Impaired GABA Neuronal Response to Acute Benzodiazepine Administration in Panic Disorder
Abstract
OBJECTIVE: Disturbances in the metabolism of the brain amino acid transmitter γ-aminobutyric acid (GABA) may contribute to the pathophysiology of human anxiety disorders. Animal studies indicate that deletions or reductions in the expression of the gene for the GABA synthetic enzyme, glutamate decarboxylase 65 (GAD65), reduce basal cortical GABA levels or stress-induced release of GABA in the cerebral cortex and increase fear behaviors. Complementing these findings, the authors recently observed lower than normal cortical GABA levels in patients with panic disorder. In the current study, the authors tested the hypothesis that panic disorder patients have a deficient GABA neuronal response to benzodiazepine (clonazepam) administration. METHOD: In a parallel-group, repeated-measures design, occipital cortex GABA responses to acute oral, open-label benzodiazepine administration were tested in 10 panic disorder patients and nine healthy comparison subjects. Occipital cortex total GABA levels were measured before and after medication administration by means of a novel proton magnetic resonance spectroscopic technique. RESULTS: Panic disorder patients had a deficient GABA neuronal response (blunted reduction of occipital cortex GABA level) to acute benzodiazepine administration, compared to the healthy subjects, who exhibited a significant decrease in occipital cortex GABA levels after this intervention. The patients also appeared to have persistently low occipital cortex GABA after chronic benzodiazepine treatment. CONCLUSIONS: Overall, these data are consistent with the hypothesis that a trait-like abnormality in GABA neuronal function contributes to the pathogenesis of human panic disorder. The data raise the possibility that GAD65 enzyme dysfunction could be a pathogenic factor in panic disorder.
There is growing interest in the potential role of the brain γ-aminobutyric acid (GABA) neuronal system in the pathophysiology of anxiety disorders. Animals with abnormalities in the regulation of GABA turnover or GABAA receptor subunit composition exhibit higher levels of fearfulness and lower than normal sensitivity to benzodiazepines. For example, deletions or reductions in the expression of the gene for the GABA synthetic enzyme, glutamate decarboxylase 65 (GAD65), reduce basal cortical GABA levels (1, 2) and decrease stress-induced release of GABA in the cerebral cortex in mice (3). GAD knockout animals exhibit higher levels of spontaneous fear behaviors (1–3), higher levels of fear conditioning (1), and a blunted behavioral sensitivity to benzodiazepines in the absence of alterations in the density of postsynaptic GABAA receptors (3). Also, mice with deletions of the γ2 subunit of the GABAA receptor display higher levels of fearfulness and benzodiazepine insensitivity (4, 5). In addition, biochemically induced deficits in GAD function predispose to lactate-induced panic responses in mice (6). Alternatively, disturbances in GABA metabolism and GAD function may also be a consequence of experimentally induced fear in animals. For example, conditioned fear stimulus exposure in freely moving mice has been associated with sustained reductions in extracellular GABA levels in the amygdala (7), while acute administration of a GABAA receptor antagonist infused into the basolateral amygdala of freely moving rats led to rapid reductions in GAD65 and GAD67 levels in amygdala-hippocampal projections (8).
Clinical studies have documented abnormalities of cortical GABA systems that tend to parallel the preclinical findings described above. For example, in the occipital cortex of patients with panic disorder, GABA levels are lower than normal, as measured by a proton magnetic resonance spectroscopic (1H-MRS) technique (9) that predominantly assays the intraneuronal pool of GABA (10). These results suggest that a trait-like disturbance in cortical GABA metabolism may be present in panic disorder, which could conceivably be due to GAD enzyme dysfunction. Similarly, neuroreceptor imaging studies have generally identified lower levels of cortical and hippocampal benzodiazepine receptor binding or affinity in panic disorder (11–15). Thus, there is appreciable direct, in vivo evidence of GABA neuronal dysfunction in panic disorder.
The present study was intended to further extend the assessment of GABA neuronal function in panic disorder. It was designed to test the hypothesis that panic disorder patients have a deficient GABA neuronal response (blunted cortical GABA suppression) to benzodiazepine administration. A previous study suggested that healthy humans and anxiety disorder patients generally exhibit reductions in plasma GABA (an indirect index of brain GABA) after acute benzodiazepine challenge (16). Although the mechanism behind this observation is unclear, one possibility is that acute benzodiazepine exposure down-modulates GAD enzyme function, resulting in an acute decrease in GABA levels. This notion is indirectly supported by preclinical observations that facilitation of GABA function (by GABA transaminase inhibition) down-regulates GAD67(17, 18) and, to a lesser extent, GAD65 expression (18). Also, withdrawal from chronic diazepam administration is associated with a marked increase in cortical GAD67 mRNA expression, indicating that benzodiazepine exposure may tend to suppress GAD gene expression (19). If panic disorder is associated with lower levels of GAD activity, and if benzodiazepine exposure normally decreases GAD function, as we propose, then the magnitude of cortical GABA responses to benzodiazepines would be lower in panic disorder patients, relative to healthy comparison subjects. The plasma GABA assessment approach referred to earlier (16) may not be sufficiently sensitive to detect differences between anxiety disorder patients and healthy comparison subjects in benzodiazepine-related GABA responses, as highlighted by our previous work, in which we detected cortical GABA abnormalities in panic disorder patients, in contrast to earlier findings of normal plasma GABA findings in this patient group (16, 20). We therefore tested our hypothesis in the following protocol utilizing an MRS procedure that directly measures occipital cortex GABA levels. We examined GABA levels in an occipital cortex region, because we had earlier developed a reliable method of assaying GABA in this region and, using this approach, had detected GABA abnormalities in this region in several neuropsychiatric diseases, including panic disorder (9, 21, 22). Also, when we began the study, our ability to reliably examine other regions of interest that have traditionally been related to anxiety (e.g., the frontal cortex) was limited because of technical issues (patient immobilization, shimming adjacent to the sinuses, and variable head shape in the frontal regions). Finally, the imaging literature, while consistently implicating frontal areas in panic, also suggested that more generalized cortical GABA abnormalities could be present (14). Since the completion of this study, other groups (23, 24) have reported preliminary data on cortical GABA level quantitation in frontal regions of interest, and it is anticipated that reliable cortical GABA data are soon likely to become routine in this pathophysiologically significant region.
Method
Design
We employed a parallel-group (patients versus healthy comparison subjects) design and a novel 1H-MRS technique (25) to quantify the effects of acute benzodiazepine (clonazepam) administration on total cortical tissue (gray and white matter) GABA levels in an occipital cortex region of interest. The details of the MR spectroscopy protocol (see description later in this section) have been described in a previous publication (9). The test-retest variability for this method of GABA assessment is 10% (26). We also gathered exploratory data on the effects of chronic clonazepam exposure/treatment on occipital cortex GABA in the panic disorder patients. To measure occipital cortex GABA levels, each patient and comparison subject received an MRS scan. The concentration of GABA was measured by comparing the integrated GABA resonance from the MRS-edited spectrum with the integrated creatine resonance obtained during the same scan.
Spectroscopy Procedures
A trained research assistant or registered nurse under the supervision of the principal investigator accompanied the patient throughout each MRS test (approximately 1.5 hours). The GABA measurements were obtained according to the method described by Rothman et al. (25). The MRS studies were performed at the Yale University MR Center with a 2.1-T Oxford Magnet (Oxford Magnetic Technology, Oxford, U.K.) with a 1-m bore, equipped with a Bruker Biospec Avance I spectrometer (Bruker Instruments, Billerica, Mass). Subjects lay with the occipital cortex against an 8-cm radiofrequency surface coil tuned to the proton nuclear magnetic resonance frequency of 89.67 MHz. Gradient-echo scout images of the subject’s brain were obtained for subject positioning. A 1.5×3×3-cm volume of interest centered on the midline of the occipital cortex, 2 cm deep from the dura, was chosen for spectroscopic measurement. An automated first- and second-order shimming routine was used to optimize B0 homogeneity in the volume of interest (27). Homonuclear editing of the 3.0-ppm (chemical shift scale [ppm]) GABA C4 resonance was performed by using the J-editing pulse sequence described previously (25). Spectral editing detects signals from hydrogen atoms that are J-coupled to hydrogen atoms on adjacent carbon atoms in the same molecule. In this case the spin-spin J-editing selected the GABA C4 triplet resonance at 3.0 ppm, which is coupled to the GABA C3 multiplet resonance at 1.9 ppm. Two subspectra of 128 scans each were subtracted to obtain a difference spectrum that isolates GABA (total) (combined measure of GABA and the GABA-containing dipeptide homocarnosine). The localization techniques included three-dimensional, image-selected, in vivo spectroscopy with outer volume suppression, selective excitation, and use of a surface spoiler coil. The spectral acquisition parameters were as follows: TR=5 seconds; TE=68 milliseconds; sweep width=15,000 Hz; and acquisition time=510 milliseconds. A chemical shift-selective 80-msec hyperbolic secant pulse followed by an inversion recovery delay and a 2-2 refocusing pulse were used for water suppression. Spectral editing of the GABA C4 resonance at 3.0 ppm was achieved by applying a 26.5-msec DANTE (delays alternating with nutations for tailored excitations) pulse to invert selectively the 1.9-ppm C3 resonance, applied symmetrically in time about the center of the refocusing pulse sequence. The free induction decay was zero-filled to 32 K, and a 3-Hz exponential filter was applied before Fourier analysis. The GABA signal was integrated over a 0.30-ppm bandwidth at 3.00 ppm. The creatine signal was integrated over a 0.20-ppm bandwidth at 3.00 ppm in the GABA-inverted spectrum. The following equation was used to calculate the GABA concentration: [GABA]=([G*/Cr*]–[M/Cr*]) (ICF) (EE) (3/2) ([Cr]), where G* is the integral in the edited spectrum, Cr* is the integral of the creatine resonance, M is the contribution to the edited GABA spectrum from edited macromolecule resonances (28, 29), ICF is the correction for the limited integral bandwidths determined from localized edited spectra of solutions of GABA and line-broadened creatine to match the in vivo processed line widths, EE is the correction for loss of intensity due to imperfect editing efficiency, 3/2 is the creatine/GABA (total) proton ratio, and [Cr] is 9 mmol/kg wet weight—the creatine concentration in the human occipital cortex (30).
The detection of N-acetylaspartate, glutamate, glutamine, and other metabolites was done with an echo delay of 12 msec, a sweep width of 15,000 Hz, and an acquisition time of 510 msec. The sequence was applied in pairs to yield subspectra that contained either macromolecules alone or macromolecules combined with the metabolites of interest. Briefly, one subspectrum was acquired without the inversion pulse. The macromolecule subspectrum was subtracted from the other subspectrum to obtain a spectrum of metabolites alone. The data were acquired in interleaved fashion, toggling between individual 48-second sets of inverted and uninverted acquisitions. Each block was stored, and the sequence ran for 20 minutes to yield 16 pairs of subspectra. A scan of unsuppressed tissue water was also acquired to evaluate the absolute level of creatine and for eddy current correction of the short-echo data. Each sub-free-induction-delay (sub-FID) was processed by using a Lorentzian-to-Gaussian conversion of –1 and 6 Hz, Fourier transformation, spectral phasing, and subtraction. The peak amplitudes at 3.03, 2.75, 2.60, 2.45, 2.29, and 2.015 ppm were measured and deconvolved by using model spectra obtained in solutions of creatine, glutamate, glutamine, aspartate, and N-acetylaspartate to determine the metabolite levels relative to the resonance of total creatine. The concentrations were determined with the assumption that the wet weight for creatine had a concentration of 9 mmol/kg.
Human Subjects
Before participating in this study, all subjects (patients and comparison subjects) had a discussion with a research psychiatrist about the risks and benefits of being in the study. If subjects agreed to participate, they were asked to sign a copy of the consent document, which was approved by the Yale Institutional Review Board (IRB #8973), and were given a copy of this document for their own records. All research procedures were carried out in accordance with Yale Institutional Review Board regulations pertaining to protection of human subjects.
All patients and comparison subjects, in good physical health (confirmed by physical examination and screening laboratory studies, including urine toxicology, HIV test, and beta HCG test in female subjects) and medication free, gave their written informed consent to participate. The patients were 10 outpatients (five women and five men; mean age=36 years, SD=11) with active panic disorder (1 panic attack/week in the month before commencement of the study) whose baseline occipital cortex GABA levels were published in a previous report (9). The patients met the clinical research criteria (DSM-IV) for a current principal diagnosis of panic disorder with or without agoraphobia, confirmed by semistructured interview (Structured Clinical Interview for DSM-IV Axis I Disorders [SCID] [31] or Anxiety Disorders Interview Schedule: Lifetime Version [32]). In addition, they did not have a lifetime history of major depressive disorder or alcohol dependence, nor did they have a substance abuse disorder within 6 months of the diagnostic interview. Five of the 10 patients had been medication naive before the study, and two had not been taking medications for 3 months. Three of 10 patients had taken occasional, as-needed doses of shorter-acting benzodiazepine medications until 1 week before the study (two had taken 0.25 mg/day or 0.50 mg/day of alprazolam, one had taken 0.25 mg/day of clonazepam).
Retrospectively matched healthy comparison subjects (four women and five men; mean age=28 years, SD=3) were recruited after the MRS data had been obtained in the patients. The patients and the comparison subjects did not differ significantly in age (Welch’s t test t=2.2, df=10, p<0.06). We were unable to obtain an age match for one older female patient. The comparison subjects were a new group of subjects recruited specifically for this project. They had no lifetime history of psychiatric illness, as confirmed by semistructured interview (SCID), nor did they have a family history of psychiatric illness in first-degree relatives, as reported in a clinical interview. We attempted to match women comparison subjects and patients for phase of menstrual cycle. Three patients were at the end of their menstrual cycle, one patient was mid-cycle, and one was postmenopausal (unmatched patient) at the time of the initial scanning session.
Each patient and comparison subject received a baseline scan. Then, the patients and comparison subjects received a single oral dose of 0.5 mg of the anxiolytic medication clonazepam. After approximately 90 minutes, subjects were rescanned to assess the effect on cortical GABA. This timing was chosen because oral clonazepam is fairly rapidly absorbed, with peak plasma concentrations achieved by 1.5 hours after a single oral dose (33, 34). Six of the 10 patients received a third scan after approximately 1 month of successful treatment with 0.5 mg of clonazepam three times a day. The patients were scanned over a 2-year period from January 1997 to February 1999, and the comparison subjects were scanned over a 1-year period from November 1999 to November 2000. MRS scanning procedures/conditions remained constant for both patients and comparison subjects over this time frame.
Statistics
The data were analyzed with a mixed-effects model with a random effect for subject and fixed effects for time (baseline versus 90 minutes after medication administration), group (panic disorder patients versus comparison subjects), and the group-by-time interaction (PROC MIXED in SAS version 8, SAS Institute, Cary, N.C.). The main analyses were performed with and without the unmatched patient with similar outcomes. Therefore, we report the results of analyses that included all patients and comparison subjects. In the F tests, Satterthwhite’s approximation for degrees of freedom for unbalanced data was used.
Results
There was a highly significant group effect (F=29.34, df=1, 29.8, p<0.0001), which was accounted for by persistent low cortical GABA in the panic disorder patients (Figure 1), as well as a significant group-by-time interaction (F=5.08, df=1, 16.3, p<0.04). Follow-up tests indicated that there were significant changes from baseline cortical GABA levels at the 90-minute postmedication time point in the comparison group (F=7.83, df=1, 16, p<0.02) but not in the panic group (F=0.14, df=1, 16.7, p=0.72) (Figure 1 shows means and raw data; Figure 2 shows representative spectra). In the healthy group, there was a 24% (1.72–1.31/1.72×100) decrease from baseline GABA levels after acute benzodiazepine exposure. An additional random-effects analysis that included the third time point (after 4 weeks of treatment) for patients revealed no significant effect of time (F=0.04, df=2, 22.6, p=0.96) (Figure 1 and Figure 2). A comparison of baseline and postmedication glutamate and glutamine levels in seven of the nine comparison subjects indicated a small but significant increase in glutamate levels because of acute clonazepam administration (baseline: mean=6.02 mmol/kg, SD=0.74; postmedication: mean=6.71 mmol/kg, SD=1.04) (paired t test t=3.3, df=6, p<0.02), but no significant change in glutamine levels (baseline: mean=2.67 mmol/kg, SD=0.33; postmedication: mean=2.70 mmol/kg, SD=0.48) (t=0.22, df=6, p=0.84).
Discussion
Our data indicate that, normally, acute benzodiazepine administration decreases human occipital cortex GABA levels. This result suggests that benzodiazepines may have the capacity to exert a direct inhibitory effect on GAD activity. Alternatively, benzodiazepine effects on other neuronal targets could modify both the excitatory drive for GABA neuronal activity and the availability of the substrates for GABA synthesis, including glutamate and glutamine (35–37). As indicated by preliminary data in the comparison subjects, acute benzodiazepine administration did not decrease glutamate or glutamine levels in the occipital cortex voxel of interest, suggesting that acute reductions in substrate availability did not account for the reductions in cortical GABA. This preliminary result is supported by published observations indicating a lack of effect of acute lorazepam (2 mg p.o.) administration on dorsolateral prefrontal cortex glutamine and glutamate levels in healthy human subjects (38). In fact, glutamate levels were slightly elevated after benzodiazepine administration, suggesting an inhibitory effect of benzodiazepine administration on glutamate turnover. The hypothesis that benzodiazepine-induced reductions in glutamatergic drive deprive GABA neurons of excitatory input could be tested in the future in humans by using 13C-MRS with infusion of appropriate 13C-labeled tracers (39–41). Other mechanisms that could account for our GABA finding in healthy subjects include benzodiazepine effects on cortical glucose metabolism and/or cortical blood flow. Previous work with [15O]H2O positron emission tomography (PET) has documented the ability of lorazepam to acutely decrease cortical blood flow in the occipital cortex and other brain regions (36). Since blood flow and metabolism tend to run parallel in the CNS, it is possible that impaired glucose metabolism resulted in less rapid production of GABA in our study. Future 13C-glucose infusion/MRS studies or within-subject [18F]fluorodeoxyglucose PET and GABA/MRS studies should help clarify these issues.
Compared to healthy individuals, patients with a diagnosis of panic disorder did not exhibit reductions in cortical GABA levels after acute or chronic benzodiazepine administration. This result is complementary to our previous work describing lower GABA levels in the occipital cortex of patients with panic disorder, relative to age- and sex-matched comparison subjects (9). The combination of lower basal cortical GABA levels and the failure to exhibit reduction in GABA levels after benzodiazepine exposure is a pattern that is somewhat reminiscent of that seen in GAD65 knockout mice (3). An important difference between the human and animal studies is that, while the knockout mice exhibited lower levels of behavioral sensitivity to benzodiazepines (which indirectly suggested lowered intrasynaptic GABA levels), their GABA levels after benzodiazepine administration were not directly measured. Thus, while the current findings could potentially reflect deficits in GAD65 levels or function in patients with panic disorder, we caution against overinterpreting the relevance of the animal model for humans, pending further investigation. The present study could not rule out GABAA receptor dysfunction in panic disorder patients as a source of the dysfunctional GABA neuronal response we observed. However, the patients exhibited satisfactory clinical responses to benzodiazepine treatment, although their occipital cortex GABA levels did not decrease. In light of evidence that the α2 subunit of the GABAA receptor substantially confers the capacity to mediate anxiolytic effects of benzodiazepines (42), it would appear that at least some GABAA receptor subtypes remain functional in panic disorder patients. The continuation of low occipital cortex GABA even after effective benzodiazepine therapy could be a risk factor for clinical relapse in some patients. Follow-up studies are needed to determine whether there is eventual normalization of occipital cortex GABA levels with effective longer-term antipanic treatment. By contrast, effective pharmacotherapy with selective serotonin reuptake inhibitors normalizes occipital cortex GABA levels in patients with major depression (43), and similar work in panic disorder patients would be of interest to ascertain whether the chronically low cortical GABA we have begun to observe in panic disorder patients is independent of medication class.
A number of limitations of the present study should also be mentioned. An important design limitation was the lack of a placebo control condition for both the acute and chronic clonazepam exposure conditions. Inclusion of a placebo control condition would have permitted us to obtain additional test-retest occipital cortex GABA data for each group as well as to control for nonspecific stress effects on GABA because of the experimental protocol. Stress or acute panic symptoms could contribute to reductions in regional cerebral blood flow (44) and therefore may have confounded our data. However, none of the patients or comparison subjects had a panic episode just before or while being scanned. In addition, on the issue of stress or state anxiety effects on occipital cortex GABA, we previously reported in our baseline study that visual analog anxiety scale scores (a measure of state anxiety) did not correlate with baseline cortical GABA levels in the panic disorder patient group (r=–0.03, p=0.9, N=13) (9). Since the patients presented here are a subgroup of that original group, it appears that state anxiety and blood flow effects secondary to state anxiety were not major confounds of the baseline occipital GABA results of the patients. Moreover, there was no significant correlation between baseline visual analog anxiety scale scores and baseline GABA scores in six of the nine comparison subjects in the present study (r=0.13, N=6, p=0.8) (three comparison subjects did not have baseline visual analog anxiety scale data). In reference to future work on longer-term medication effects on occipital cortex GABA in panic disorder, the use of a placebo control group would permit delineation of placebo responders from medication responders and nonresponders, which would improve the interpretation of this type of data set. In the light of these issues, our present data on longer-term effects of clonazepam therapy on occipital cortex GABA must be considered preliminary.
This study was conducted with a small number of subjects, and therefore the possibility of type I error must also be considered. While the effect size for the comparison subjects was substantial, relative to the test-retest variability of our MRS method, it is conceivable that the effect could be diluted or washed out in a larger group of subjects. Another related concern is the stability of occipital cortex GABA measurement during the period of the study (approximately 3 years). The test-retest variation of our GABA/MRS method over a 2-year period is approximately 10% (26), so there is some potential for test-retest error to have biased our results. Although patients were scanned over a 2-year period and the comparison subjects were scanned over the subsequent 1-year period, scanning procedures remained constant during the entire study period. Another problem that could have been confounding was the issue of recent benzodiazepine exposure in three of the panic disorder patients. However, when we performed a post hoc, nonparametric analysis of the panic disorder group data, excluding the data for those three patients (baseline GABA scores versus 90-minutes postmedication scores), we noted that the pattern of results was similar to that observed in the entire group of panic disorder patients (baseline GABA level: mean=0.98 mmol/kg, SD=0.19, N=7; 90-minutes postmedication GABA level: mean=1.06 mmol/kg, SD=0.19, N=6) (Wilcoxon statistic=–7.0, p=0.56, N=6 pairs). In addition, although the difference in age between groups approached but did not reach significance, the patient group was older. Thus, it is conceivable that age effects could have complicated the interpretation of our data. Post hoc Pearson product-moment correlation analyses of age and baseline cortical GABA level in each group, however, suggested no significant relationship between age and GABA level in either group (healthy comparison group: r=0.06, p=0.88, N=8; panic disorder group: r=0.25, p=0.49, N=10).
Another potential problem with the comparability of the two data sets (patient group versus comparison group) is the possibility that a “floor effect” in the patient group biased against discernment of additional medication-related decreases in occipital cortex GABA. Other patient populations, such as those with severe melancholic major depression, have been reported to have extremely low occipital cortex GABA levels (mean=0.71 mmol/kg, SD=0.27, N=14) (21), suggesting that the levels of occipital cortex GABA measured by our MRS technique in this study were not at the lower limit of detection. However, it remains an open question whether the relatively low occipital cortex GABA levels of the panic disorder patients were at some kind of physiological floor at which acute perturbations of GABA function are incapable of producing further downward change because of counterregulatory mechanisms. This possibility could be tested in follow-up investigations, such as a dose-response protocol in healthy subjects.
Another design issue in the present study was that we did not have a reliable method of monitoring between-group pharmacokinetic differences in reference to clonazepam metabolism. Future studies that use this paradigm would benefit from either plasma monitoring of benzodiazepine levels or the use of an intravenous benzodiazepine administration protocol to enhance standardization of protocol procedures. It is of interest that the GABA data for the panic disorder group displayed more variability than that for the comparison group, as variation in drug metabolism could have contributed to this phenomenon. Other factors such as a family history of mood or anxiety psychopathology (45) or illness subtypes (46) could also have contributed to variability in the patient data.
Finally, there remain issues of interpretation in reference to the meaning of cortical GABA level data obtained in a relatively large volume of cortical tissue. Since GABA subserves many different CNS functions, it may be problematic to infer that changes in our integrated measure of intraneuronal GABA directly reflect GABA neuronal changes related to a specific clinical syndrome or medication. In this regard, future assessment of additional cortical areas more traditionally associated with anxiety, e.g., the frontal cortex, should be illuminating, as will efforts to quantitate GABA levels in white matter pathways that connect anxiety-relevant cortical regions.
Overall, these results strengthen the hypothesis that panic disorder is associated with GABA neuronal dysfunction. Our work thus far suggests that the GABA dysfunction in panic disorder may be a trait-like abnormality, since acute anxiolytic therapy with the benzodiazepine clonazepam was not associated with normalization of low occipital cortex GABA in panic disorder patients. In addition, there does not seem to be a correlation between illness severity and GABA nor between state anxiety and GABA, as we have previously reported (9). Furthermore, preliminary data point to an association between the extent of the lower than normal GABA levels in the occipital cortex in panic disorder and a family history of mood or anxiety psychopathology (45, 47). Preclinical studies that directly assess benzodiazepine effects on cortical GAD65 and GAD67 function and gene expression promise to clarify the biochemical basis of the effects that we have reported in this preliminary study. In addition, future molecular genetic studies of panic disorder, probing genes that could account for lower levels of GAD activity, would facilitate the interpretation of the current data and further advance our understanding of the contributions of GABA systems to panic disorder.
Received Nov. 25, 2002; revision received Dec. 29, 2003; accepted Jan. 18, 2004. From the Departments of Psychiatry, Biomedical Engineering, Internal Medicine, Radiology, and Epidemiology and Public Health, Yale University School of Medicine, New Haven, Conn.; and the Department of Psychiatry, Indiana University School of Medicine, Indianapolis. Address reprint requests to Dr. Goddard, Indiana University Department of Psychiatry, Adult Psychiatry Clinic, UH 3124A, 550 North University Blvd., Indianapolis, IN 46202-5266; [email protected] (e-mail). Supported by NIMH grants MH-01322 and MH-58657 (Dr. Goddard); NIMH Mental Health Clinical Research Center grant MH-30929 (Drs. Goddard, Krystal, and Mason); a grant from the National Alliance for Research on Schizophrenia and Depression (Dr. Mason); grant K02 1 AA-00261-01 from the National Institute on Alcohol Abuse and Alcoholism to the VA-Yale Alcohol Research Center and the National Center for Post-Traumatic Stress Disorder (Dr. Krystal); grant R29-N5032126 from the National Institute for Neurological Disorders and Stroke (Dr. Rothman); and the Connecticut Department of Mental Health and Addiction Services. The authors thank the staff of the Yale Anxiety Clinic and Program for their contributions to this work.
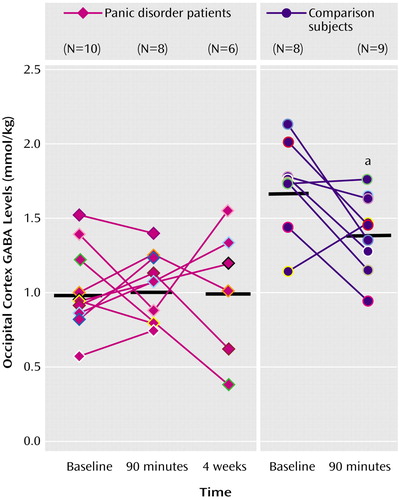
Figure 1. Occipital Cortex GABA Levels at Baseline and After Administration of Clonazepam in Panic Disorder Patients and Healthy Comparison Subjects
aStatistically significant change from baseline levels (p<0.02, F test).
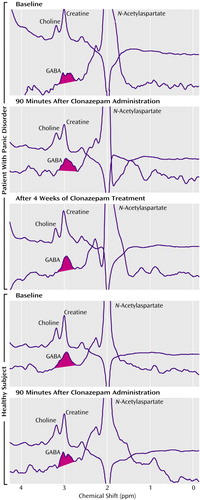
Figure 2. Representative GABA Spectra Obtained Before and After Clonazepam Exposure in a Panic Disorder Subject and an Unpaired Healthy Comparison Subjecta
aIn each panel, the top spectrum was obtained without the application of the DANTE (delays alternating with nutations for tailored excitations) pulse, and the bottom spectrum is a subtraction spectrum (values with DANTE pulse minus values without DANTE pulse), which highlights the GABA peak.
1. Kaneko K, Obata K: An increase in excitatory synaptic transmission in the lateral amygdala of adult GAD65 knockout mice. Abstr Soc Neurosci 2000; 30:1392Google Scholar
2. Stork O, Ji FY, Kaneko K, Stork S, Yoshinobu Y, Moriya T, Shibata S, Obata K: Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res 2000; 865:45–58Crossref, Medline, Google Scholar
3. Kash SF, Tecott LH, Hodges C, Baekkeskov S: Increased anxiety and altered responses to anxiolytics in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA 1999; 96:1698–1703Crossref, Medline, Google Scholar
4. Gunther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, Crestani F, Aguzzi A, Arigoni M, Lang Y, et al: Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA 1995; 92:7749–7753Crossref, Medline, Google Scholar
5. Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy JM, Luscher B, Mohler H: Decreased GABAA–receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci 1999; 2:833–839Crossref, Medline, Google Scholar
6. Shekhar A, Keim SR, Simon JR, McBride WJ: Dorsomedial hypothalamic GABA dysfunction produces physiological arousal following sodium lactate infusions. Pharmacol Biochem Behav 1996; 55:249–256Crossref, Medline, Google Scholar
7. Stork O, Ji FY, Obata K: Reduction of extracellular GABA in the mouse amygdala during and following confrontation with a conditioned fear stimulus. Neurosci Lett 2002; 327:138–142Crossref, Medline, Google Scholar
8. Berretta S, Munno DW, Benes FM: Amygdalar activation alters the hippocampal GABA system: “partial” modelling for postmortem changes in schizophrenia. J Comp Neurol 2001; 431:129–138Crossref, Medline, Google Scholar
9. Goddard AW, Mason GF, Almai A, Rothman DL, Behar KL, Petroff OAC, Charney DS, Krystal JH: Reductions in occipital cortex GABA levels in panic disorder detected with 1H-magnetic resonance spectroscopy. Arch Gen Psychiatry 2001; 58:556–561Crossref, Medline, Google Scholar
10. Waagepetersen HS, Sonnewald U, Schousboe A: The GABA paradox: multiple roles as metabolite, neurotransmitter, and neurodifferentiative agent. J Neurochem 1999; 73:1335–1342Crossref, Medline, Google Scholar
11. Schlegel S, Steinert H, Bockisch A, Hahn K, Schloesser R, Benkert O: Decreased benzodiazepine receptor binding in panic disorder measured by IOMAZENIL-SPECT: a preliminary report. Eur Arch Psychiatry Clin Neurosci 1994; 244:49–51Crossref, Medline, Google Scholar
12. Kaschka W, Feistel H, Ebert D: Reduced benzodiazepine receptor binding in panic disorders measured by iomazenil SPECT. J Psychiatr Res 1995; 29:427–434Crossref, Medline, Google Scholar
13. Kuikka JT, Pitkanen A, Lepola U, Partanen K, Vainio P, Bergstrom KA, Wieler HJ, Kaiser KP, Mittelbach L, Koponen H, et al: Abnormal regional benzodiazepine receptor uptake in the prefrontal cortex in patients with panic disorder. Nucl Med Commun 1995; 16:273–280Crossref, Medline, Google Scholar
14. Malizia AL, Cunningham VJ, Bell CJ, Liddle PF, Jones T, Nutt DJ: Decreased brain GABA (A)-benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Arch Gen Psychiatry 1998; 55:715–720Crossref, Medline, Google Scholar
15. Bremner JD, Innis RB, White T, Fujita M, Sibersweig D, Goddard AW, Staib L, Stern E, Cappiello A, Woods S, Baldwin R, Charney DS: SPECT [I-123]iomazenil measurement of the benzodiazepine receptor in panic disorder. Biol Psychiatry 2000; 47:96–106Crossref, Medline, Google Scholar
16. Roy-Byrne PP, Cowley DS, Hommer D, Greenblatt DJ, Kramer GL, Petty F: Effect of acute and chronic benzodiazepines on plasma GABA in anxious patients and controls. Psychopharmacology (Berl) 1992; 109:152–156Crossref, Google Scholar
17. Mason GF, Martin DL, Martin SB, Manor D, Sibson NR, Patel A, Rothman DL, Behar KL: Decrease in GABA synthesis rate in rat cortex following GABA-transaminase inhibition correlates with the decrease in GAD67 protein. Brain Res 2001; 914:81–91Crossref, Medline, Google Scholar
18. Sheikh S, Martin DL: Elevation of brain GABA levels with vigabatrin differentially affects GAD65 and GAD67 expression in various regions of rat brain. J Neurosci Res 1998; 52:736–741Crossref, Medline, Google Scholar
19. Izzo E, Auta J, Impagnatiello F, Pesold C, Guidotti A, Costa E: Glutamic acid decarboxylase and glutamate receptor changes during tolerance and dependence to benzodiazepines. Proc Natl Acad Sci USA 2001; 98:3483–3488Crossref, Medline, Google Scholar
20. Goddard AW, Narayan M, Woods SW, Germine M, Kramer GL, Davis LL, Petty F: Plasma levels of gamma-aminobutyric acid and panic disorder. Psychiatry Res 1996; 63:223–225Crossref, Medline, Google Scholar
21. Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH: Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 1999; 56:1043–1047Crossref, Medline, Google Scholar
22. Behar KL, Rothman DL, Petersen KF, Hooten M, Delaney R, Petroff OAC, Shulman GI, Navarro V, Petrakis IL, Charney DS, Krystal JH: Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. Am J Psychiatry 1999; 156:952–954Link, Google Scholar
23. Ke Y, Cohen BM, Bang JY, Yang M, Renshaw PF: Assessment of GABA concentration in human brain using two-dimensional proton magnetic resonance spectroscopy. Psychiatry Res Neuroimaging 2000; 100:169–178Crossref, Medline, Google Scholar
24. Wang PW, Dieckmann N, Sailasuta N, Adalsteinsson E, Spielman D, Ketter TA:3–T 1H-magnetic resonance spectroscopic measurements of prefrontal cortical gamma-aminobutyric acid (GABA) levels in bipolar disorder patients and healthy volunteers, in Abstracts of the 57th Annual Convention of the Society for Biological Psychiatry. Jacksonville, Fla, SOBP, 2002, p 197SGoogle Scholar
25. Rothman DL, Petroff OAC, Behar KL, Mattson RH: Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci USA 1993; 90:5662–5666Crossref, Medline, Google Scholar
26. Petroff OAC, Rothman DL: Measuring human brain GABA in vivo. Mol Neurobiol 1998; 16:97–121Crossref, Medline, Google Scholar
27. Shen J, Rycyna RE, Rothman DL: Improvements on an in vivo automatic shimming method (FASTERMAP). Magn Reson Med 1997; 38:834–839Crossref, Medline, Google Scholar
28. Behar KL, Ogino T: Characterization of macromolecule resonances in the 1H-NMR spectrum of rat brain. Magn Reson Med 1993; 30:38–44Crossref, Medline, Google Scholar
29. Hofmann L, Slotboom J, Boesch C, Kreis R: Characterization of macromolecular baseline in localized (1)H-MR spectra of human brain. Magn Reson Med 2001; 46:855–863Crossref, Medline, Google Scholar
30. Petroff OAC, Spencer DD, Alger JR, Prichard JW: High-field proton magnetic resonance spectroscopy of human cerebrum obtained during surgery for epilepsy. Neurology 1989; 39:1197–1202Crossref, Medline, Google Scholar
31. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
32. DiNardo PA, Brown TA, Barlow DH: Anxiety Disorders Interview Schedule: Lifetime Version (ADIS-IV-L). New York, Graywind, 1994Google Scholar
33. Wang L, Wang XD: Pharmacokinetic and pharmacodynamic effects of clonazepam in children with epilepsy treated with valproate: a preliminary study. Ther Drug Monit 2002; 24:532–536Crossref, Medline, Google Scholar
34. Chauhan BL, Sane SP, Revankar SN, Rammamurthy L, Doshi B, Bhatt AD, Bhate VR, Kulkarni RD: Comparative bioavailability study of clonazepam after oral administration of two tablet formulations. J Assoc Physicians India 2000; 48:985–987Medline, Google Scholar
35. Battaglioli G, Martin DL: Glutamine stimulates γ-aminobutyric acid synthesis in synaptosomes but other putative astrocyte-to-neuron shuttle substrates do not. Neurosci Lett 1996; 209:129–133Crossref, Medline, Google Scholar
36. Matthew E, Andreason P, Pettigrew K, Carson RE, Herscovitch P, Cohen R, King C, Johanson CE, Greenblatt DJ, Paul SM: Benzodiazepine receptors mediate regional blood flow changes in the living human brain. Proc Natl Acad Sci USA 1995; 92:2775–2779Crossref, Medline, Google Scholar
37. Patel AB, Rothman DL, Cline GW, Behar KL: Glutamine is the major precursor for GABA synthesis in rat neocortex in vivo following acute GABA-transaminase inhibition. Brain Res 2001; 919:207–220Crossref, Medline, Google Scholar
38. Brambilla P, Stanley JA, Nicoletti M, Harenski K, Wells KF, Mallinger AG, Keshavan MS, Soares JC: 1H MRS brain measures and acute lorazepam administration in healthy human subjects. Neuropsychopharmacology 2002; 26:546–551Crossref, Medline, Google Scholar
39. Sibson NR, Dhankhar A, Mason GF, Behar KL, Rothman DL, Shulman RG: In vivo 13C NMR measurements of cerebral glutamine synthesis as evidence for glutamate-glutamine cycling. Proc Natl Acad Sci USA 1997; 94:2699–2704Crossref, Medline, Google Scholar
40. Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, Petroff OA, Shulman GI, Shulman RG, Rothman DL: Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Nat Acad Sci USA 1999; 96:8235–8240Crossref, Medline, Google Scholar
41. Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL: Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci 2002; 22:1523–1531Crossref, Medline, Google Scholar
42. Uwe R, Crestani F, Mohler H: GABAA receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci 2001; 22:188–194Crossref, Medline, Google Scholar
43. Sanacora G, Mason GF, Rothman DL, Krystal JH: Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry 2002; 159:663–665Link, Google Scholar
44. Boshuisen ML, Ter Horst GJ, Paans AM, Reinders AA, den Boer JA: rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. Biol Psychiatry 2002; 52:126–135Crossref, Medline, Google Scholar
45. Goddard AW, Mason GF, Rothman DL, Behar KL, Petroff OAC, Krystal JH: Family psychopathology and magnitude of reductions in occipital cortex GABA levels in panic disorder (letter). Neuropsychopharmacology 2004; 29:639–640Crossref, Medline, Google Scholar
46. Sanacora G, Mason GF, Anand A, Epperson CN, Wu YT, Krystal JH: Occipital cortex GABA concentrations differentiate depressive subtypes, in Abstracts of the 58th Annual Convention of the Society for Biological Psychiatry. Jacksonville, Fla, SOBP, 2003, p 171SGoogle Scholar
47. Goddard AW, Mason GF, Rothman DL, Behar KL, Krystal JH: Influence of medication and family history on cortical GABA levels in panic disorder, in Abstracts of the 57th Annual Convention of the Society for Biological Psychiatry. Jacksonville, Fla, SOBP, 2002, p 48SGoogle Scholar



