Effect of Abrupt Change From Standard to Low Serum Levels of Lithium: A Reanalysis of Double-Blind Lithium Maintenance Data
Abstract
OBJECTIVE: Growing evidence suggests that abrupt lithium discontinuation increases the risk of recurrence for patients with bipolar disorder. To assess the effect of abrupt change in lithium dose, the authors reanalyzed data from a previously reported, randomized, double-blind trial of standard- versus low-dose lithium for maintenance therapy in bipolar disorder. METHOD: In the original study, serum lithium levels were obtained during a 2-month open stabilization period for 94 patients with bipolar disorder who were then randomly assigned to be maintained on a low (serum level=0.4–0.6 meq/liter) or a standard (0.8–1.0 meq/liter) level of lithium therapy. Patients were then followed for up to 182 weeks. This reanalysis examined the potential confounding influence of prerandomization lithium level and change in lithium level on the outcome of subjects assigned to a standard or low maintenance dose of lithium. RESULTS: In a Cox proportional hazards model incorporating pre- and postrandomization lithium levels and the interaction of these factors, only the interaction term remained significantly associated with time to recurrence. CONCLUSIONS: The findings indicate that change in serum lithium level may be a more powerful predictor of recurrence of bipolar disorder than the absolute assignment to a low or a standard dose of lithium and suggest that an abrupt decrease in lithium level should be avoided. This reanalysis did not directly address optimal maintenance lithium levels but raises questions about the original study’s finding of superiority for lithium levels ≥0.8 meq/liter. The results underscore the importance of accounting for the possible confounding effects of changes in the intensity of pharmacotherapy in studies of maintenance therapies for bipolar disorder.
Lithium discontinuation may have significant negative consequences for patients with bipolar disorder, particularly in the first year after treatment is terminated (1, 2). After discontinuation, the risk of recurrence of affective illness, and particularly of manic episodes, appears to be greater than that expected in untreated patients (3). Overall morbidity and suicidal behavior in particular are also greater (4). This elevated risk has been demonstrated in a group of pregnant women and nonpregnant comparison subjects (5) and in adolescents with bipolar I disorder (6). However, not all studies have shown this elevation (7).
Abrupt lithium discontinuation may yield a particularly great risk for relapse (2). In two studies that addressed this concern, gradual taper (>14 days) significantly reduced morbidity compared with a rapid taper (8, 9). A later study noted a similar effect (5).
The consequences of rapid change in lithium levels during treatment have not been investigated in detail. Fluctuations in serum lithium levels are quite common even during long-term treatment, particularly as compliance with treatment may be erratic (10, 11). Poor compliance has been independently associated with poorer outcome (12).
The purpose of this investigation was to examine the apparent risk posed by an abrupt change in lithium level. We reanalyzed data from a study of the efficacy of low- versus standard-dose lithium in maintenance therapy for patients with bipolar disorder (13) to examine the potential confounding influence of rapid change in lithium dose on the effects of the random assignment to treatment groups. In that study, stable, euthymic patients with bipolar disorder who were being treated with lithium were randomly assigned to maintenance treatment with either standard or low serum lithium levels. However, as that study was performed before the effects of abrupt discontinuation were described, the original analysis did not include an interaction effect between the baseline and the randomly assigned lithium levels. On the basis of the more recent data of Faedda and Baldessarini and colleagues (8, 9), we hypothesized that the patients who experienced a dose reduction would be at greater risk for recurrence than those who continued to receive their original dose. We investigated this hypothesis by examining the interactive effects of previous dose levels on the outcome of a standard- versus a low-dose strategy for maintenance treatment.
Method
Original Study Method
Participants in the original study by Gelenberg et al. (13) met Research Diagnostic Criteria (RDC) and DSM-III criteria for bipolar illness with at least one episode of mania; were age 18–75 years; and were clinically stable, with at least 6 months elapsed from the onset of their last mood episode and 2 months or more of recovery. Subjects with rapid cycling (four or more episodes per year) or those who had not tolerated lithium levels of at least 0.6 meq/liter for 2 months were excluded. After complete description of the study to the subjects, written informed consent was obtained.
To establish serum lithium level at study entry, the 94 outpatients in the original study were initially treated for a baseline period of 2 months without changing their dose of lithium. During the baseline period, lithium was prescribed in capsules of the same appearance as those to be used in the randomization phase of the study so there would be no change in the appearance of the medication at the time of random assignment to treatment groups. After the patients were randomly assigned to a treatment group (0.4–0.6 meq/liter versus 0.8–1.0 meq/liter), a study physician who was not blinded to the subjects’ treatment group assignment adjusted each patient’s lithium dose to produce the designated serum lithium level. For patients with a prerandomization lithium level in the standard range who were assigned to the low lithium level group, the 300-mg lithium study capsules were replaced by identical 150-mg capsules, thus halving the dose while keeping the number of capsules constant.
Assignment was stratified and blocked at each treatment center on the basis of three clinical variables: length of remission since the last affective episode (less than 1 year versus 1 year or more), polarity of the last episode (mania versus depression), and number of prior episodes in the past 3 years (none, one, or two versus three or more).
Reanalysis Method
In the original report, the potential influence of the change in dose status was not examined. To assess this potential confounding variable, we treated the prerandomization dose as if it were a stratification variable, defined as a low (producing a serum lithium level <0.6 meq/liter) or a standard (≥0.6 meq/liter) dose range. We used a Cox proportional hazards model to investigate the influence of the randomized treatment condition (low versus standard maintenance dose) relative to the main and interactive effects of prerandomization serum level. We also included the original stratification terms (length of remission, polarity of last episode, and number of episodes in the past 3 years) in this model as possible markers of recurrence risk.
In our outcome analysis, we used the same outcome criteria that were used in the original study: patients were defined as depressed or manic if they met either the appropriate DSM-III or the appropriate RDC criteria as assessed by clinicians blinded to treatment status. Hypomania in that study was defined as meeting RDC criteria for hypomania for 4 consecutive weeks, and occurrence of hypomania was also considered an endpoint. All analyses used an intent-to-treat design, with patients who did not relapse censored at the end of the follow-up period.
We subsequently illustrated the outcome of this analysis by presenting Kaplan-Meier survival curves for each of the following groups identified in relation to the point of random assignment:
1. Standard before and after: patients with prerandomization serum lithium levels in the standard range who were assigned to the standard range treatment group; at entry to the treatment phase, their lithium dose was not changed.
2. Standard before and low after: patients with prerandomization lithium levels in the standard range who were assigned to the low-range treatment group; at entry to treatment phase, their lithium dose was decreased.
3. Low before and after: patients with prerandomization serum lithium levels in the low range who were assigned to the low range treatment group; at entry to treatment phase, their lithium dose was not changed.
4. Low before and standard after: patients with prerandomization lithium levels in the low range who were assigned to the standard-range treatment group; at entry to treatment phase, their lithium dose was increased.
For this analysis, results from patients who did not relapse were censored at end of follow-up or after 52 weeks, whichever was less. This latter endpoint was selected because we believed that, given the substantial rates of discontinuation in the original study, survival curves would be unreliable beyond 1 year.
Survival curves were compared with the log-rank (Mantel-Cox) chi-square test. To better describe the four groups, baseline demographic and clinical characteristics of the groups were compared by using Kruskal-Wallis analysis of variance (ANOVA) for age and the Pearson chi-square test for all other variables. Statview for Windows, version 5.0 (SAS Institute, Cary, N.C.) was used for all analyses. All statistical tests were two-tailed, with significance set at p<0.05.
Results
Population and Baseline Characteristics
At baseline 94 patients were randomly assigned to treatment groups, including 28 men and 28 women at Massachusetts General Hospital and 14 men and 24 women at Hillside Hospital.
Likelihood of Relapse
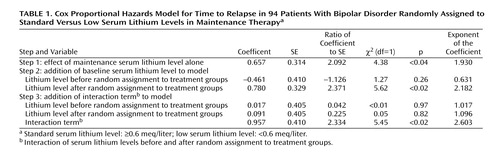
Consistent with the original results of Gelenberg et al. (13), random assignment to the low serum lithium level group was associated with greater risk of relapse (χ2=4.36, df=1, p<0.05) in a Cox proportional hazards model when the prerandomization lithium level was ignored (Table 1). Entry of the main effect of the prerandomization lithium level did not alter this result and was not, by itself, a significant predictor of outcome (χ2=1.27, df=1, p>0.25). However, when the interaction between the preexisting lithium level and the assigned level was considered, only this term significantly predicted longitudinal outcome (χ2=5.45, df=1, p<0.02). The exponentiated Cox regression coefficient for this term was 2.60 (95% confidence interval [CI]=1.17–5.82), indicating that the effect of reduction to low serum lithium levels was nearly three times greater for patients with prerandomization levels in the standard range than it was for patients with prerandomization levels in the low range.
To further control for the effects of illness severity at baseline, each of the three clinical severity measures was incorporated in turn into the Cox model. The interaction term remained significant for the duration of remission (χ2=5.85, df=1, p<0.02), polarity of the prior episode (χ2=8.25, df=1, p<0.01), and number of prior episodes (χ2=7.58, df=1, p<0.01). Duration of remission was also significantly associated with relapse (χ2=4.93, df=1, p<0.05), as was the prior number of episodes (χ2=4.97, df=1, p<0.05); the polarity term was not significant (χ2=0.53, df=1, p>0.4).
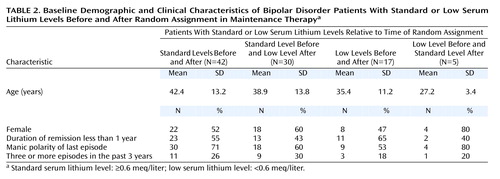
In an effort to better understand the groups formed by considering serum lithium levels before and after random assignment, demographic and illness severity characteristics were compared for the following four groups: standard serum lithium levels before and after randomization (N=42), standard level before and low level after (N=30), low before and after (N=17), and low before and standard after (N=5). The unequal group sizes resulted from the subjects’ original assignment to the low and standard treatment groups, which was not block-randomized by the stabilization-phase lithium level. Table 2 shows the gender, mean age, and clinical severity variables for each of the four comparison groups. There were no statistically significant differences between groups at baseline for any of the demographic or severity variables (p>0.5 for all comparisons except age by Pearson chi-square test; p>0.2 for age by Kruskal-Wallis ANOVA).
Kaplan-Meier survival curves for each of the four groups created by consideration of the interaction term are presented in Figure 1. Subjects were censored at end of follow-up or 52 weeks, whichever was less; analysis without the latter criterion yielded similar results (results not shown). The relapse rate did not differ significantly between patients whose lithium doses were unchanged after randomization in either the standard or the low lithium level groups (Pearson χ2=0.76, df=1, p>0.3). We found a significantly greater relapse rate in the patients with a prerandomization standard dose who were assigned to the low lithium level group, relative to those who were assigned to the standard level group (log rank [Mantel-Cox] χ2=5.55, df=1, p<0.02). There was also a significantly greater relapse rate among patients with a prerandomization standard lithium level who were assigned to the low level group, compared with to those who continued in the low level group (χ2=5.17, df=1, p<0.03). Only five patients with a low prerandomization level were assigned to the standard level group, and comparisons with the other three groups yielded no statistically significant differences in relapse rate.
Discussion
In our reanalysis of the data from a prospective, randomized trial of lithium maintenance in bipolar disorder (13), we found that the assigned dose of maintenance treatment appeared to have a dramatically different effect depending on whether this dose represented a change in treatment intensity. Patients whose lithium levels were abruptly decreased were more likely to suffer a recurrence than those whose levels were maintained in the same range as their prerandomization level. Indeed, when the change in dose level was taken into account in our reanalysis, the absolute level of maintenance therapy (low versus standard) was no longer a significant predictor of longitudinal outcome.
Our finding that a rapid decrease in serum lithium level may substantially increase the risk for episode recurrence is consistent with earlier studies (2, 5) that have demonstrated a similar risk after abrupt lithium discontinuation. It also extends the findings of a small study in which patients with decreases in serum lithium level of greater than 0.2 meq/liter had a higher recurrence rate (14).
The major limitation of this study is that it represents a post hoc analysis, addressing a question the original study was not designed to answer. Many factors in the original study, as well as our use of an intent-to-treat analysis, could bias our results towards the null hypothesis. For example, perhaps because of poorer compliance, lithium levels in the standard level group tended to deviate toward lower-than-target levels, which could obscure a true dose-response relationship. However, as it is unlikely that such a study could now be done prospectively for both ethical and logistical reasons, our results may represent the best opportunity to evaluate the consequences of abrupt dose change.
With these caveats in mind, a central implication of our findings is that conclusions about the negative effects of low maintenance doses in the Gelenberg et al. study may actually be specific to the change in dose level. It is possible that some lithium-treated patients may be safely maintained at lower serum lithium levels than standard guidelines would suggest. This interpretation is consistent with the results of several prospective maintenance trials (15–17) recently reviewed by Hopkins and Gelenberg (18). In addition, in a crossover study that did discern a benefit of higher compared to lower lithium levels, many of the relapses occurred within 2 months after an abrupt decrease in lithium dose (14). In our analysis, patients who continued to receive their original low dose of lithium did as well as patients who continued to receive their higher dose, although the small groups generated by our post hoc analysis yielded insufficient statistical power to detect a significant difference.
A complementary interpretation of these results is that greater baseline lithium level was a marker for greater disease severity, an example of confounding by indication. Patients came to their prerandomization lithium dose as a result of their personal history of treatment. However, it is noteworthy that we were unable to identify such differences in severity by examining three stratification variables from the original study: the number of prior episodes, duration of euthymia, and the polarity of the last episode. Moreover, statistical control of these severity variables in our proportional hazards models did not eliminate the interaction between pre- and postrandomization serum lithium levels on longitudinal outcome.
Even if it is not a marker of severity per se, serum lithium level at study entry could represent a marker for a given patient’s lithium requirement. A magnetic resonance spectroscopy study suggested only a weak correlation between serum lithium levels in the 0.6–1.0 meq/liter range and brain lithium levels (19). This finding alone challenges the idea that high and low serum lithium levels may be adequate a priori for judging effective levels of the drug. Once an effective lithium dose has been established clinically for a particular patient, any change, not just abrupt change, could yield greater risk of recurrence.
Medication discontinuation strategies are relatively commonplace in studies of maintenance treatments of bipolar disorder (20). Our study underscores the importance of considering current dose levels and taper effects, when examining the effects of maintenance treatments. In particular, we suggest that studies utilizing rapid discontinuation strategies may not provide an unambiguous accounting of treatment effects. For example, in many early placebo-controlled studies of the effects of lithium, lithium was abruptly discontinued on study entry (21–24), which may have artificially elevated the relapse rate in the placebo group, exaggerating the observed comparative benefit of lithium. As the optimum taper duration is unknown, even more recent studies in which lithium was tapered over 2 weeks (20) may not represent true comparisons of treatment effects. Until the discontinuation effect is better characterized, we concur with the recommendations of Baldessarini et al. (9) and Viguera et al. (5) that lithium should be tapered over at least 2 weeks.
Our study was not able to answer questions about the effects of an abrupt increase in lithium dose for patients already receiving a low dose of medication. Only five patients underwent such a change, precluding confidence in the recurrence rates obtained for this cohort. Nonetheless, the relatively high recurrence rate we observed in this group bears watching in future studies where rapid dose escalation is used for otherwise stable patients.
The neurobiological mechanisms underlying the effects of rapid lithium dose change also merit further investigation. Lithium may yield both acute and chronic changes in central neurotransmission (25, 26). In particular, adaptation to long-term lithium treatment may occur, rendering patients more vulnerable to fluctuations in lithium levels. One single photon emission computed tomography study (27) of acute lithium withdrawal in stable euthymic bipolar disorder patients identified profound changes in perfusion: an increase in inferior posterior regions and a decrease in anterior cingulate cortex and other limbic areas. Similar studies after changes in lithium level may help to clarify the means by which recurrence risk is increased.
In summary, our study provides further evidence that abrupt decrease in lithium dose is associated with an elevated risk of recurrence in bipolar disorder. Past trials of lithium maintenance should be interpreted cautiously with this potential confounder in mind, and future trials should be designed to avoid or account for this effect.
 |
 |
Received April 24, 2001; revision received Dec. 7, 2001; accepted Jan. 22, 2002. From the Department of Psychiatry, Massachusetts General Hospital. Address reprint requests to Dr. Perlis, Department of Psychiatry, Massachusetts General Hospital, 50 Staniford St., 5th Floor, Boston, MA 02114; [email protected] (e-mail). Supported in part by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression and a Daland Fellowship in Clinical Investigation from the American Philosophical Society (Dr. Perlis). The authors thank the other principal authors of the original lithium trial, including Alan J. Gelenberg, M.D., Martin B. Keller, M.D., and Phillip Lavori, Ph.D.
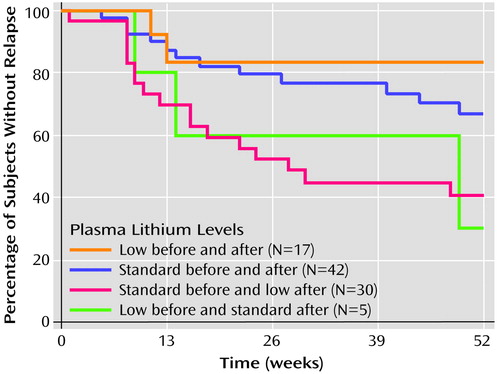
Figure 1. Kaplan-Meier Survival Curves Showing Percentage of Bipolar Disorder Patients Without Relapse in Four Groups Defined by Standard or Low Serum Lithium Levels Before and After Random Assignment in Maintenance Therapya
aStandard serum lithium level: ≥0.6 meq/liter; low serum lithium level: <0.6 meq/liter. Patients were censored at 52 weeks or end of follow-up, whichever was less.
1. Baldessarini RJ, Tondo L, Faedda G, Suppes T, Floris G, Rudas N: Effects of the rate of discontinuing lithium maintenance treatment in bipolar disorders. J Clin Psychiatry 1996; 57:441-448Crossref, Medline, Google Scholar
2. Baldessarini RJ, Tondo L: Recurrence risk in bipolar manic-depressive disorders after discontinuing lithium maintenance treatment: an overview. Clin Drug Invest 1998; 15:337-351Crossref, Medline, Google Scholar
3. Suppes T, Baldessarini RJ, Faedda GL, Tohen M: Risk of recurrence following discontinuation of lithium treatment in bipolar disorder. Arch Gen Psychiatry 1991; 48:1082-1088Crossref, Medline, Google Scholar
4. Baldessarini RJ, Tondo L, Hennen J: Effects of lithium treatment and its discontinuation on suicidal behavior in bipolar manic-depressive disorders. J Clin Psychiatry 1999; 60(suppl 2):77-84Google Scholar
5. Viguera A, Nonacs R, Cohen L, Tondo L, Murray L, Baldessarini RJ: Risk of recurrence in bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry 2000; 157:179-184Link, Google Scholar
6. Strober M, Morrell W, Lampert C, Burroughs J: Relapse following discontinuation of lithium maintenance therapy in adolescents with bipolar I illness: a naturalistic study. Am J Psychiatry 1990; 147:457-461Link, Google Scholar
7. Sashidharan S, McGuire R: Recurrence of affective illness after withdrawal of long-term lithium treatment. Acta Psychiatr Scand 1983; 68:126-133Crossref, Medline, Google Scholar
8. Faedda GL, Tondo L, Baldessarini RJ, Suppes T, Tohen M: Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry 1993; 50:448-455Crossref, Medline, Google Scholar
9. Baldessarini RJ, Tondo L, Floris G, Rudas N: Reduced morbidity after gradually discontinuing lithium in bipolar I and II disorders: a replication study. Am J Psychiatry 1997; 154:551-553Link, Google Scholar
10. Guscot R, Taylor L: Lithium prophylaxis in recurrent affective illness: efficacy, effectiveness and efficiency. Br J Psychiatry 1994; 164:741-746Crossref, Medline, Google Scholar
11. Johnson R, McFarland B: Lithium use and discontinuation in a health maintenance organization. Am J Psychiatry 1996; 153:993-1000Link, Google Scholar
12. Kulhara P, Basu D, Mattoo S, Sharan P, Chopra R: Lithium prophylaxis of recurrent bipolar affective disorder: long-term outcome and its psychosocial correlates. J Affect Disord 1999; 54:87-96Crossref, Medline, Google Scholar
13. Gelenberg AJ, Kane JM, Keller MB, Lavori P, Rosenbaum JF, Cole K, Lavelle J: Comparison of standard and low serum levels of lithium for maintenance treatment of bipolar depression. N Engl J Medicine 1989; 321:1489-1493Crossref, Medline, Google Scholar
14. Waters B, Lapierre Y, Gagnon A, Cahudhry R, Tremblay A, Sarantidis D, Gray R: Determination of the optimal concentration of lithium for the prophylaxis of manic-depressive disorder. Biol Psychiatry 1982; 17:1323-1329Medline, Google Scholar
15. Jerram T, McDonald R: Plasma lithium control with particular reference to minimum effective levels, in Lithium in Medical Practice. Edited by Johnson F, Johnson S. Baltimore, University Park Press, 1978, pp 407-413Google Scholar
16. Coppen A, Abou-Saleh M, Milln P, Bailey J, Wood K: Decreasing lithium dosage reduces morbidity and side-effects during prophylaxis. J Affect Disord 1983; 5:353-362Crossref, Medline, Google Scholar
17. Vestergaard P, Licht RW, Brodersen A, Rasmussen N, Christensen H, Arngrim T, Gronwall B, Kristensen E, Poulstrup I: Outcome of lithium prophylaxis: a prospective follow-up of affective disorder patients assigned to high and low serum levels. Acta Psychiatr Scand 1998; 98:310-315Crossref, Medline, Google Scholar
18. Hopkins H, Gelenberg A: Serum lithium levels and the outcome of maintenance therapy of bipolar disorder. Bipolar Disord 2000; 2:174-179Crossref, Medline, Google Scholar
19. Sachs GS, Renshaw PF, Lafer B, Stoll AL, Guimaraes AR, Rosenbaum JF, Gonzalez RG: Variability of brain lithium levels during maintenance treatment: a magnetic resonance spectroscopy study. Biol Psychiatry 1995; 38:422-428Crossref, Medline, Google Scholar
20. Bowden C, Calabrese J, McElroy S, Gyulai L, Wassef A, Petty F, Pope HJ, Chou J, Keck PJ, Rhodes L, Swann A, Hirschfeld R, Wozniak P (Divalproex Maintenance Study Group): A randomized, placebo-controlled 12-month trial of divalproex and lithium treatment of outpatients with bipolar I disorder. Arch Gen Psychiatry 2000; 57:487-489Crossref, Google Scholar
21. Baastrup P, Poulsen J, Schou M, Thomsen K, Amdisen A: Prophylactic lithium: double blind discontinuation in manic-depressive and recurrent-depressive disorders. Lancet 1970; 2:326-330Crossref, Medline, Google Scholar
22. Melia P: Prophylactic lithium: a double-blind trial in recurrent affective disorders. Br J Psychiatry 1970; 116:621-624Crossref, Medline, Google Scholar
23. Prien R, Caffey EJ, Klett C: Prophylactic efficacy of lithium carbonate in manic-depressive illness: report of the Veterans Administration and National Institute of Mental Health collaborative study group. Arch Gen Psychiatry 1973; 28:337-341Crossref, Medline, Google Scholar
24. Stallone F, Shelley E, Mendlewicz J, Fieve R: The use of lithium in affective disorders, 3: a double-blind study of prophylaxis in bipolar illness. Am J Psychiatry 1973; 130:1006-1010Link, Google Scholar
25. Lennox R, McNamara R, Papke R, Manji H: Neurobiology of lithium: an update. J Clin Psychiatry 1998; 59(suppl 6):37-47Google Scholar
26. Post R, Weiss S, Clark M, Chuang D, Hough C, He L: Lithium, carbamazepine, and valproate in affective illness: biochemical and neurobiologival mechanisms, in Bipolar Medications: Mechanisms of Action. Edited by Manji H, Bowden C, Belmaker R. Washington, DC, American Psychiatric Press, 2000, pp 219-248Google Scholar
27. Goodwin G, Cavanaugh J, Glabus M, Kehoe R, O’Carroll R, Ebmeier K: Uptake of 99mTc-exametazime shown by single photon emission computed tomography before and after lithium withdrawal in bipolar patients: associations with mania. Br J Psychiatry 1997; 170:426-430Crossref, Medline, Google Scholar



