Cannabis Use and Age at Onset of Schizophrenia
Abstract
OBJECTIVE: The purpose of the study was to assess the independent influences of gender and cannabis use on milestones of early course in schizophrenia. METHOD: In this population-based, first-contact incidence study conducted in The Hague, the Netherlands, patients (N=133) were interviewed with the Comprehensive Assessment of Symptoms and History, and key informants were interviewed with the Instrument for the Retrospective Assessment of the Onset of Schizophrenia. Milestones of early course were 1) first social and/or occupational dysfunction, 2) first psychotic episode, and 3) first negative symptoms. RESULTS: Male patients were significantly younger than female patients at first social and/or occupational dysfunction, first psychotic episode, and first negative symptoms. Cannabis-using patients were significantly younger at these milestones than were patients who did not use cannabis. Multivariate analyses showed that cannabis use, but not gender, made an independent contribution to the prediction of age at first psychotic episode: male cannabis users were a mean of 6.9 years younger at illness onset than male nonusers. In contrast, age at first social and/or occupational dysfunction and the risk of developing negative symptoms before the first contact with a physician for treatment of possible psychotic disorder were predicted by gender, but not by cannabis use. CONCLUSIONS: The results indicate a strong association between use of cannabis and earlier age at first psychotic episode in male schizophrenia patients. Additional studies examining this possibly causal relationship are needed.
The gender difference in age at onset is one of the most consistent findings in schizophrenia research (1–5). According to a study in Mannheim, Germany, for example, women were 3–4 years older than men at illness onset by any definition of onset, including impaired social or occupational functioning, emergence of positive symptoms, or emergence of negative symptoms (6, 7).
Another variable that has been suggested to influence the timing of onset is illicit substance abuse, mainly use of cannabis, but the results of the relevant studies are mixed. Some studies found that substance-using patients are younger than their nonusing counterparts (8–11); one study reported an earlier onset for female, but not for male users (12); and other studies found no significant effect of drug use (13, 14). However, these studies were all hospital based and, with one exception (14), failed to adjust for gender differences.
The objective of the present study was to assess the independent influences of gender and cannabis use on milestones of early course in a Dutch population-based incidence cohort of patients with schizophrenia. Since the sale and use of cannabis are condoned in the Netherlands, the information on its use provided by patients and key informants may be highly reliable.
Method
Recruitment of Patients
The procedures of our first-contact incidence study have been reported previously (15). Briefly, all residents of the city of The Hague, age 15–54 years, who made a first-in-lifetime contact with a physician for a (suspected) psychotic disorder during the period from April 1997 to April 1999 were referred for study. After the procedures had been fully explained, the researchers asked the patients to provide written informed consent for a diagnostic interview (with the Comprehensive Assessment of Symptoms and History [16]), an interview with a key informant (with the Instrument for the Retrospective Assessment of the Onset of Schizophrenia [17]), and access to the patient’s medical file. Information from all sources was used to make a consensus DSM-IV diagnosis and to assess retrospectively the onset of the early signs and symptoms of schizophrenia. The researchers performed a diagnostic reassessment of all patients 2.5 years after the first contact. To this end, the researchers reinterviewed the patients using a follow-up version of the Comprehensive Assessment of Symptoms and History (18), collected key informant information with a modified version of the Instrument for the Retrospective Assessment of the Onset of Schizophrenia, and obtained information from the medical file. The follow-up rate was 89%. Using all available information from the two assessments, two psychiatrists made a (second) consensus DSM-IV diagnosis. The present study included all patients with a diagnosis of a “schizophrenic disorder” (DSM-IV diagnostic code 295.xx, i.e., schizophrenia, schizophreniform disorder, and schizoaffective disorder) at the second assessment (N=126) and seven patients who had been lost to follow-up but who had received any of these diagnoses at the initial assessment. Of these 133 patients, 97 (73%) were male and 36 (27%) were female. The group was ethnically diverse and included natives of the Netherlands (32%), first- and second-generation immigrants from Surinam (20%) and Morocco (17%), and others (32%).
Assessment of Onset of Schizophrenia
During the incidence study the following milestones of early course were assessed: 1) onset of social and/or occupational dysfunction, as defined in DSM-IV, to include one or more major areas of functioning such as work, school, or interpersonal relations that are markedly below the prior level, or failure to achieve the expected level of functioning; 2) first psychotic episode, i.e., onset of delusions, hallucinations, disorganized speech, or disorganized or catatonic behavior that leads to behavioral changes (this latter criterion was included because 23 patients [17%] had reported transient psychotic experiences during childhood or youth, without concomitant functional deterioration); and 3) the onset of negative symptoms (before first contact with a physician), i.e., affective flattening, alogia, avolition, or social withdrawal. Whenever no information was available on the exact day on which the milestone occurred (or if this day was not known), we chose the middle of the month or year (e.g., “June 1993” was recorded as June 15, 1993, and “1986” was recorded as July 1, 1986).
A total of 110 patients (83%) were assessed by using the Comprehensive Assessment of Symptoms and History or the Instrument for the Retrospective Assessment of the Onset of Schizophrenia (for 67% of patients, both instruments had been administered). Twenty-three patients (17%) refused contact with the researchers, and information on their milestones was obtained from their physician. Inconsistencies between information from the patient and information from the key informant were discussed by the research team, and the most reliable source was selected. In some cases the most reliable source was judged to be the key informant (for instance, a mother who said that her son had been dysfunctional at school since October 1995 and gave clear examples, whereas the patient said he functioned well), and in other cases the patient was the most reliable source (for example, a patient who told us that he began hearing voices in February 1997, but his mother had not discovered until May 1997 that her son had experienced acoustic hallucinations). Since many schizophrenia patients are only somewhat aware of their negative symptoms (19), our assessments of the onset of negative symptoms relied heavily on the reports of family members. In five cases our information was insufficient to determine the onset of social and/or occupational dysfunction, and in seven cases the start of the first psychotic episode was unknown. In seven of the total of 73 patients with negative symptoms, the time of onset of these symptoms was unclear.
Assessment of Cannabis Use
Using information from the patient, the key informant, the medical file, and drug screens, we determined whether the patient had used cannabis, how often the patient had used cannabis, and when this use had occurred in relation to the three milestones of early course. Again, inconsistencies were discussed by the research team, and the most reliable source was selected. Patients who had used cannabis less than four times were considered nonusers.
Some patients had also used other illicit substances. If one defines “use” as the consumption of these substances at least once a month during the year before first contact, 16 patients (12%) had “used” one or more of these substances (amphetamines: N=9; cocaine: N=12; heroin: N=1). Misuse, defined as daily use for a period of at least 2 weeks during the year before inclusion, had been present in only three patients. All of these patients had also used cannabis (20). In 13 patients, the onset of cannabis use had preceded the onset of use of amphetamines or cocaine by a median of 2 years, and in three cases the use of cannabis and other substances had begun simultaneously. When cannabis was used together with other substances, cannabis was used more frequently than any other substance. Daily use of cannabis occurred in 13 of 16 patients, and daily use of cocaine and amphetamines occurred in two and one of 16 patients, respectively.
Statistical Analysis
Median ages at first social and/or occupational dysfunction and at the first psychotic episode were calculated for male and female patients and for cannabis users and nonusers. Next, median age differences (not differences between median ages) with 95% confidence intervals (CIs) between male and female patients and between cannabis users and nonusers were determined (21).
Since not all patients had negative symptoms, a Kaplan-Meier curve was estimated for cumulative probability of freedom from negative symptoms for male and female patients and for cannabis users and nonusers. We determined the age at which 50% of the group was free from negative symptoms (i.e., median age) for male and female patients and for cannabis users and nonusers. After that, we calculated median age differences with 95% CIs between male and female patients and between cannabis users and nonusers (21).
Multiple linear regression analyses were used to examine the independent contribution of gender and cannabis use to the prediction of age at first social and/or occupational dysfunction and age at first psychotic episode. Finally, Cox’s proportional hazards regression analysis was used to look at the independent contribution of gender and cannabis use to the risk of negative symptoms. These effects were expressed as hazard ratios. Since very few women used cannabis, there was collinearity between the variables “gender” and “cannabis use.” For that reason, in each regression model we used two dummy variables; the reference group was male patients who did not use cannabis before a specific milestone, and the comparison groups were male patients who used cannabis before a specific milestone and female patients who did not use cannabis before a specific milestone. Thus, we examined the main effect of gender, adjusted for cannabis use, and the main effect of cannabis use, adjusted for gender. Given the small number of cannabis-using women, it was not possible to examine an interaction effect. Since we hypothesized a causal relationship between cannabis use and early onset, we determined for each patient whether cannabis had been used before the emergence of the milestone in question. At the moment of first contact with a physician, 64 (66%) men and six (17%) women used cannabis. Forty men and five women had used it before the onset of social and/or occupational dysfunction, 55 men and five women before the first psychotic episode, and 29 men and one woman before the onset of negative symptoms.
Results
First Social and/or Occupational Dysfunction and First Psychotic Episode
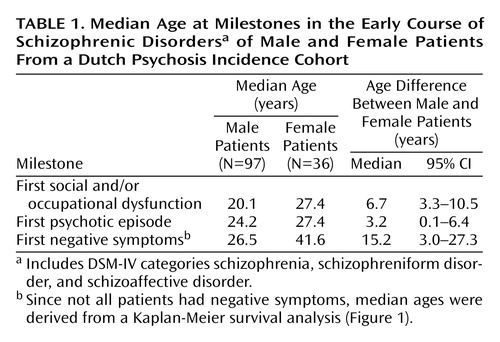
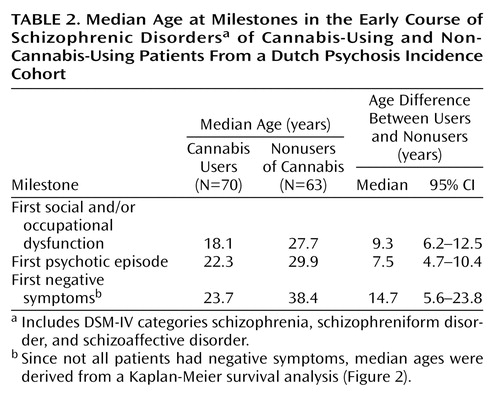
The median age at first social and/or occupational dysfunction and at first psychotic episode, as well as the median age differences with 95% CIs, for male and female patients are presented in Table 1. In Table 2 we report the same outcome measures for cannabis-using and nonusing patients.
First Negative Symptoms
Seventy-three patients (55%) had developed negative symptoms before their first contact with a physician. Figure 1 shows the Kaplan-Meier survival curves for cumulative probability of freedom from negative symptoms in male and female patients. The median age at onset of negative symptoms was 26.5 years for male patients and 41.6 years for female patients (Table 1). A log-rank test revealed that this difference was significant (χ2=7.4, df=1, p=0.007).
The corresponding curves for cannabis-using versus nonusing patients are shown in Figure 2. For cannabis users the median age at onset of negative symptoms was 23.7 years, compared with 38.4 years for nonusers (Table 2). This difference was also statistically significant (χ2=11.0, df=1, p<0.001, log-rank test).
Multiple Regression Analyses
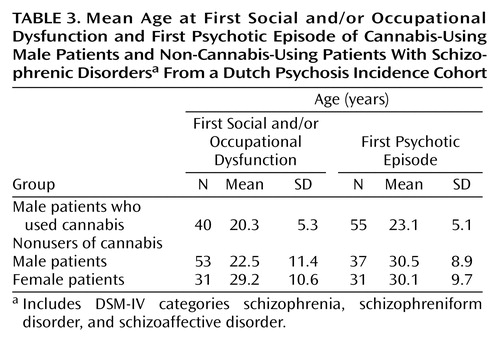
Mean ages at first social and/or occupational dysfunction and at first psychotic episode for the three groups based on dummy variables are presented in Table 3. A linear regression analysis showed that gender, adjusted for cannabis use, made an important contribution to the prediction of age at social and/or occupational dysfunction (B=6.4, 95% CI=2.1 to 10.7, p=0.004). Thus, non-cannabis-using women were a mean of 6.4 years older than nonusing men at the onset of social and/or occupational dysfunction. The contribution of cannabis use, adjusted for gender, was not significant (B=2.4, 95% CI=–1.4 to 6.3, p=0.20).
When a similar analysis was done for age at first psychotic episode, however, cannabis used made the largest contribution (B=6.9, 95% CI=3.7 to 10.0, p<0.001). It is important to note that the contribution of gender was not significant (B=2.1, 95% CI=–3.4 to 3.9, p=0.90). Male cannabis users were found to have their first psychotic episode a mean of 6.9 years earlier than male nonusers.
Cox proportional hazards regression analysis showed that the risk of developing negative symptoms was predicted by gender but not by cannabis use (for male patients, compared to female patients: hazard ratio [adjusted for cannabis use]=2.5, 95% CI=1.1 to 5.0, p=0.02; for cannabis users, compared to nonusers: hazard ratio [adjusted for gender]=1.3, 95% CI=0.7 to 2.2, p=0.40). In other words, male patients who did not use cannabis were 2.5 times more likely than female nonusers to develop negative symptoms in the early course of schizophrenia.
Discussion
We assessed the independent influences of gender and cannabis use on milestones of early course of schizophrenia in a Dutch population-based incidence cohort. Most univariate findings of this study were in line with those of previous studies: male gender was found to be a predictor of an earlier age at onset of social and/or occupational dysfunction and of a higher risk of developing negative symptoms before first contact with a physician for treatment (5). The salient finding, however, was that cannabis use was a much stronger predictor than gender of age at first psychotic episode. In male patients there was a 7-year age difference between users and nonusers of this substance.
Interpretation
When trying to formulate an interpretation of these findings, one should be aware of some trends in the epidemiology of psychiatric disorders over the past few decades, including increasing rates of illicit drug use in the general population and, possibly, a changing sex ratio for schizophrenia. Although some textbooks report that the risk for the disorder is equal for both sexes (22), a recent meta-analysis of incidence studies published after 1980 found a significant excess of male patients (23). Since the use of illicit drugs is more common in males than in females, increasing drug use might be one of the possible explanations for this finding. It is equally important to recognize, however, that the gender difference in age at psychosis onset was already present in previous periods when cannabis use was uncommon and that use of this substance cannot be the explanation for this phenomenon. Furthermore, it is uncertain whether the age at onset is decreasing or whether the gender difference in this respect is on the increase.
One can think of at least three mechanisms that could explain the relationship between cannabis use and earlier age at psychosis onset in males. These mechanisms are not mutually exclusive, and one can raise several arguments for and against the plausibility of each mechanism. First, it is possible that cannabis has no influence on risk or age at onset and that younger patients, compared with older patients, are more likely to use this substance before the first psychotic episode because the use is age related. However, the prevalence of cannabis use in the general population was substantially lower than in this incidence cohort. The proportions of Dutch people who had used cannabis in the last month were 7% for the 20–24-year age group, 5% for the 25–29-year age group, and 2% for the 30–34-year age group (24). A second possibility is that cannabis hastens the onset of psychosis in subjects who would have also developed the disorder if they had never used this substance. This possibility is supported by the observation that cannabis may trigger or exacerbate psychotic symptoms in healthy subjects and in schizophrenia patients (25–28). However, as noted earlier, there is no definitive evidence for a decreasing age at onset in schizophrenia nor for an increasing gender difference in this respect. Third, it is possible that cannabis makes manifest schizophrenia in young subjects who are genetically at risk for developing the disorder. According to this point of view, which has recently gained support from studies in the Netherlands (29) and Sweden (30), some of these individuals would never have developed schizophrenia had they not used cannabis. This mechanism could account for a higher schizophrenia risk for males, because cannabis is more commonly used by males. Finally, all three mechanisms could be relevant.
Validity and Limitations
This investigation has several strengths. First, the study was population based. Second, DSM-IV diagnoses for most patients were based on two assessments, separated by an interval of 2.5 years. Third, for most assessments, the researchers had three sources of information: a semistructured diagnostic interview, a key informant interview, and the medical file. It could be hypothesized that the results were biased by ethnicity, but we found no significant age differences in the milestones of early course between the four largest ethnic groups (natives of the Netherlands, Surinamese, Moroccans, and others) (Kruskal-Wallis tests used for analysis of age at first social and/or occupational dysfunction and age at first psychotic episode, and log-rank tests used for analysis of age at first negative symptoms). It is also implausible that the findings have been confounded by other factors that have been reported to be associated with early onset, including a history of obstetric complications and/or a family history of schizophrenia (31, 32). A higher prevalence of obstetric complications in male or female schizophrenia patients has not been demonstrated (5), and a higher prevalence of these complications in patients who use or who do not use cannabis seems unlikely. The presence of a family history of schizophrenia was not examined here, but the validity of the distinction between genetic and sporadic subtypes of the disorder is controversial (33). It is unlikely that the use of cocaine or amphetamines had an additional effect on age at first psychosis. The median age at first psychotic episode of the 14 patients (all male) who had used amphetamines and/or cocaine in addition to cannabis before the first psychosis was 23.2 years. For males who had only used cannabis, the median age at first psychotic episode was 22.1 years.
The reliability of our assessments of milestones of early course was not evaluated, but our data were similar to those of the ABC (Age, Beginning, Course) Schizophrenia Study in Mannheim, Germany (6), suggesting that our assessments were quite accurate. Is it unlikely that we mistook cannabis-induced apathy or depressive symptoms for negative symptoms. If these symptoms had been confounded, one would expect cannabis use to be a risk factor for the development of these symptoms. Likewise, one would expect that female gender would be a risk factor for the development of these symptoms, because women have a greater risk for depression in schizophrenia than men (5).
Finally, an important limitation of all studies on this topic, including the present one, is the reliance on verbal reports of patients and relatives about cannabis use. The liberal Dutch attitude toward this substance is likely to have led to reliable reports on its use, but this reliability is not entirely certain.
Comparison to ABC Schizophrenia study
It is important to note that the milestones of early course, as reported in this study, were derived from a different context (the ABC Schizophrenia Study). In the ABC study, the male-to-female ratio of the subjects was close to 1.0, and the prevalence of cannabis use was low (14%, compared with 53% in our study). Also, the authors of the ABC study presented means instead of medians for age at milestones. When we calculated mean values for age at milestones for our study participants, we found that the first psychotic episode occurred about 2 years later in male as well as in female patients in the ABC study, compared with our study, and that the age at which negative symptoms emerged was similar in the ABC study and in our study (data available on request).
Conclusions
The present study shows that in Dutch male schizophrenia patients, the use of cannabis is associated with a much earlier onset of the disorder. The explanation for this association remains unclear. Since early onset is associated with a poorer prognosis of the disorder, the relationship between cannabis use and the risk of developing an early-onset type of schizophrenia is an important focus for future research.
 |
 |
 |
Received Dec. 10, 2002; revision received July 22, 2003; accepted July 31, 2003. From the Rudolf Magnus Institute of Neuroscience, Department of Psychiatry, University Medical Center Utrecht; the Center for Biostatistics, Utrecht University, Utrecht, the Netherlands; and Parnassia Psychomedical Center, The Hague, the Netherlands. Address reprint requests to Dr. Veen, Department of Psychiatry, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, Netherlands; [email protected] (e-mail). Partly supported by the Theodore and Vada Stanley Foundation. The authors thank psychiatrists Jan Dirk Blom, Winfried Laan, and Diede Schols for their participation in the diagnostic meetings.
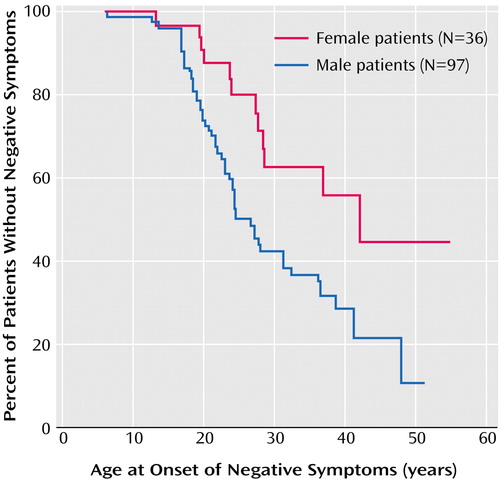
Figure 1. Kaplan-Meier Survival Curve for Cumulative Probability of Freedom From Negative Symptoms Before First Contact With a Physician for Male and Female Patients With Schizophrenic Disordersa From a Dutch Psychosis Incidence Cohort
aIncludes DSM-IV categories schizophrenia, schizophreniform disorder, and schizoaffective disorder.
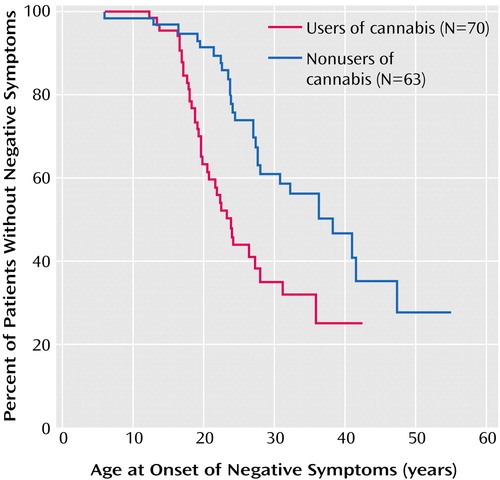
Figure 2. Kaplan-Meier Survival Curve for Cumulative Probability of Freedom From Negative Symptoms Before First Contact With a Physician for Cannabis-Using and Non-Cannabis-Using Patients With Schizophrenic Disordersa From a Dutch Psychosis Incidence Cohort
aIncludes DSM-IV categories schizophrenia, schizophreniform disorder, and schizoaffective disorder.
1. Dahlberg G, Sternberg S: Eine statistische Untersuchung ueber die Wahrscheinlichkeit der Erkrankung an verschiedenen Psychosen und ueber die demographische Haeufigkeit van Geisteskranken. Zeitschrift fuer die gesammte Neurologie und Psychiatrie 1931; 133:447–482Crossref, Google Scholar
2. Sjøgren T: Genetic-statistical and psychiatric investigations of a West-Swedish population. Acta Psychiatr Neurol Suppl 1948; 52:11–83Google Scholar
3. Angermeyer MC, Kühn L: Gender differences in age of onset of schizophrenia: an overview. Eur Arch Psychiatry Neurol Sci 1988; 237:351–364Crossref, Medline, Google Scholar
4. DeLisi LE: The significance of age of onset for schizophrenia. Schizophr Bull 1992; 18:209–215Crossref, Medline, Google Scholar
5. Leung A, Chue P: Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand 2000; 101:3–38Crossref, Google Scholar
6. Häfner H, Maurer K, Löffler W, an der Heiden W, Munk-Jørgensen P, Hambrecht M, Riecher-Rössler A: The ABC Schizophrenia Study: a preliminary overview of the results. Soc Psychiatry Psychiatr Epidemiol 1998; 33:380–386Crossref, Medline, Google Scholar
7. Häfner H, Maurer K, Löffler W, Riecher-Rössler A: The influence of age and sex on the onset and early course of schizophrenia. Br J Psychiatry 1993; 162:80–86Crossref, Medline, Google Scholar
8. Addington J, Addington D: Effect of substance misuse in early psychosis. Br J Psychiatry 1998; 172:134–136Crossref, Google Scholar
9. DeQuardo JR, Carpenter CF, Tandon R: Patterns of substance abuse in schizophrenia: nature and significance. J Psychiatr Res 1994; 28:267–275Crossref, Medline, Google Scholar
10. Weller MP, Ang PC, Latimer-Sayer DT, Zachary A: Drug abuse and mental illness (letter). Lancet 1988; 1:997Crossref, Medline, Google Scholar
11. Hambrecht M, Häfner H: Substance abuse and the onset of schizophrenia. Biol Psychiatry 1996; 40:1155–1163Crossref, Medline, Google Scholar
12. Rabinowitz J, Bromet EJ, Lavelle J, Carlson G, Kovasznay B, Schwartz JE: Prevalence and severity of substance use disorders and onset of psychosis in first-admission psychotic patients. Psychol Med 1998; 28:1411–1419Crossref, Medline, Google Scholar
13. Sevy S, Robinson DG, Solloway S, Alvir JM, Woerner MG, Bilder R, Goldman R, Lieberman J, Kane J: Correlates of substance misuse in patients with first-episode schizophrenia and schizoaffective disorder. Acta Psychiatr Scand 2001; 104:367–374Crossref, Medline, Google Scholar
14. Cantor-Graae E, Nordstrøm LG, McNeil TF: Substance abuse in schizophrenia: a review of the literature and a study of correlates in Sweden. Schizophr Res 2001; 48:69–82Crossref, Medline, Google Scholar
15. Selten JP, Veen N, Feller W, Blom JD, Schols D, Camoenië W, Oolders J, van der Velden M, Hoek HW, Rivero VM, van der Graaf Y, Kahn R: Incidence of psychotic disorders in immigrant groups to the Netherlands. Br J Psychiatry 2001; 178:367–372Crossref, Medline, Google Scholar
16. Andreasen NC, Flaum M, Arndt S: The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 1992; 49:615–623Crossref, Medline, Google Scholar
17. Häfner H, Riecher-Rössler A, Hambrecht M, Maurer K, Meissner S, Schmidtke A, Fätkenheuer B, Löffler W, van der Heiden W: IRAOS: an instrument for the assessment of onset and early course of schizophrenia. Schizophr Res 1992; 6:209–223Crossref, Medline, Google Scholar
18. Ho B-C, Nopoulos P, Flaum M, Arndt S, Andreasen NC: Two-year outcome in first-episode schizophrenia: predictive value of symptoms for quality of life. Am J Psychiatry 1998; 155:1196–1201Link, Google Scholar
19. Selten JP, Gernaat HBPE, Nolen WA, Wiersma D, van den Bosch RJ: Experience of negative symptoms: comparison of schizophrenic patients to patients with a depressive disorder and to normal subjects. Am J Psychiatry 1998; 155:350–354Link, Google Scholar
20. Veen N, Selten JP, Hoek HW, Feller W, van der Graaf Y, Kahn RS: Use of illicit substances in a psychosis incidence cohort: a comparison among different ethnic groups in the Netherlands. Acta Psychiatr Scand 2002; 105:440–443Crossref, Medline, Google Scholar
21. Altman DG, Machin D, Bryant TN, Gardner MJ (eds): Statistics With Confidence: Confidence Intervals and Statistical Guidelines. Bristol, UK, BMJ Books, 2001Google Scholar
22. Hales RE, Yudofsky SC, Talbott JA (eds): The American Psychiatric Press Textbook of Psychiatry, 3rd ed. Washington, DC, American Psychiatric Press, 1999, p 447Google Scholar
23. Aleman A, Kahn RS, Selten JP: Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry 2003; 60:565–571Crossref, Medline, Google Scholar
24. van der Laar M, Cruts G, Korf D, Knibbe R, Willemse M, Rigter H, van Alem V (eds): National Drug Monitor. Utrecht, the Netherlands, Trimbos-Instituut, 2001Google Scholar
25. Linszen DH, Dingemans PM, Lenior ME: Cannabis abuse and the course of recent-onset schizophrenic disorders. Arch Gen Psychiatry 1994; 51:273–279Crossref, Medline, Google Scholar
26. Verdoux H, Gindre C, Sorbara F, Tournier M, Swendsen JD: Cannabis use and the expression of psychosis vulnerability in daily life (abstract). Schizophr Res 2002; 53:225Google Scholar
27. Hall W, Solowij N: Adverse effects of cannabis. Lancet 1998; 352:1611–1616Crossref, Medline, Google Scholar
28. D’Souza DC, Abi-Saab W, Madonick S, Wray Y, Forselius K, MacDougall L, Brush L, Castello K, Krystal J: Cannabinoids and psychosis: evidence from studies with iv THC in schizophrenic patients and controls (abstract). Schizophr Res 2000; 47:33Crossref, Google Scholar
29. van Os J, Bak M, Hanssen M, van Bijl R, de Graaf R, Verdoux H: Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol 2002; 156:319–327Crossref, Medline, Google Scholar
30. Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G: Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. Br Med J 2002; 325:1199–1201Crossref, Medline, Google Scholar
31. Verdoux H, Geddes JR, Takei N, Lawrie SM, Bovet P, Eagles JM, Heun R, McCreadie RG, McNeil TF, O’Callaghan E, Stöber G, Willinger U, Wright P, Murray RM: Obstetric complications and age at onset in schizophrenia: an international collaborative meta-analysis of individual patient data. Am J Psychiatry 1997; 154:1220–1227Link, Google Scholar
32. Gorwood P, Leboyer M, Jay M, Payan C, Feingold J: Gender and age at onset in schizophrenia: impact of family history. Am J Psychiatry 1995; 152:208–212Link, Google Scholar
33. Roy MA, Crowe RR: Validity of the familial and sporadic subtypes of schizophrenia. Am J Psychiatry 1994; 151:805–814Link, Google Scholar



