Increased Brain GABA Concentrations Following Acute Administration of a Selective Serotonin Reuptake Inhibitor
Abstract
OBJECTIVE: The authors used magnetic resonance spectroscopy (MRS) to assess the effect of acute administration of the selective serotonin reuptake inhibitor (SSRI) citalopram on cortical levels of γ-aminobutyric acid (GABA). METHOD: Ten healthy volunteers received either intravenous citalopram (10 mg) or saline in a randomized, double-blind, crossover design. The occipital GABA/creatine ratio was measured with a proton MR spectral editing technique. RESULTS: In comparison with saline, citalopram produced a mean increase of 35% in relative brain GABA concentration in the occipital cortex. CONCLUSIONS: These findings extend previous work showing that SSRI treatment increases cortical GABA in depressed patients and suggest that this results from an action of SSRIs on GABA neurons rather than as a secondary consequence of mood improvement.
Studies using proton magnetic resonance spectroscopy (MRS) have shown that unmedicated patients with major depression have decreased total concentrations of the neurotransmitter γ-aminobutyric acid (GABA) in the occipital cortex (1). Repeated treatment of depressed subjects with either electroconvulsive therapy (2) or selective serotonin reuptake inhibitors (SSRIs) (3) increases total occipital GABA levels, suggesting that antidepressant treatments may have a common ability to increase GABA neurotransmission. An alternative explanation, however, is that clinical recovery itself is associated with normalization of cortical GABA levels in depressed subjects.
One way of resolving this issue is to determine whether treatment of nondepressed subjects with antidepressant medication also increases cortical GABA levels. Animal experimental studies suggest that acute challenge with serotonin (5-HT)-enhancing agents increases GABA release in the cortex (4). The aim of the present study therefore was to test the hypothesis that acute administration of the SSRI citalopram would increase occipital GABA levels in healthy subjects.
Method
We recruited 10 healthy volunteers (nine men and one woman; mean age=41.6 years, range=20–67) who had no history of any DSM-IV axis I disorder according to the Structured Clinical Interview for DSM-IV. None acknowledged a history of depression in a first-degree relative. All subjects had been free of psychotropic medication for at least 3 months and gave written informed consent for the study, which was approved by the local ethics committee.
Subjects were scanned (eyes open) on two occasions (mean test interval=12.2 days, range=4–37) after receiving either intravenous citalopram (10 mg) or saline over 30 minutes in a randomized, double-blind, crossover design. MRS spectra were acquired from subjects between 40 and 65 minutes after completion of the infusion. The 10-mg dose of citalopram was chosen on the basis of a previous dose-response study that showed reliable 5-HT neuroendocrine responses in the absence of significant subjective side effects (5).
To measure occipital total GABA (GABA and the GABA dipeptide homocarnosine), we applied a proton MR spectral editing technique based on a spatially localized (point-resolved spectroscopy [6]) double-quantum filter (7). This single-shot technique (256 averages, TR=3 seconds) allowed us to detect the GABA-CH2 resonance (3 ppm) with suppression of uncoupled resonances from choline, creatine, and N-acetylaspartate. The localized double-quantum filter method was implemented on a 3-T Varian-Inova spectrometer with a standard birdcage head coil; 27 cm3 (3×3×3 cm3) voxels were placed in the occipital lobe region guided by T1-weighted axial images. We studied the occipital lobe because it offers clear landmarks for consistent placement of the spectroscopic voxel and is removed from major sources of static magnetic field inhomogeneities in the head, allowing narrower spectroscopic line widths to be achieved. We also acquired a standard point-resolved spectroscopy spectrum (TE=68 msec, TR=3 seconds) in order to measure signal intensities from choline, creatine, and N-acetylaspartate. Three-dimensional structural images (T1-weighted, gradient echo imaging providing 32 32-mm slices) were used for voxel segmentation to estimate gray matter content in the MRS voxel.
Spectra were analyzed by using Magnetic Resonance User Interface software (MRUI project: http://carbon.uab.es/mrui/) with frequency-selective, time domain-fitting VARPRO (8). We calculated GABA/creatine ratios after correcting for editing efficiency and number of equivalent protons in GABA and creatine; we assumed that the saturations of GABA and creatine resonances were similar. These GABA/creatine ratios can be converted to absolute concentrations of GABA, given known concentrations of creatine in the brain (assumed to be 7 mM, based on measured, approximately equal voxel concentrations of gray and white matter). Differences in GABA levels after pretreatment with saline and citalopram were compared by using a paired t test, and Pearson’s product-moment correlation analyses were performed. Scan/rescan reliability, calculated as twice the standard deviation of the difference in measurements on two occasions without drug administration, was 26.7%.
Results
There was a mean rise of 34.5% (SE=13.5) in the GABA/creatine ratio after the citalopram infusion (mean=0.36, SE=0.10) compared with saline (mean=0.28, SE=0.005) (t=2.51, df=9, p<0.04) (Figure 1). Estimated mean GABA concentrations were 1.96 mmol/kg (SE=0.35) after placebo and 2.52 mmol/kg (SE=0.70) after citalopram. There was no correlation between the change in GABA/creatine ratio following citalopram and age, the interval between the start of the infusion and acquisition of the spectrum, number of days between the scans, or body weight (all p values >0.10). Visual analogue scales measuring anxiety and somatic symptoms did not distinguish citalopram from placebo administration.
Discussion
Our data indicate that acute intravenous administration of the SSRI citalopram increases total occipital GABA levels in healthy volunteers. The magnitude of this increase was very similar to that reported by Sanacora and colleagues (3), who studied depressed patients before and after an average of 8 weeks of SSRI treatment. Our findings therefore suggest that SSRI treatment can increase occipital GABA levels independent of clinical changes in mood. Although three subjects did not show an increase in occipital GABA levels after citalopram, most biological responses to 5-HT challenge show substantial interindividual variation. In a previous study (3), two of 11 depressed patients did not show an increase in brain GABA after chronic SSRI treatment. Genetic polymorphisms in 5-HT receptors may determine some of these individual differences (9).
The increase in occipital GABA levels after acute citalopram administration could reflect interactions of 5-HT pathways with GABAergic neurons. The ascending 5-HT innervation of the cortex preferentially targets GABA interneurons, and consistent with this, electrophysiological studies have shown increased activity of GABA interneurons following administration of 5-HT2A and 5-HT3 receptor agonists (10). It must be acknowledged, however, that there are many other less specific ways in which citalopram might alter brain GABA levels, including effects on the function of synthetic enzymes, glucose metabolism, or glutamate/glutamine cycling.
Networks of GABA interneurons can synchronize activity within local populations of pyramidal neurons and perhaps also across distributed neural networks (11). It has been proposed that disruption of this synchrony may underlie various kinds of neurological and psychological dysfunction (12), so modulatory effects of SSRIs on cortical GABA may provide new insights into therapeutic mechanisms.
Received Jan. 24, 2003; revision received July 9, 2003; accepted July 15, 2003. From the University Department of Psychiatry, Warneford Hospital; and the Centre for Functional Magnetic Imaging of the Brain, Department of Clinical Neurology, John Radcliffe Hospital, Oxford, U.K. Address reprint requests to Dr. Cowen, University Department of Psychiatry, Warneford Hospital, Oxford, OX3 7JX, U.K.; [email protected]. Supported by the Medical Research Council (U.K.).
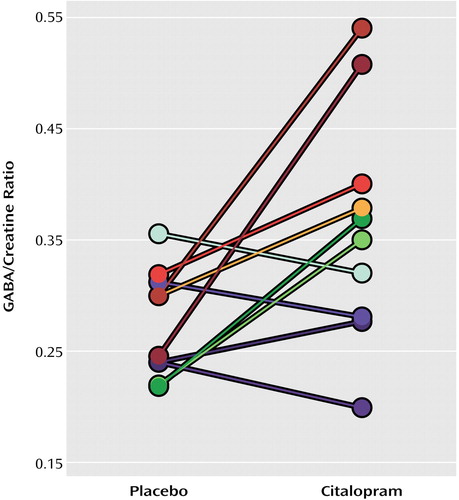
Figure 1. Occipital Cortex GABA/Creatine Ratios in 10 Healthy Volunteers Following Intravenous Administration of Citalopram (10 mg) and Placeboa
aSubjects received both citalopram and placebo (saline) in a randomized, double-blind, crossover manner.
1. Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH: Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 1999; 56:1043–1047Crossref, Medline, Google Scholar
2. Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB, Berman RM, Krystal JH: Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry 2003; 160:577–579Link, Google Scholar
3. Sanacora G, Mason GF, Rothman DL, Krystal JH: Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry 2002; 159:663–665Link, Google Scholar
4. Abi-Saab WM, Bubser M, Roth RH, Deutch AY: 5-HT2 receptor regulation of extracellular GABA levels in the prefrontal cortex. Neuropsychopharmacology 1999; 20:92–96Crossref, Medline, Google Scholar
5. Attenburrow MJ, Mitter PR, Whale R, Terao T, Cowen PJ: Low-dose citalopram as a 5-HT neuroendocrine probe. Psychopharmacology (Berl) 2001; 155:323–326Crossref, Medline, Google Scholar
6. Bottomley PA: US Patent 4, 1984, 480, 228Google Scholar
7. Keltner JR, Wald LW, Frederick B, Renshaw PJ: In vivo detection of GABA in human brain using a localized double-quantum filter technique. Magn Reson Med 1997; 37:366–371Crossref, Medline, Google Scholar
8. Knijn A, de Beer R, Luyten PR, van Ormondt D: Accurate quantification of in-vivo 31-P NMR signals using a variable projection method and prior knowledge. Magn Reson Med 1988; 6:92–98Crossref, Medline, Google Scholar
9. Whale R, Quested DJ, Laver D, Harrison PJ, Cowen PJ: Serotonin transporter (5-HTT) promoter genotype may influence the prolactin response to clomipramine. Psychopharmacology (Berl) 2000; 150:120–122Crossref, Medline, Google Scholar
10. Gellman RL, Aghajanian GK: Pyramidal cells in piriform cortex receive a convergence of inputs from monoamine activated GABAergic interneurons. Brain Res 1993; 600:63–73Crossref, Medline, Google Scholar
11. McBain CJ, Fisahn A: Interneurons unbound. Nat Rev Neurosci 2001; 2:11–23Crossref, Medline, Google Scholar
12. Varela F, Lachaux JP, Rodriguez E, Martinerie J: The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci 2001; 2:229–239Crossref, Medline, Google Scholar



