasing antipsychotics.
Negative symptoms (Positive and Negative Syndrome Scale) showed a significant correlation with bone mineral density of the lumbar region (rs=–0.29, N=75, p<0.04) and the femoral neck (rs=–0.29, N=75, p<0.03) (z scores). Furthermore, Positive and Negative Syndrome Scale total score was correlated with bone mineral density of the right proximal femur (rs=–0.31, N=57, p<0.02) in male patients (z scores). When investigating the correlation between the depression factor of the Positive and Negative Syndrome Scale (following the five-factor model [17]) and bone mineral density, we found significant results for the femoral neck (rs=–0.49, N=75, p<0.001), Ward’s area (rs=–0.34, N=75, p<0.02), and the right proximal femur (rs=–0.37, N=75, p=0.006).
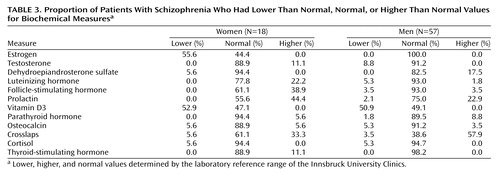
Biochemical measures are shown in Table 3. 25-Hydroxy-vitamin D3 deficiency was found in 50.9% of male and in 52.9% of female patients. When calculating the relationship between bone mineral density and serum levels of 25-hydroxy-vitamin D3, a positive correlation was found for the z scores of the femur (rs=0.26, N=57, p<0.05) and the trochanteric region (rs=0.30, N=57, p<0.03) in male patients.
A body mass index greater than 25 kg/m was found in 23 (42.1%) of the male patients and five (27.8%) of the female patients. A body mass index greater than 30 kg/m2 was found in 15 (26.3%) of the men and six (33.3%) of the women. Positive correlations (p<0.05) between body mass index and bone mineral density of the lumbar region (z scores) were found in both male (rs=0.3, N=57) and female (rs=0.46, N=18) patients. In addition, z scores of the femoral neck and body mass index were positively correlated in patients of both sexes (male: rs=0.59, N=57, p<0.01; female: rs=0.57, N=18, p<0.05). This relationship was approximately linear in the range of the body mass index values covered: neither scatterplots nor confirmatory statistics (test for significance of the quadratic term) gave an indication for a nonlinear relationship.
Nicotine consumption, duration of illness, plasma levels of hormones (gonadal, thyroid, cortisol, and prolactin levels) (Table 3), and liver and kidney function had no influence on bone mineral density.
Discussion
Our data demonstrate that even young patients with schizophrenia suffer from low bone mineral density, as indicated by Halbreich et al. (1) in a smaller group of patients. Also in line with this report, male patients were more severely affected than female patients. A similar finding has been reported in patients suffering from major depression (18).
Bergemann et al. (19) investigated women with schizophrenia and found a high bone turnover but normal bone mineral density. One explanation for these sex differences may be that the beginning of the disorder in male patients is about 5 years earlier than in female patients (20). Therefore, potential illness-related factors contributing to a loss of bone mineral density have had longer lasting impact in male patients. On the other hand, at least in our study, duration of illness had no influence on bone mineral density. An alternative explanation could be that women with schizophrenia take better care of themselves with regard to adequate nutrition and exercise than men and therefore suffer less from osteoporosis. To our knowledge, this has not yet been explored.
We were not able to confirm the hypothesis that treatment with prolactin-increasing antipsychotics enhances the risk for osteoporosis. The trend we found in the direction of confirming this hypothesis has to be interpreted with caution. It disappeared when one patient who was an extreme outlier concerning duration of treatment was excluded from the analysis. In addition, this weak association was found only in the lumbar region L2 of male patients. It is also important to note that several patients suffered from osteopenia or osteoporosis despite the fact that they had never been treated with prolactin-increasing antipsychotics.
In contrast to our findings, Abraham et al. (2) reported an inverse relationship between prolactin level and bone mass in 16 patients (seven receiving typical antipsychotics, three risperidone, and six clozapine). Subjects had been receiving their respective antipsychotic for at least 6 months. In a cross-sectional study, Becker et al. (21) investigated 26 premenopausal female patients with schizophrenia who had been treated with either risperidone (N=12) or olanzapine (N=14) for at least 2 years. Whereas bone mineral density was similar in both groups, bone speed was found to be lower in the risperidone-treated group than the olanzapine-treated group, indicating that the relative risk for fragility fracture of women treated with risperidone might be higher.
We found an influence of body mass index and psychopathology on bone mineral density. The relationship between body mass index and bone mineral density is a well-known phenomenon; for instance, people suffering from nutritional impairment may show hypogonadism (22). The influence of psychopathology on bone mineral density might be a consequence of the behavior of patients caused by negative, depressive, and positive symptoms. Patients with negative and depressive symptoms are likely to be physically inactive and show less tendency to go outside, which could in turn lead to lower 25-hydroxy-vitamin D3 levels. Both types of behavior, therefore, could have a negative impact on bone mineral density. We are not able to differentiate whether the high negative symptom score is due to primary or secondary symptoms, the latter potentially related to extrapyramidal motor side effects. In both instances one would expect inactivity, including staying indoors. Positive symptoms such as paranoid delusions, on the other hand, can lead to an erratic intake of food, leading to nutritional deficits.
We do not have any information about bone mineral density in unmedicated patients with schizophrenia. Many lifestyle factors found in patients suffering from schizophrenia are known to contribute to a loss of bone mineral density. Therefore, it is difficult to tease out the significance of single variables, such as an increase of prolactin levels, on the loss of bone mineral density in patients with schizophrenia.
A number of limitations need to be considered when interpreting the findings of our study. First, the number of patients studied, although larger than in comparable studies, is modest, especially with respect to female patients. Nevertheless, the signal, especially in men with schizophrenia, is strong. Second, female sexual hormones can be reliable judged only when blood sample collection is corrected to phase of menstrual cycle. Finally, our results need to be confirmed in a prospective longitudinal setting, ideally involving first-episode patients.
A large number of patients showed a vitamin D deficiency. In man, the supply of vitamin D is mainly secured by the formation of cholecalciferol (vitamin D3) in the skin through the action of ultraviolet light. A small fraction is obtained from the diet. Therefore, malnutrition, a reduced duration of exposure to sunlight, or both have to be discussed as possible mechanisms responsible for a vitamin D3 deficiency in patients with schizophrenia (23, 24).
Vitamin D therapy is recommended clinical practice in patients suffering from a decrease of bone mineral density (25, 26). Whether a prophylactic addition of vitamin D to the treatment of patients with schizophrenia who suffer from vitamin D deficiency would avoid loss of bone mineral density, as shown in subjects without schizophrenia (27, 28), has to be the subject of further studies. Moreover, our finding of a relationship between low vitamin D levels and reduced bone mineral density resulted from an exploratory analysis and therefore needs to be replicated with a priori hypothesis testing.
The question arises of the clinical significance of the high rate of bone mineral density aberrations found here. As Kanis (22) stated, the clinical significance of osteoporosis is driven by the fractures that occur as a consequence of the condition. The ability to predict hip fractures by the measurement of bone mineral density is at least as good as the predictive power of blood pressure readings for stroke and may be even better than predicting coronary artery disease by measuring serum cholesterol (29–32). The predictive value of bone mineral density can be enhanced by including other factors in the risk equation. Biochemical factors (33, 34) as well as clinical variables enhance the risk for osteoporotic fractures. Many of these risks are commonly found in patients with schizophrenia. They include cigarette smoking, excessive alcohol consumption, vitamin D deficiency, immobility, and low levels of gonadal hormones (35). In this context, we have found a considerable percentage of patients with estrogen and vitamin D3 deficiency. Since we did not control for phase of the menstrual cycle when drawing blood, estrogen levels have to be interpreted with caution.
In summary, patients with schizophrenia suffer from low bone density. Despite the fact that there is no universally accepted screening policy to identify individuals at high risk, this study and other reports lend support to directing more attention to bone metabolism in schizophrenia patients. In addition, a number of different factors enhancing the risk for osteoporotic fractures are found in patients with schizophrenia. Additional studies are needed to investigate the individual contribution of these variables to the decrease of bone mineral density and the actual incidence of osteoporosis-induced fractures in patients with schizophrenia.
Preventive measures should be part of any treatment of patients with schizophrenia. These include information about a balanced diet with sufficient amounts of calcium and vitamin D; motivation to regular weight-bearing exercise (walking, biking, etc.); avoidance of tobacco, caffeine, and alcohol; and sufficient exposure to sunlight. Once osteoporosis is diagnosed, treatment should be initiated in close cooperation with endocrinologists. Clearly, the efficacy of preventive measures and treatment strategies to avoid or treat osteoporosis in patients with schizophrenia has to be the subject of further studies.
 |
 |
 |
Received March 10, 2003; revision received Sept. 3, 2003; accepted Jan. 30, 2004. From the Department of Biological Psychiatry, Department of Internal Medicine, and Department of Nuclear Medicine, Innsbruck University Hospital. Address correspondence and reprint requests to Dr. Hummer, Department of Biological Psychiatry, Innsbruck University Hospital, Anichstrasse 35, A-6020 Innsbruck, Austria; [email protected] (e-mail).
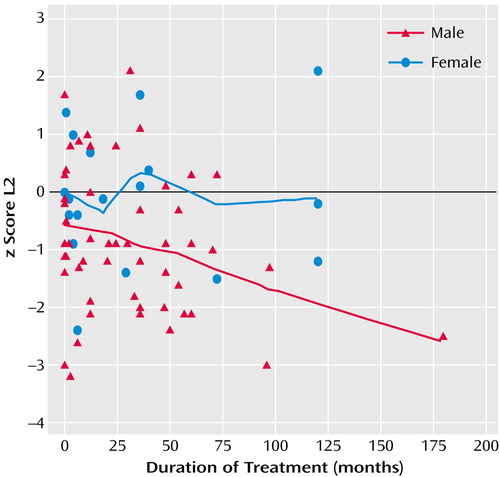
Figure 1. Relationship in 75 Patients With Schizophrenia Between Bone Mineral Density in Lumbar Region L2 and Duration of Treatment With Prolactin-Level-Increasing Antipsychotic Medicationsa
aSignificant correlation among men (rs=–0.25, N=57, p<0.08) but not among women.
1. Halbreich U, Rojansky N, Palter S, Hreshchyshyn M, Kreeger J, Bakhai Y, Rosan R: Decreased bone mineral density in medicated psychiatric patients. Psychosom Med 1995; 57:485–491Crossref, Medline, Google Scholar
2. Abraham G, Halbreich U, Friedman RH, Josiassen RC: Bone mineral density and prolactin associations in patients with chronic schizophrenia. Schizophr Res 2003; 59:17–18Crossref, Medline, Google Scholar
3. Parfitt AM: Quantum concept of bone remodeling and turnover: implications for the pathogenesis of osteoporosis. Calcif Tissue Int 1979; 28:1–5Crossref, Medline, Google Scholar
4. Wolinsky-Friedland M: Drug-induced metabolic bone disease. Endocrinol Metab Clin North Am 1995; 24:395–420Crossref, Medline, Google Scholar
5. Johnston CC, Melton LJ, Lindsdy R: Clinical indications for bone mass measurements: report of the Scientific Advisory Committee of the National Osteoporosis Foundation. J Bone Miner Res 1989; 4(suppl 2):1–28Google Scholar
6. Glaser DL, Kaplan FS: Osteoporosis: definition and clinical presentation. Spine 1997; 22(24 suppl):12S-16SGoogle Scholar
7. Halbreich U, Kinon BJ, Gilmore JA, Kahn LS: Elevated prolactin levels in patients with schizophrenia: mechanisms and related adverse effects. Psychoneuroendocrinology 2003; 28:53–67Crossref, Medline, Google Scholar
8. Klibanski A, Neer RM, Beitins IZ, Ridgway EC, Zervas NT, McArthur JW: Decreased bone density in hyperprolactinemic women. N Engl J Med 1980; 303:1511–1514Crossref, Medline, Google Scholar
9. Halbreich U, Palter S: Accelerated osteoporosis in psychiatric patients: possible pathophysiological processes. Schizophr Bull 1996; 22:447–454Crossref, Medline, Google Scholar
10. Licinio J, Seibyl JP, Altemus M, Charney DS, Krystal JH: Elevated CSF levels of interleukin-2 in neuroleptic-free schizophrenic patients. Am J Psychiatry 1993; 150:1408–1410Link, Google Scholar
11. Cantor-Graae E, Nordstrom LG, McNeil TF: Substance abuse in schizophrenia: a review of the literature and a study of correlates in Sweden. Schizophr Bull 2001; 48:69–82Crossref, Google Scholar
12. Kavanagh DJ, McGrath J, Saunders JB, Dore G, Clark D: Substance misuse in patients with schizophrenia: epidemiology and management. Drugs 2002; 62:743–755Crossref, Medline, Google Scholar
13. DeLeon J, Verghese C, Tracy JI, Josiassen RC, Simpson GM: Polydipsia and water intoxication in psychiatric patients: a review of the epidemiological literature. Biol Psychiatry 1994; 35:408–419Crossref, Medline, Google Scholar
14. Weltgesundheitsorganisation: Internationale Klassifikation psychischer Störungen: ICD 10. Bern, Germany, Verlag Hans Huber, 1993Google Scholar
15. World Health Organization: Guidelines for Preclinical Evaluation and Clinical Trials in Osteoporosis. Geneva, WHO, 1998, pp 5–6Google Scholar
16. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
17. White L, Harvey PD, Opler LA, Lindenmayer JP (PANSS Study Group): Empirical assessment of the factorial structure of clinical symptoms in schizophrenia: a multisite, multimodel evaluation of the factorial structure of the Positive and Negative Syndrome Scale. Psychopathology 1997; 3:263–274Crossref, Google Scholar
18. Schweiger U, Weber B, Deuschle M, Heuser I: Lumbar bone mineral density in patients with major depression: evidence of increased bone loss at follow-up. Am J Psychiatry 2000; 157:118–120Link, Google Scholar
19. Bergemann N, Auler B, Parzer P, Resch F, Mundt C, Ziegler R, Seibel M: High bone turnover but normal bone mineral density in women with schizophrenia (abstract). Bone 2001; 28:248Google Scholar
20. Häfner H, Maurer K, Loffler W, Fatkenheuer B, An der Heiden W, Riecher-Rössler A, Behrens S, Gattaz WF: The epidemiology of early schizophrenia: influence of age and gender on onset and early course. Br J Psychiatry 1994; 23:29–38Google Scholar
21. Becker D, Liver O, Mester R, Rapoport M, Weizman A, Weiss M: Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry 2003; 64:761–766Crossref, Medline, Google Scholar
22. Kanis JA: Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002; 359:1929–1936Crossref, Medline, Google Scholar
23. Webb AR, Kline L, Holick MF: Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 1988; 67:373–378Crossref, Medline, Google Scholar
24. Holick MF: Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr 1995; 61(3 suppl):638S-645SGoogle Scholar
25. Sherman S: Preventing and treating osteoporosis: strategies at the millennium. Ann NY Acad Sci 2001; 949:188–197Crossref, Medline, Google Scholar
26. Watts NB: Therapies to improve bone mineral density and reduce the risk of fracture: clinical trial results. J Reprod Med 2002; 47(1 suppl):82–92Google Scholar
27. Ovesen L: Vitamin therapy in the absence of obvious deficiency: what is the evidence? Drugs 1984; 27:148–170Crossref, Medline, Google Scholar
28. Rodrigues-Martinez MA, Garcia-Cohen EC: Role of Ca(2+) and vitamin D in the prevention and treatment of osteoporosis. Pharmacol Ther 2002; 93:37–49Crossref, Medline, Google Scholar
29. Kanis JA (WHO Study Group): Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporos Int 1994; 4:368–381Crossref, Medline, Google Scholar
30. WHO Study Group: Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group. World Health Organ Tech Rep Ser 1994; 843:1–129Medline, Google Scholar
31. Cooper C, Aihie A: Osteoporosis: recent advances in pathogenesis and treatment. Q J Med 1994; 87:203–209Medline, Google Scholar
32. Kanis JA, Johnell O, Oden A, DeLae C, Jonsson B, Dawson A: Ten-year risk of osteoporotic fracture and the effect of risk factors on screening strategies. Bone 2002; 30:251–258Crossref, Medline, Google Scholar
33. Garnero P, Sornay-Rendu E, Clustrat B, Delmas PD: Biochemical markers of bone turnover, endogenous hormone and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 2000; 15:1526–1536Crossref, Medline, Google Scholar
34. Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J (Committee of Scientific Advisors of the International Osteoporosis Foundation): The use of biochemical markers of bone turnover in osteoporosis. Osteoporos Int 2000; 11(suppl 6):S2-S17Google Scholar
35. Kanis JA, McCloskey EV: Evaluation of the risk of hip fracture. Bone 1996; 18(3 suppl):127S-132SGoogle Scholar



