Impairment of Social Perception Associated With Lesions of the Prefrontal Cortex
Abstract
OBJECTIVE: Behavioral and social impairments have been frequently reported after damage to the prefrontal cortex in humans. This study evaluated social perception in patients with prefrontal cortex lesions and compared their performance on a social perception task with that of healthy volunteers. METHOD: Thirty-three patients with prefrontal cortex lesions and 31 healthy volunteers were tested with the Interpersonal Perception Task. In this task, subjects viewed videotaped social interactions and relied primarily on nonverbal cues to make interpersonal judgments, such as determining the degree of intimacy between two persons depicted in the videotaped scene. Patients with prefrontal cortex lesions were classified according to lesion involvement of specific regions, including the orbitofrontal cortex, dorsolateral prefrontal cortex, and anterior cingulate cortex. RESULTS: Relative to the comparison subjects, patients whose lesions involved the orbitofrontal cortex demonstrated impaired social perception. Contrary to predictions, patients with lesions in the dorsolateral prefrontal cortex also showed deficits in using social cues to make interpersonal judgments. All patients, particularly those with lesions in the dorsolateral prefrontal cortex, showed poorer insight into their deficits, relative to healthy volunteers. CONCLUSIONS: These findings of deficits in social perception after damage to the orbitofrontal cortex extend previous clinical and experimental evidence of damage-related impairment in other aspects of social cognition, such as the ability to accurately evaluate emotional facial expressions. In addition, the results suggest that the dorsolateral prefrontal cortex is recruited when inferences about social interactions are made on the basis of nonverbal information.
Behavioral changes and disturbances in social interaction occur frequently after lesions of the prefrontal cortex, particularly those that involve the ventromedial prefrontal cortex or orbitofrontal sectors. Case reports of patients with orbitofrontal cortex lesions, such as those of Phineas Gage (1, 2) and E.V.R. (3), described striking behavioral changes leading to social dysfunction. These dysfunctions include inappropriate affect, disinhibition and lack of restraint, impaired insight and self-monitoring, social withdrawal, and failure to perceive and respond to interpersonal cues. Patients who exhibit impaired social behavior may have preserved intellectual functions, such as language, memory, and perception, and may perform with limited or no deficits on standard neuropsychological tests that are believed to be sensitive to frontal lobe dysfunction (3, 4).
Various mechanisms have been proposed to explain the aberrant social behavior observed after orbitofrontal cortex lesions. These explanations have focused on deficits in decision making because of lack of a “somatic marker” (5, 6), inability to alter behavior appropriately in response to a change in reinforcement contingencies (7, 8), difficulties in representing the mental states of others or in “theory of mind” (9, 10), and impaired ability to gain access to social knowledge (11–13). In the current study, we investigated whether patients with orbitofrontal cortex lesions show deficits in social perception, or the ability to use verbal and nonverbal cues to interpret social interactions. Deficits in perception of social cues have been demonstrated in other neuropsychiatric disorders that involve prefrontal cortex abnormalities, such as schizophrenia (for a recent review, see reference 14).
In this study, we used a standardized task of social cognition, the Interpersonal Perception Task (15), to compare the performance of patients with prefrontal cortex lesions with that of healthy volunteers. The Interpersonal Perception Task consists of 30 different videotaped social interactions for which subjects are required to make judgments regarding different aspects of the relationships of the persons depicted, such as level of intimacy and social status. This task is unique in that the scenes in the Interpersonal Perception Task show unrehearsed, spontaneous behavior of nonactor individuals. Thus, the Interpersonal Perception Task is more representative of real-life social situations, compared with other social cognitive tasks such as evaluating depictions of emotions in static pictures of faces.
Studies of patients with lesions confined to the frontal lobes demonstrate involvement of the ventromedial/orbitofrontal prefrontal sectors in socially relevant behaviors (5, 6, 8, 9, 12, 13, 16–29). Inability to monitor behavior and decreased insight have also been reported after prefrontal cortex damage (e.g., reference 30), but there is little empirical support for these clinical observations (but see references 30, 31). We hypothesized that, relative to healthy volunteers, patients with lesions involving the orbitofrontal cortex would demonstrate deficits in social perception on the Interpersonal Perception Task. No differences in performance were expected between the healthy volunteers and patients with prefrontal cortex lesions that did not involve the orbitofrontal cortex. We also hypothesized that patients with lesions in the prefrontal cortex would have decreased awareness of their social cognitive ability, relative to healthy comparison subjects. To better understand any observed deficits in social cognition, we compared performance on the Interpersonal Perception Task with standard neuropsychological measures and scores on the Neurobehavioral Rating Scale (32), an observer-rated measure of neuropsychiatric symptoms. We were specifically interested in the relationship between Interpersonal Perception Task performance and measures of executive function and working memory, because some studies have demonstrated an association between executive function and performance on social cognitive tasks (22, 26). We hypothesized that the ability to shift attention from one individual to another or from one conceptualization of a situation to another on the basis of new information would facilitate accurate interpersonal perception.
Method
Subjects
Thirty-three consecutive outpatients (30 male patients, three female patients) with nonprogressive prefrontal cortex lesions and 31 healthy comparison subjects (23 male subjects, eight female subjects) matched for age and educational level were included in the study. The patients had a mean age of 52.5 years (SD=7.5) and 14.1 years of education (SD=2.5), and the comparison subjects had a mean age of 54.5 years (SD=9.8) and 14.9 years of education (SD=2.0). The patients and the comparison subjects did not differ in age (t=0.91, df=62, p=0.37) or number of years of education (t=1.40, df=62, p=0.17).
Lesion Analysis
All patients had cerebral lesions restricted to the frontal lobes. Most of the patient group had acquired prefrontal cortex lesions secondary to penetrating head injury from missiles or shrapnel during the Vietnam War (N=29). Other etiologies were similarly nonacute and included tumor resection (N=1) and stroke from aneurysmal subarachnoid hemorrhage (N=3). The latter group was tested between 9 months and 11 years after the neurological event (mean=6.25 years, SD=5.17).
Lesion analyses were performed by using the program Analysis of Brain Lesions (ABLe) implemented in Medx (Medical Numerics, Sterling, Va.). ABLe characterizes brain lesions in magnetic resonance imaging (MRI) and computerized tomography (CT) scans of the adult human brain by spatially normalizing the lesioned brain into Talairach space using the Montreal Neurological Institute template brain. It reports anatomical structures in the normalized brain by using an interface to the Talairach Daemon (33) and quantifies Brodmann’s area involvement. CT scans were used for patients who had penetrating head injuries in Vietnam because these patients had retained ferromagnetic particles from shrapnel in their brains; MRI scans were used for the remaining subjects. Images were available for 26 of the 33 patients; data for the remaining seven patients were not included in analyses based on specific anatomical regions but were included in analyses comparing the entire group of patients with prefrontal cortex lesions with the healthy volunteers. Registration accuracy of the scans to the Montreal Neurological Institute template ranged from 90.40% to 95.34%, with a mean of 93.19% (SD=1.34).
Three prefrontal cortex sectors were identified as regions of interest and were quantified by using ABLe: 1) the ventromedial/orbitofrontal cortex (Brodmann’s area 25, lower 24, 32, medial aspect of 11, 12, and 10, and 13, 14, 47) (34–36), 2) the dorsolateral prefrontal cortex (Brodmann’s area 9/46) (37), and 3) the anterior cingulate cortex (Brodmann’s area 24, 25, 32). The dorsolateral prefrontal cortex was selected as a prefrontal cortex control region. The anterior cingulate cortex was identified because many of the lesions involved this region and previous studies have indicated a role for the anterior cingulate cortex in social cognition (29, 38–40). Because the majority of patients had damage involving all three regions, lesion groups were arbitrarily formed so that the prefrontal cortex region (orbitofrontal cortex, dorsolateral prefrontal cortex, or anterior cingulate cortex) made up at least two-thirds of the lesion volume and neither of the other two regions of interest contributed more than one-fourth of the total lesion volume. Twelve patients formed the orbitofrontal cortex lesion group, and four patients were included in the dorsolateral prefrontal cortex lesion group. Two other groups were formed. One included patients whose lesions involved primarily orbitofrontal cortex and anterior cingulate cortex regions (N=5). The remaining patients (N=5) included those with lesions involving all three areas in roughly equal proportions and one patient with both dorsolateral prefrontal cortex and anterior cingulate cortex lesions but no ventromedial/orbitofrontal damage. Composite images of the brain regions affected in the four lesion groups are presented in Figure 1. The volume of lesions ranged from 7.43 cm3 to 120.53 cm3 (mean=56.57 cm3, SD=35.37) and did not differ among the four lesion groups (F=1.06, df=3, 22, p=0.39). There were also no differences in age, number of years of education, or IQ among the four groups. Lesion groups were not studied according to laterality, because the numbers of subjects were too small to allow meaningful analyses. Because most patients had damage to all three regions of interest (orbitofrontal cortex, dorsolateral prefrontal cortex, anterior cingulate cortex), they were also studied according to the percentage of volume each region of interest contributed to the total lesion volume.
All participating subjects understood the study procedures and gave their written informed consent to participate in the study. This work was approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke, Bethesda, Md.
Measures
Interpersonal Perception Task
The Interpersonal Perception Task (41) is a standardized method of assessing the accuracy of social judgments regarding videotaped interpersonal situations. Validation studies demonstrate that performance on the Interpersonal Perception Task correlates with self- and peer-ratings of sociability and social competence (41–44). Test-retest reliability for the Interpersonal Perception Task over a 5-week period was 0.70 (41).
In the Interpersonal Perception Task, subjects view 30 brief, real-life scenes. Each scene is preceded by a question that requires the subject to reach a conclusion about the people who are interacting with one another in the scene. The Interpersonal Perception Task was designed to assess perception of nonverbal aspects of social exchange, including facial expression and body language, as well as the social meaning of statements. In the development of the Interpersonal Perception Task, videotaped scenes were edited to omit specific verbal content that would lead the viewer to the correct answer without the need to attend to other aspects of the exchange. For example, a scene with two adult women and a child would not include the child addressing one of the women as “mother,” if the question required subjects to determine which woman was the mother of the child. Thus, subjects need to “read between the lines” of verbal dialogue and interpret nonverbal cues to accurately make interpersonal judgments on the Interpersonal Perception Task.
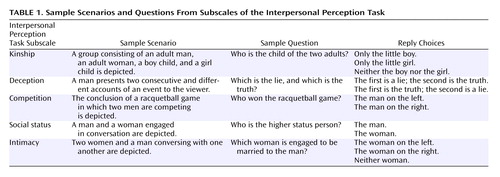
Five different aspects of social interaction are assessed in the Interpersonal Perception Task—social status, intimacy, kinship, competition, and deception. Each area is depicted in six scenarios. Each scenario is preceded by a question requiring subjects to make a judgment about the relationship between individuals involved in the interaction, and two or three response options are provided. Sample scenarios and questions are presented in Table 1.
To assess the accuracy of self-judgments of social cognitive ability, we asked subjects to estimate their score on the Interpersonal Perception Task after they completed the task. Estimates were obtained for 25 of the 33 patients with prefrontal cortex lesions and 26 of the 31 healthy comparison subjects. There were no significant differences in Interpersonal Perception Task performance between subjects for whom we had estimates of performance and those for whom we did not.
Additional neuropsychological measures
We examined the relationship between social cognitive deficits and other neuropsychological variables in the patients with prefrontal cortex lesions by using the following tests: 1) the WAIS-R (45) or WAIS-III (46), 2) Wechsler Memory Scale—Revised (WMS-R) (47) or Wechsler Memory Scale, 3rd ed. (WMS-III) (48), and 3) Wisconsin Card Sorting Test (49). Subjects enrolled before 1997 (N=8) received the WAIS-R and WMS-R; the remaining subjects (N=25) received the WAIS-III and WMS-III. There were no differences in performance on the Interpersonal Perception Task for subjects who received earlier versus more recent versions of the intelligence and memory tests.
To examine the relationship between Interpersonal Perception Task performance and measures of executive function and working memory, we calculated correlation coefficients between Interpersonal Perception Task scores and both the number of categories completed on the Wisconsin Card Sorting Test and scores on the working memory factor of the WMS-III.
Behavioral measures
To determine whether performance on the Interpersonal Perception Task was indicative of observable, everyday behavior, patients were assessed with the Neurobehavioral Rating Scale (32). The Neurobehavioral Rating Scale is a validated measure of neuropsychiatric symptoms resulting from head injury, including loss of insight, disinhibition, and impaired attention. The behavior of the patients with prefrontal cortex lesions was observed and rated with the Neurobehavioral Rating Scale by the research assistant over a 1-week evaluation period. Data were available for 28 of the 33 patients. There were no differences in performance on the Interpersonal Perception Task for patients who had a Neurobehavioral Rating Scale score and those who did not.
All tasks were administered individually.
Statistical Analysis
Scores for each of the five subscales of the Interpersonal Perception Task (kinship, deception, competition, social status, and intimacy) and a total Interpersonal Perception Task score based on the number of correct items on all subscales were submitted for analysis. In addition, a discrepancy score—the difference between the actual and estimated Interpersonal Perception Task scores—was calculated. The maximum score for each subscale was 6, and the maximum total Interpersonal Perception Task score was 30. Individual subscale and total Interpersonal Perception Task scores were rank-transformed for between-group comparisons because the scores were not normally distributed and there were unequal variances between groups.
Differences in the ranked total Interpersonal Perception Task scores between lesion groups and the healthy comparison group were analyzed by using planned independent t tests (each patient group versus the comparison group). To determine differences on individual Interpersonal Perception Task subscales, analysis of variance of ranked scores was performed. Post hoc analyses were conducted only if the omnibus F value was significant. Dunnett’s test was used to reduce the number of comparisons and control for family-wise error. The probability threshold for group comparisons was set at p<0.05, one-tailed, because the patients were expected to perform more poorly than the healthy comparison subjects.
The patients with prefrontal cortex lesions were also studied according to the extent of orbitofrontal cortex, dorsolateral prefrontal cortex, and anterior cingulate cortex damage, quantified as the percentage of each region involved in the entire lesion. The percentages were calculated by using volumes from ABLe (volume of the specific region/total lesion volume × 100). The association between these measures and Interpersonal Perception Task scores was analyzed by using Spearman’s correlation coefficient, because these data were not normally distributed. Spearman’s correlation coefficient was also used to examine correlations between Interpersonal Perception Task scores, neuropsychological measures, and the Neurobehavioral Rating Scale rating. The alpha level was set at 0.05, two-tailed, for these analyses.
Results
Effects of Lesion Location on Interpersonal Perception Task Performance
Between-group comparisons
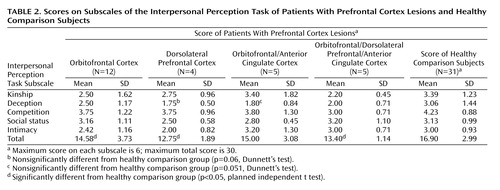
Scores on the Interpersonal Perception Task and its subscales for the patient groups and the healthy comparison subjects are summarized in Table 2. The comparison subjects had a mean total score of 16.90 (SD=2.99) of a possible 30 on the Interpersonal Perception Task. Their average score was consistent with published norms (41). The mean total scores for the patient groups showed that all patient groups performed at a level greater than chance (16 Interpersonal Perception Task scenes with three response options and 14 Interpersonal Perception Task scenes with two alternatives=12.3/30 by chance).
As hypothesized, the patients with lesions in the orbitofrontal cortex had a lower mean total Interpersonal Perception Task score (mean=14.58, SD=3.73) than the healthy comparison subjects (t=–1.98, df=41, p<0.03). However, the patients with lesions in the dorsolateral prefrontal cortex also performed significantly more poorly than the healthy subjects (t=–2.80, df=33, p<0.005), as did the patients with lesions in the orbitofrontal/dorsolateral prefrontal/anterior cingulate cortex (t=–2.83, df=34, p<0.004). Patients with lesions in the orbitofrontal/anterior cingulate cortex also scored lower than the comparison subjects on the Interpersonal Perception Task, but this difference fell short of statistical significance (t=–1.42, df=34, p=0.08). Analysis of variance of ranked Interpersonal Perception Task subscale scores for the comparison subjects and the four lesion groups showed that only the deception subscale score was significantly different among the groups (F=2.81, df=4, 52, p<0.04). In post hoc tests for the deception subscale scores, the group with lesions in the dorsolateral prefrontal cortex and the group with lesions in the orbitofrontal/anterior cingulate cortex were impaired relative to the comparison group, but the differences only approached statistical significance (dorsolateral prefrontal cortex group: p=0.06, Dunnett’s test; orbitofrontal/anterior cingulate cortex group: p=0.051, Dunnett’s test). Post hoc comparisons were not conducted for the other Interpersonal Perception Task subscale scores, which did not differ statistically among the groups according to the overall F value.
Thus, contrary to hypotheses, all four lesion groups, including patients with dorsolateral prefrontal cortex lesions only, showed poorer performance on the Interpersonal Perception Task, relative to the healthy comparison group. In particular, patients with lesions in the dorsolateral prefrontal cortex and patients with lesions in the orbitofrontal/anterior cingulate cortex had difficulty detecting lies (as measured by the score on the deception subscale).
Correlational analyses
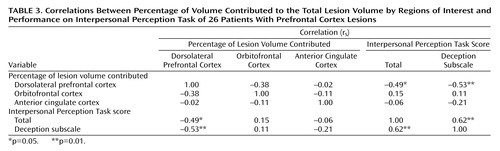
Correlations were calculated between the percentages of lesion volume contributed by each of the three prefrontal cortex regions and total Interpersonal Perception Task scores for all patients. The results are presented in Table 3. Consistent with the finding of poorer performance in the dorsolateral prefrontal cortex group on the Interpersonal Perception Task, relative to the comparison subjects, greater dorsolateral prefrontal cortex damage predicted lower Interpersonal Perception Task scores (p<0.02). In addition, lesions with greater dorsolateral prefrontal cortex involvement were associated with poorer performance in detecting lies (p=0.005). In contrast, the extent of orbitofrontal cortex or anterior cingulate cortex involvement was not correlated with performance on the overall Interpersonal Perception Task or the deception subscale.
Effects of Lesion Location on Awareness of Social Perception Deficits
Self-estimates of Interpersonal Perception Task performance and actual scores by group are illustrated in Figure 2. All patients with prefrontal cortex lesions overestimated their performance on the Interpersonal Perception Task, relative to the healthy volunteers (t=3.38, df=49, p=0.0007). The patients with lesions in the dorsolateral prefrontal cortex and those with lesions in the orbitofrontal/dorsolateral prefrontal/anterior cingulate cortex overestimated their Interpersonal Perception Task scores, relative to the healthy comparison subjects. The healthy comparison subjects had a mean discrepancy score of 3.42 (SD=4.39), compared with 9.33 (SD=5.86) for the patients with lesions in the dorsolateral prefrontal cortex (t=2.15, df=27, p=0.02) and 13.0 (SD=5.35) for the patients with lesions in the orbitofrontal/dorsolateral prefrontal/anterior cingulate cortex (t=3.96, df=28, p=0.002). The patients with lesions in the orbitofrontal cortex were also less accurate in estimating their score on the Interpersonal Perception Task (mean discrepancy score=6.00, SD=4.91), relative to the comparison group, but this difference fell short of significance (t=1.27, df=30, p=0.11). In contrast, there was no difference in discrepancy score between the patients with lesions in the orbitofrontal/anterior cingulate cortex (mean discrepancy score=4.00, SD=3.81) and the healthy volunteers (t=0.27, df=29, p=0.39).
Relationship Between Interpersonal Perception Task and Other Measures
As hypothesized, performance of all patients with prefrontal cortex lesions on the Interpersonal Perception Task was significantly correlated with measures of working memory (WMS working memory score) (rs=0.58, df=23, p=0.003) and executive function (number of categories completed on the Wisconsin Card Sorting Test) (rs=0.60, df=24, p=0.001). These results suggest that some cognitive processes involved in interpreting social and emotional cues may be shared with working memory and problem-solving ability.
The performance on the Interpersonal Perception Task of the patients with prefrontal cortex lesions was negatively correlated with ratings of behavior on the Neurobehavioral Rating Scale (rs=–0.53, df=26, p=0.003). These results indicate that deficits in social perception in the patients with prefrontal cortex lesions were associated with greater psychopathology.
Discussion
The objective of this study was to examine the effects of prefrontal cortex lesions on perception of social cues. Detailed lesion analysis of brain images with computer software (ABLe) allowed us to identify specific Brodmann’s areas and to quantify the extent of damage to structures involved in the lesion. As hypothesized, we found that patients with damage involving the orbitofrontal sectors were impaired on the Interpersonal Perception Task, relative to healthy volunteers. However, contrary to hypotheses, we also found that patients with primarily dorsolateral prefrontal cortex damage showed significantly poorer performance on the Interpersonal Perception Task. This finding was supported by an association between extent of dorsolateral prefrontal cortex damage across all patient groups and poorer social perception ability, as measured by the Interpersonal Perception Task. As expected, patients with lesions in the orbitofrontal/anterior cingulate cortex and patients with lesions in the orbitofrontal/dorsolateral prefrontal/anterior cingulate cortex also showed deficits on the Interpersonal Perception Task, relative to healthy comparison subjects. Analyses of Interpersonal Perception Task subscale scores showed that patients with dorsolateral prefrontal cortex or orbitofrontal/anterior cingulate cortex lesions were most impaired in detection of lies. Across the group with prefrontal cortex lesions, social perceptual ability was correlated with measures of working memory and executive function. In addition, their performance on the Interpersonal Perception Task was associated with observer ratings of neuropsychiatric symptoms. Finally, consistent with clinical observations, subjects with prefrontal cortex lesions were poorer at judging their ability to interpret social behavior. In particular, patients with lesions involving dorsolateral prefrontal cortex regions had marked impairment in awareness of social perception ability.
Our findings for the patients with lesions in the orbitofrontal cortex are consistent with previous studies that have demonstrated other deficits in social perception in patients after ventral frontal damage, namely, deficits in identifying emotional expression by using face or voice stimuli (12, 50). Other investigators have proposed that social cognitive deficits after orbitofrontal cortex damage are attributable to impairment in complex processes that involve the manipulation or control of thoughts and behaviors, such as decision making (5, 6, 51), appropriate alteration of behavior in response to environmental cues (7, 8), or representation of the mental states of others (theory of mind) (9, 10, 16). However, our results and those of other studies suggest that the behavioral disturbances observed in patients with lesions in the orbitofrontal cortex may be partly caused by a disruption in more fundamental processes that involve the perception of social cues or emotional cues and that misinterpretation of these cues results in inappropriate behavioral responses. Our finding of an association between impaired social perceptual ability, as measured by the Interpersonal Perception Task, and greater observer-rated neuropsychiatric disturbance provides support for this notion.
Contrary to our hypotheses, the patients with dorsolateral prefrontal cortex lesions also had impaired Interpersonal Perception Task performance and had specific deficits in detection of lies. Across all patients with prefrontal cortex lesions, greater dorsolateral prefrontal cortex damage was associated with poorer performance on the Interpersonal Perception Task, but the extent of orbitofrontal cortex or anterior cingulate cortex damage was not correlated with performance on this measure. Other studies have demonstrated that patients with dorsolateral or dorsomedial damage are impaired on measures of social cognition, including the Gambling Task, a decision-making task considered to be sensitive to orbitofrontal cortex damage (22, 26). In further support of social cognitive deficits in patients with dorsolateral prefrontal cortex damage, we found a moderate to strong association between Interpersonal Perception Task performance and cognitive deficits associated with dorsolateral prefrontal cortex lesions, including deficits in working memory and executive function. Similarly, other investigators have concluded that working memory ability and other executive skills appear to influence decision making in the Gambling Task both in patients with lesions in the orbitofrontal cortex and in patients with lesions in the dorsolateral prefrontal cortex (22, 26).
The poorer performance on the Interpersonal Perception Task of patients with lesions in the dorsolateral prefrontal cortex may also be interpreted as reflecting deficits in socio-cognitive skills necessary for theory of mind. Theory of mind, or the ability to attribute mental states to self and others, has been reported to be impaired in patients with prefrontal cortex lesions (9, 10, 16, 52, 53). The Interpersonal Perception Task requires subjects to use primarily nonverbal cues to make inferences about the nature of relationships between individuals; thus, it requires both accurate perception of social cues as well as perspective-taking (e.g., the subject must have knowledge of how a mother would behave toward her own child and must be able to accurately interpret the observed behavior). Previous investigators have suggested that ventromedial or orbitofrontal cortex lesions are associated with theory-of-mind deficits (e.g., reference 10), but few studies have analyzed prefrontal cortex lesions by specific subregions. One study of the effect of damage to specific prefrontal cortex regions on theory of mind showed that patients with bilateral orbitofrontal cortex lesions were impaired on tasks that required more subtle social reasoning (i.e., detecting a social faux pas) but not on typical false-belief theory-of-mind tasks (9). In contrast, patients with unilateral left dorsolateral prefrontal cortex lesions were not impaired on these tasks. In the current study, patients with strictly left-sided lesions were not included in the study group. However, our data, together with previous investigations, suggest that damage to the bilateral or right unilateral prefrontal cortex contributes to impairment in theory of mind. Analyses of Interpersonal Perception Task subscale scores showed that patients with lesions involving dorsolateral prefrontal cortex regions were particularly impaired in detection of lies, a process requiring intact theory of mind. Our finding of an association between performance on the Interpersonal Perception Task and measures of working memory and executive function is also consistent with findings from studies of children that indicate that the acquisition of theory of mind coincides with the development of executive control (54) (although there are conflicting reports regarding the relation between execution function and theory of mind; e.g., references 16, 55). Our findings suggest that cognitive flexibility and the ability to keep concepts in mind may contribute to the perception or utilization of social cues to make interpersonal judgments regarding a dynamic social interaction.
We also compared the self-assessment of social cognitive ability in patients with prefrontal cortex lesions with that in healthy comparison subjects. Disturbances in self-awareness and level of insight have frequently been reported in case studies of patients with prefrontal cortex lesions (30), but these clinical observations have received little systematic evaluation. One study demonstrated that patients with prefrontal cortex damage were impaired in estimating their performance on a temporal ordering task (31). In the current study, we found evidence for deficits in judging social perceptual ability in patients with prefrontal cortex lesions. In particular, patients with lesions involving the dorsolateral prefrontal cortex (both the group with lesions in the dorsolateral prefrontal cortex and the group with lesions in the orbitofrontal/dorsolateral prefrontal/anterior cingulate cortex) were significantly poorer at estimating their scores on the Interpersonal Perception Task, compared with the healthy volunteers. In contrast, patients with lesions that did not involve dorsolateral prefrontal cortex regions (the group with lesions in the orbitofrontal cortex and the group with lesions in the orbitofrontal/anterior cingulate cortex) were not significantly impaired in monitoring their social cognitive skills. Thus, it appears that patients with prefrontal cortex lesions are impaired in their self-judgments of social cognitive, as well as general cognitive, ability (31) and that the deficit may be specific to damage to dorsolateral prefrontal cortex areas. Our finding, while requiring replication, implicates the dorsolateral prefrontal cortex in mediating awareness of self and the ability to self-monitor social cognitive skills.
As in most studies of human brain lesions, the patients in our study had prefrontal cortex damage that was not restricted to one subregion of the prefrontal cortex. However, a major strength of our study is that we were able to identify specific Brodmann’s areas and quantify damaged regions by analyzing structural brain images with computer software. In addition to providing greater accuracy in classifying lesions, quantification of specific structures allowed us to study the patients with prefrontal cortex damage as a group in order to examine associations between social perceptual ability and the extent of damage in specific prefrontal cortex subregions. Using the Interpersonal Perception Task, we found that patients with lesions in the orbitofrontal cortex showed deficits in social perceptual ability, or the capacity to perceive social and emotional cues to make interpersonal judgments or inferences. Our findings extend previous explanations for disrupted social behavior that focus on the control or manipulation of thoughts and behavior (decision making, response reversal) and support the idea that impaired perception of emotional cues contributes to inappropriate social responses in patients with orbitofrontal cortex lesions. Our study also showed that dorsolateral prefrontal cortex lesions were associated with deficits in social perception and with decreased awareness of impairment in social cognition. These findings may be interpreted within the context of theory of mind and its association with executive control. Although the dorsolateral prefrontal cortex has traditionally been ascribed “nonsocial” higher cognitive functions, our results, and those of others, suggest that this brain region may also be involved in social cognitive processes, including perception of emotional cues, interpersonal judgments, and self-awareness of social cognitive ability.
 |
 |
 |
Presented in part at the 13th annual meeting of the American Neuropsychiatric Association, San Diego, March 9–12, 2002. Received July 2, 2003; revision received Nov. 18, 2003; accepted Nov. 21, 2003. From the Cognitive Neuroscience Section, National Institute of Neurological Disorders and Stroke, Bethesda, Md. Address reprint requests to Dr. Grafman, Cognitive Neuroscience Section, NINDS/NIH, Building 10, Room 5C205, 10 Center Dr., MSC 1440, Bethesda, MD 20892–1440; [email protected] (e-mail). The authors thank Molly K. Colvin for assistance with data collection and data entry, and Charlotte F. Manly, Ph.D., for guidance in analyzing the data and for comments on a previous version of the manuscript.
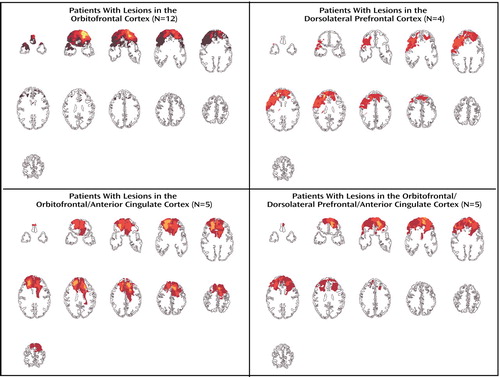
Figure 1. Composite Images of Damaged Brain Regions in Four Groups of Patients With Lesions in the Prefrontal Cortex Who Participated in a Study of Social Perceptiona
aLighter colors denote areas of greatest overlap of lesions among patients.
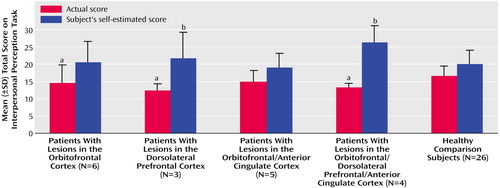
Figure 2. Actual and Self-Estimated Total Scores on the Interpersonal Perception Task in Four Groups of Patients With Lesions in the Prefrontal Cortex and Healthy Comparison Subjects
aSignificantly different from healthy comparison group (p<0.05, planned independent t test).
bOverestimation of score is significantly different from overestimation in the healthy comparison group (p<0.05, t test).
1. Harlow JM: Passage of an iron rod through the head. Boston Medical and Surgical Journal 1848; 39:389–393Crossref, Google Scholar
2. Harlow JM: Recovery from the passage of an iron bar through the head. Publications of the Massachusetts Medical Society 1868; 2:239–347Google Scholar
3. Eslinger PJ, Damasio AR: Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology 1985; 35:1731–1741Crossref, Medline, Google Scholar
4. Dimitrov M, Phipps M, Zahn T, Grafman J: A thoroughly modern Gage. Neurocase 1999; 5:345–354Crossref, Google Scholar
5. Damasio AR, Tranel D, Damasio H: Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res 1990; 41:81–94Crossref, Medline, Google Scholar
6. Bechara A, Damasio AR, Damasio H, Anderson SW: Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994; 50:7–15Crossref, Medline, Google Scholar
7. Rolls ET: The orbitofrontal cortex. Phil Trans R Soc Lond B Biol Sci 1996; 351:1433–1443; discussion 1443–1444Crossref, Medline, Google Scholar
8. Rolls ET, Hornak J, Wade D, McGrath J: Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry 1994; 57:1518–1524Crossref, Medline, Google Scholar
9. Stone VE, Baron-Cohen S, Knight RT: Frontal lobe contributions to theory of mind. J Cogn Neurosci 1998; 10:640–656Crossref, Medline, Google Scholar
10. Stuss DT, Gallup GG Jr, Alexander MP: The frontal lobes are necessary for “theory of mind.” Brain 2001; 124(part 2):279–286Google Scholar
11. Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM: Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology 1996; 46:1231–1238Crossref, Medline, Google Scholar
12. Hornak J, Rolls ET, Wade D: Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia 1996; 34:247–261Crossref, Medline, Google Scholar
13. Mah L, Arnold MC, Grafman J: Deficits in social knowledge following damage to ventromedial prefrontal cortex. J Neuropsychiatry Clin Neurosci (in press)Google Scholar
14. Pinkham AE, Penn DL, Perkins DO, Lieberman J: Implications for the neural basis of social cognition for the study of schizophrenia. Am J Psychiatry 2003; 160:815–824Link, Google Scholar
15. Archer D, Costanzo M: Guide to The Interpersonal Perception Task. Berkeley, University of California Extension Center, 1988Google Scholar
16. Rowe AD, Bullock PR, Polkey CE, Morris RG: “Theory of mind” impairments and their relationship to executive functioning following frontal lobe excisions. Brain 2001; 124(part 3):600–616Google Scholar
17. Happe F, Malhi GS, Checkley S: Acquired mind-blindness following frontal lobe surgery? a single case study of impaired “theory of mind” in a patient treated with stereotactic anterior capsulotomy. Neuropsychologia 2001; 39:83–90Crossref, Medline, Google Scholar
18. Shammi P, Stuss DT: Humour appreciation: a role of the right frontal lobe. Brain 1999; 122(part 4):657–666Google Scholar
19. Dimitrov M, Grafman J, Hollnagel C: The effects of frontal lobe damage on everyday problem solving. Cortex 1996; 32:357–366Crossref, Medline, Google Scholar
20. Bechara A, Tranel D, Damasio H, Damasio AR: Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex 1996; 6:215–225Crossref, Medline, Google Scholar
21. Bechara A, Damasio H, Tranel D, Damasio AR: Deciding advantageously before knowing the advantageous strategy. Science 1997; 275:1293–1295Crossref, Medline, Google Scholar
22. Bechara A, Damasio H, Tranel D, Anderson SW: Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci 1998; 18:428–437Crossref, Medline, Google Scholar
23. Bechara A, Tranel D, Damasio H: Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 2000; 123(part 11):2189–2202Google Scholar
24. Bechara A, Damasio H, Damasio AR, Lee GP: Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci 1999; 19:5473–5481Crossref, Medline, Google Scholar
25. Milne E, Grafman J: Ventromedial prefrontal cortex lesions in humans eliminate implicit gender stereotyping. J Neurosci 2001; 21:RC150Google Scholar
26. Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T: Decision-making processes following damage to the prefrontal cortex. Brain 2002; 125(part 3):624–639Google Scholar
27. Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW: Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology 1999; 20:322–339Crossref, Medline, Google Scholar
28. Clark L, Manes F, Antoun N, Sahakian BJ, Robbins TW: The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia 2003; 41:1474–1483Crossref, Medline, Google Scholar
29. Hornak J, Bramham J, Rolls ET, Morris RG, O’Doherty J, Bullock PR, Polkey CE: Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain 2003; 126(part 7):1691–1712Google Scholar
30. Price BH, Daffner KR, Stowe RM, Mesulam MM: The comportmental learning disabilities of early frontal lobe damage. Brain 1990; 113(part 5):1383–1393Google Scholar
31. Jurado MA, Junque C, Vendrell P, Treserras P, Grafman J: Overestimation and unreliability in “feeling-of-doing” judgements about temporal ordering performance: impaired self-awareness following frontal lobe damage. J Clin Exp Neuropsychol 1998; 20:353–364Crossref, Medline, Google Scholar
32. Levin H, High W, Goethe K, Sisson R, Overall J, Rhoades H, Eisenberg H, Kalisky Z, Gary H: The neurobehavioural rating scale: assessment of the behavioural sequelae of head injury by the clinician. J Neurol Neurosurg Psychiatry 1987; 50:183–193Crossref, Medline, Google Scholar
33. Talairach Daemon. San Antonio, Tex, University of Texas Health Science Center, Research Imaging Center, 1997. (http://ric.uthscsa.edu/RIC_WWW.data/Components/talairach/talairachdaemon.html)Google Scholar
34. Bechara A: The neurology of social cognition. Brain 2002; 125(part 8):1673–1675Google Scholar
35. Ongur D, Ferry AT, Price JL: Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 2003; 460:425–449Crossref, Medline, Google Scholar
36. Ongur D, Price JL: The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 2000; 10:206–219Crossref, Medline, Google Scholar
37. Petrides M, Pandya DN: Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci 1999; 11:1011–1036Crossref, Medline, Google Scholar
38. Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J: Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting Stroop. Biol Psychiatry 1999; 45:1542–1552Crossref, Medline, Google Scholar
39. Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL: The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 1998; 44:1219–1228Crossref, Medline, Google Scholar
40. Yamasaki H, LaBar KS, McCarthy G: Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci USA 2002; 99:11447–11451Crossref, Medline, Google Scholar
41. Costanzo M, Archer D: Interpreting the expressive behavior of others: the Interpersonal Perception Task. J Nonverb Behav 1989; 13:225–245Crossref, Google Scholar
42. Schroeder JE, Ketrow SM: Social anxiety and performance in an interpersonal perception task. Psychol Rep 1997; 81(3, part 1):991–996Google Scholar
43. Schroeder JE: Interpersonal perception skills: self-concept correlates. Percept Mot Skills 1995; 80:51–56Crossref, Google Scholar
44. Miczo N, Sergin C, Allspach LE: Relationship between nonverbal sensitivity, encoding, and relational satisfaction. Communication Reports 2001; 14:39–48Crossref, Google Scholar
45. Wechsler D: Wechsler Adult Intelligence Scale—Revised. San Antonio, Tex, Psychological Corp, 1981Google Scholar
46. Wechsler D: Wechsler Adult Intelligence Scale, 3rd ed. San Antonio, Tex, Psychological Corp (Harcourt), 1997Google Scholar
47. Wechsler D: The Wechsler Memory Scale—Revised. San Antonio, Tex, Psychological Corp, 1974Google Scholar
48. Wechsler D: The Wechsler Memory Scale, 3rd ed. San Antonio, Tex, Psychological Corp (Harcourt), 1997Google Scholar
49. Heaton RK: The Wisconsin Card Sorting Test Manual. Odessa, Fla, Psychological Assessment Resources, 1981Google Scholar
50. Blair RJ, Cipolotti L: Impaired social response reversal: a case of “acquired sociopathy.” Brain 2000; 123(part 6):1122–1141Google Scholar
51. Bar-On R, Tranel D, Denburg NL, Bechara A: Exploring the neurological substrate of emotional and social intelligence. Brain 2003; 126(part 8):1790–1800Google Scholar
52. Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J: Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. J Cogn Neurosci 2003; 15:324–337Crossref, Medline, Google Scholar
53. Channon S, Crawford S: The effects of anterior lesions on performance on a story comprehension test: left anterior impairment on a theory of mind-type task. Neuropsychologia 2000; 38:1006–1017Crossref, Medline, Google Scholar
54. Perner J, Lang B: Development of theory of mind and executive control. Trends Cogn Sci 1999; 3:337–344Crossref, Medline, Google Scholar
55. Fine C, Lumsden J, Blair RJ: Dissociation between “theory of mind” and executive functions in a patient with early left amygdala damage. Brain 2001; 124(part 2):287–298Google Scholar



