Prospective Long-Term Follow-Up of 44 Patients Who Received Cingulotomy for Treatment-Refractory Obsessive-Compulsive Disorder
Abstract
OBJECTIVE: Long-term outcome associated with cingulotomy for obsessive-compulsive disorder (OCD) was prospectively assessed. Findings are reported for 18 patients previously described in 1995 and for 26 new patients. METHOD: An open preoperative and follow-up assessment was conducted at multiple time points for 44 patients undergoing one or more cingulotomies for treatment-refractory OCD. The patients were assessed by using the Structured Clinical Interview for DSM-III-R preoperatively and with the Yale-Brown Obsessive Compulsive Scale, the Beck Depression Inventory, and the Sickness Impact Profile both preoperatively and at all follow-up assessments. The patients completed clinical global improvement scales at all follow-up assessments. RESULTS: At mean follow-up of 32 months after one or more cingulotomies, 14 patients (32%) met criteria for treatment response and six others (14%) were partial responders. Thus, 20 patients (45%) were at least partial responders at long-term follow-up after one or more cingulotomies. Few adverse effects were reported. CONCLUSIONS: Thirty-two percent to 45% of patients previously unresponsive to medication and behavioral treatments for OCD were at least partly improved after cingulotomy. Cingulotomy remains a viable treatment option for patients with severe treatment-refractory OCD.
Obsessive-compulsive disorder (OCD) has a lifetime prevalence of 1%–3% (1, 2). Although most patients with OCD are successfully treated with pharmacotherapy and/or behavior therapy, a small fraction remain incapacitated despite numerous medication trials and intensive behavior therapy. Since the 1960s, neurosurgical treatment has been used for patients with especially severe and treatment-refractory OCD.
We previously reported the results of a retrospective follow-up of 33 patients who had undergone cingulotomy for treatment-refractory OCD at Massachusetts General Hospital between 1965 and 1986 (3). Using the criteria of improvement of at least 35% in the Yale-Brown Obsessive Compulsive Scale score and a clinical global improvement rating of 1 (“much better”) or 2 (“moderately better”), we estimated that 25% to 30% of these patients benefited substantially from this procedure.
Next, we reported a prospective study, including pre- and postoperative assessments, of 18 patients with OCD undergoing cingulotomy at Massachusetts General Hospital (4). The prospective study used the same criteria for improvement that were used in the 1991 study. At a mean follow-up of 26.8 months, 25% to 30% of the patients, who had previously been unresponsive to medication and behavior treatments, were significantly improved after cingulotomy.
We now report the results of an extended prospective longitudinal follow-up of 44 patients, including the 18 patients in our initial prospective study (4), who have had cingulotomy for OCD at Massachusetts General Hospital since 1989. This study included more than double the number of patients who were initially followed and increased the length of follow-up.
Our specific research questions were:
1. What proportion of patients undergoing cingulotomy for OCD will be classified as responders at follow-up approximately 6 months after the operation and at the most recent follow-up?
2. How will patients who undergo multiple cingulotomies respond at the initial and most recent follow-up?
3. Will patients undergoing cingulotomy show significant changes in functional status at the most recent follow-up?
4. What is the nature and frequency of adverse effects associated with cingulotomy?
Method
Evaluation of Candidacy
Guidelines for assessing candidacy for clinical intervention specified that each patient must have 1) met DSM-III-R criteria for OCD, 2) had severe OCD symptoms and functional impairment, and 3) failed a specific and rigorous regimen of medication and behavior therapy trials. In addition, the referring psychiatrist was required to agree to follow the patient after release from the hospital after cingulotomy. The patients were referred by their local psychiatrists to the Massachusetts General Hospital Cingulotomy Assessment Committee for limbic system surgery for severe OCD. The assessment committee, which consisted of three psychiatrists, a neurosurgeon, and a neurologist, reviewed each patient’s records and determined whether preliminary approval for surgery was appropriate. Next, patients were directly interviewed and examined by a psychiatrist, a neurologist, and a neurosurgeon, and they completed additional tests and evaluations. They were then assessed at the Massachusetts General Hospital OCD Clinic and Research Unit, where the Structured Clinical Interview for DSM-III-R for diagnosis of axis I disorders (5) was administered. They were also assessed with additional standardized instruments, including the Yale-Brown Obsessive Compulsive Scale (6), the Beck Depression Inventory (7), and the Sickness Impact Profile (8).
Most surgical candidates have OCD that has been unresponsive to all available and appropriate psychotropic medication trials and behavior treatments. Each patient was required to have had adequate trials (at least 10 weeks at the maximally tolerated dose) of at least three of the serotonin reuptake inhibitors (clomipramine, fluoxetine, sertraline, paroxetine, fluvoxamine, or citalopram) and augmentation of at least one of the previous drugs for 1 month with at least two of the following medications: lithium, clonazepam, buspirone, or a neuroleptic. All patients also must have had an adequate trial of behavior therapy consisting of at least 20 hours of exposure and response prevention. Information regarding medication compliance history was obtained from the patient’s referring psychiatrist and the patient’s records, in addition to the history provided by the patient. The maximum dose tolerated was a subjective measure, in large part influenced by the patient’s ability or willingness to tolerate adverse effects. The team attempted to ensure, through its communication with the patient and the referring physician, that previous treatment trials were not abandoned prematurely due solely to mild side effects. In a more general sense, patients who were characterized as poorly compliant (e.g., missing appointments, not taking their medications as prescribed, not genuine in their effort to participate in behavior therapy) were not deemed appropriate candidates for surgery. Patients who were accepted for cingulotomy provided informed consent for surgery and clinical follow-up.
Patient Characteristics
Forty-four patients (28 men and 16 women) were approved for and underwent one or more cingulotomy procedures at Massachusetts General Hospital. The mean age of the patients at the time of the operation was 34.4 years (SD=11.8, range=16–69).
Operative Technique
Through bilateral burr holes, 1.2 cm in diameter, 9.5 cm posterior to the nasion, and 1.5 cm to either side of the midline, electrically insulated thermistor electrodes were positioned in each cingulate gyrus with magnetic resonance imaging (MRI) stereotactic guidance. The initial targets were located 0.7 cm lateral to the midline bilaterally, 2 cm posterior to the most anterior portions of the frontal horns, and 1.0 mm above the roof of the ventricles. Typically lesions are created by heating the uninsulated 1.0-cm tip of the electrode to 80–85 degrees Centigrade for 90 seconds by radiofrequency current. The electrode is then withdrawn 1.0 cm and the lesion is enlarged superiorly by using the same lesion parameters. These steps are repeated for the opposite hemisphere. This produces symmetrical lesions in the anterior cingulate cortex bilaterally. A recent morphometric magnetic resonance imaging study involving a subset of patients (N=9) undergoing cingulotomy for treatment-refractory OCD at Massachusetts General Hospital demonstrated that the mean total lesion volume for the cohort was 3.58 cm3 (SD=1.24, range=1.97–5.83), with the average center of the lesions lying 9 mm lateral from the midsagittal plane, 18 mm anterior to the anterior commissure, and 30 mm superior to the intercommissural plane (9). When repeated cingulotomies were required, additional lesions were placed 8–10 mm anterior to the initial lesions.
Follow-Up Assessments
MRI scans were obtained for all patients within 48–72 hours after the cingulotomy to confirm placement of lesions. An attempt was made to follow-up all patients at approximately 6 months after their first cingulotomy, and follow-up of the entire cohort has been initiated approximately every 5 years. The initial 6-month follow-up point was chosen because it corresponds to the clinical practices of the Massachusetts General Hospital Cingulotomy Assessment Committee. Specifically, the committee typically will not consider a patient for a second cingulotomy until 6 postoperative months have passed. Consequently, a patient whose referring psychiatrist suggests a second cingulotomy will typically initiate contact with the assessment committee at the 6-month time point, thereby facilitating the follow-up assessment. At this re-evaluation point, the assessment committee reviews the patient’s progress since the first evaluation, reviews the referring physician’s report, and determines whether a second cingulotomy is indicated because of enduring severe symptoms. Because almost all patients lived some distance from Massachusetts General Hospital, only those patients who were scheduled to return to the Neurosurgery Service for evaluation before a second cingulotomy procedure were followed-up in person. All other patients were followed-up through interviews by telephone. The following rating scales were administered during these follow-up visits or telephone calls: the Yale-Brown Obsessive Compulsive Scale, the clinical global improvement scale, the Beck Depression Inventory, and the Sickness Impact Profile.
Rating Scales
The Yale-Brown Obsessive Compulsive Scale (6) score has been used as the main dependent variable in multicenter treatment trials for OCD and has demonstrated reliability and validity.
To assess outcome in a variety of areas, we used a series of clinical global improvement scales that had been used in our previous studies (3, 4). Patients were asked, “Compared to how you felt before your first neurosurgical operation, circle a number to describe how you are feeling now in each of the following areas.” The three areas included in the assessment were OCD symptoms, depression, and anxiety. Each area was rated on the following scale: 1=much better, 2=moderately better, 3=slightly better, 4=no change, 5=slightly worse, 6=moderately worse, 7=much worse.
The Beck Depression Inventory (7), a widely used 21-item self-report scale, was used to assess severity of depression. This scale has demonstrated good reliability and validity for this purpose.
The Sickness Impact Profile (8), a 136-item self-administered checklist, was used to assess functional status. The Sickness Impact Profile yields a total score, 12 subscale scores for individual categories of functional status, and three dimensional scores (including physical, psychosocial, and other effects, such as impact on work, rest, recreational pastimes, and sleep). The total, subscale, and dimensional scores have demonstrated excellent reliability, discriminant validity, and sensitivity to change.
The major clinical criterion pertaining to severity of illness was functional impairment, with patients’ preoperative functioning typically corresponding to a DSM-III-R Global Assessment of Functioning score of less than 60. Although the Yale-Brown Obsessive Compulsive Scale score is a general guide to symptom severity, the score on this scale may underestimate the severity of symptoms in some patients, such as those with obsessions only, for whom the scale score would reflect approximately one-half of their true symptom severity. In general, however, most patients who are approved for cingulotomy have Yale-Brown Obsessive Compulsive Scale scores of greater than 25.
Data Analysis
For clinical ratings of OCD and depression symptoms, we analyzed data at four times: 1) at baseline, before the first cingulotomy; 2) at the first postsurgical follow-up (a mean of 6.7 months after the first cingulotomy), at which point no patient had yet undergone a second cingulotomy; 3) at the first postsurgical follow-up after a second cingulotomy (a mean of 7.0 months after the second cingulotomy) for those patients who had multiple cingulotomies; and 4) at the most recent follow-up (a mean of 32 months after the first cingulotomy), at which time 17 patients (39%) had undergone two cingulotomies and one patient (2%) had undergone three cingulotomies. Analyses of functional status, measured by the Sickness Impact Profile, at the most recent follow-up compared to the baseline incorporated two-tailed, paired t tests.
To classify patient response we employed the same conservative criteria that were used in our previous prospective studies (3, 4). Responders had an improvement of 35% in the Yale-Brown Obsessive Compulsive Scale score and a clinical global improvement score less than or equal to 2 and did not attribute the improvement to a treatment other than cingulotomy. Partial responders had either improvement of 35% in the Yale-Brown Obsessive Compulsive Scale score or a clinical global improvement score less than or equal to 2 or had adequate improvement on both scales but attributed improvement to another treatment.
Results
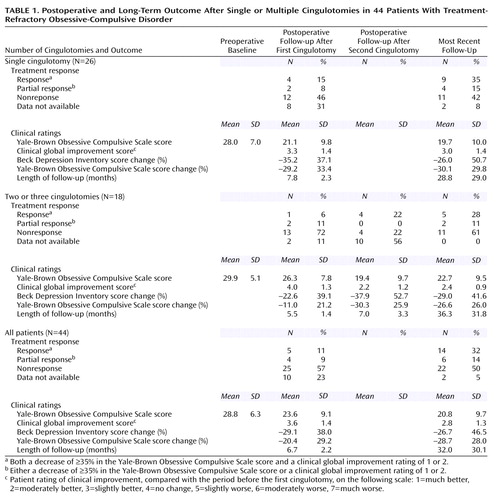
Outcome After Single Cingulotomy
Table 1 summarizes the treatment response categories and clinical rating scale results for all patients at baseline, the first follow-up, and long-term follow-up after one cingulotomy. Five of the 44 patients (11%) met the criteria for being a responder at the first follow-up point. Another four patients (9%) were classified as partial responders at the first follow-up. At the first follow-up, mean improvement in OCD as measured by the Yale-Brown Obsessive Compulsive Scale score was 20.4%, and the mean clinical global improvement score for OCD was 3.6. Correlations of the clinical global improvement ratings for OCD with the scores for depression (r=0.54, N=26, p=0.004) and the scores for anxiety (r=0.63, N=26, p=0.001) were also significant.
At the most recent follow-up, 14 of the 44 patients (32%) met the conservative criteria for responders at a mean follow-up time of 32 months. Six additional patients (14%) met the more liberal criteria for partial responders. Thus, a total of 20 patients (45%) met criteria for at least partial response. At the most recent follow-up, the mean improvement in OCD as measured by the Yale-Brown Obsessive Compulsive Scale score was 28.7%, and the mean clinical global improvement score for OCD was 2.8. The correlations of the clinical global improvement scores for OCD with the scores for depression (r=0.55, N=39, p<0.001) and the scores for anxiety (r=0.75, N=39, p<0.001) were significant.
Outcome After Multiple Cingulotomies
Among the patients who received more than one cingulotomy, only one responder (6%) and two partial responders (11%) were seen at initial follow-up after the first cingulotomy. However, in the initial follow-up after the second cingulotomy, there were four responders (22%) and no partial responders (Table 1). Both patients who were partial responders after a single cingulotomy became full responders after a second cingulotomy.
At the most recent follow-up for the 18 patients who underwent multiple cingulotomies, five patients (28%) were responders and two (11%) were partial responders. Thus, seven patients (39%) met criteria for at least partial response.
A two-by-three chi-square analysis of single versus multiple cingulotomies by responder status (full response, partial response, no response) revealed no difference in responder status at the most recent follow-up between those who received a single cingulotomy and those who received multiple cingulotomies (χ2=0.54, df=2, p=0.76).
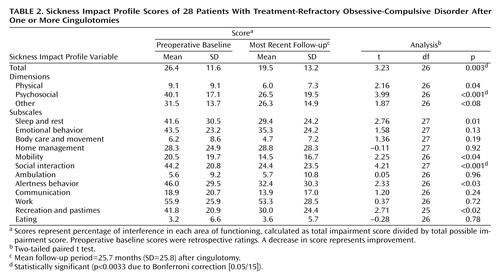
Functional Status
Table 2 shows patients’ scores on the Sickness Impact Profile at baseline (rated retrospectively) and at the most recent follow-up (N=28) for each specific area of functional status and for overall status. For the group as a whole, there were improvements in most functional areas measured by subscales of the Sickness Impact Profile as well as in the total score. The improvement was significant for one of the 11 subscales, for one of the three dimensions, and for the total score. Moreover, no individual area of functioning showed significant worsening.
Adverse Effects
Nine patients (20%) reported at least one adverse effect after cingulotomy. Two patients (5%) reported memory deficits, which resolved within 6–12 months. One patient demonstrated resolution of memory deficits as measured by neuropsychological testing, and one patient self-reported resolution of memory deficits. One patient (2%) exhibited apathy with decreased energy after cingulotomy. This effect resolved within 6 months. In addition, three patients (7%) had urinary disturbances after surgery. Two patients (5%) experienced urinary retention. Both cases of urinary retention resolved within a few days postoperatively; one patient required catheterization, and the other did not. The third patient had received a diagnosis of prostate cancer before undergoing the cingulotomy and had been experiencing urinary incontinence before the surgery; this patient reported that the urinary incontinence worsened after the cingulotomy. After cingulotomy one patient (2%) developed a seizure disorder that has required ongoing treatment with anticonvulsant medication. One patient (2%) developed postoperative edema with resulting hydrocephalus. The hydrocephalus resolved after placement of a ventriculostomy. One patient (2%) committed suicide approximately 6 years after undergoing cingulotomy. Before the suicide, this patient’s OCD symptoms had improved after cingulotomy. However, this patient had a long history of major depression with almost continuous suicidal ideation for more than 8 years and a history of one previous suicide attempt. Although nine patients (20%), excluding the patient who committed suicide, reported at least one adverse effect, only two (5%) patients reported enduring sequelae.
Discussion
The current finding that 32% of patients met conservative criteria for clinical improvement at long-term follow-up is consistent with our previous retrospective and prospective studies (3, 4). This extended follow-up also showed that the patients had overall improvement in functional status and a relatively low incidence of significant adverse effects. These results indicate that cingulotomy may be a useful treatment for patients with severe, treatment-refractory OCD.
Because this data set included a substantial number of patients who had single and multiple cingulotomies and because follow-up was conducted at multiple time points, some speculation about the clinical implications of these findings is possible. Most patients who undergo a second cingulotomy do so after not responding to the initial procedure within approximately 6 months. These data indicated that although 23% of the patients who underwent a single cingulotomy were at least partial responders at the initial follow-up (a mean of 7.8 months after the operation), by the most recent follow-up (a mean of 28.8 months after the operation) this figure had increased to 50%. Thus, it may be prudent to delay considering subsequent cingulotomies until after a longer period of time than 6 months. Improvement over time was also evident in patients who underwent a second cingulotomy. Although 22% of these patients were at least partial responders at initial follow-up (a mean 7.0 months after the second cingulotomy), at the most recent follow-up (a mean 36.3 months after the second cingulotomy) this figure had increased to 39%.
Although it is difficult to compare the relative efficacy of medications to that of cingulotomy for the treatment of OCD, one can use the meta-analytic statistic of effect size to compare different studies. The within-treatment effect size statistic is calculated as the baseline mean minus the end-of-study mean divided by the baseline standard deviation. For the Yale-Brown Obsessive Compulsive Scale, the within-subject effect size statistic for all patients in the current study was 1.27. Dividing the patients into groups of those undergoing a single cingulotomy and those undergoing multiple cingulotomies yielded effect size statistics of 1.18 and 1.41, respectively. These within-subject effect size statistics were comparable to those found for the active treatment groups in medication trials (including trials of fluvoxamine, fluoxetine, and clomipramine) conducted at our institution, where the Yale-Brown Obsessive Compulsive Scale within-subject effect size statistic has ranged from 1.09 to 1.53 (10, 11). Of course, the patients who have undergone cingulotomy, by definition, have OCD that has been refractory to pharmacotherapy.
Although the pathophysiology of OCD and the mechanisms by which antiobsessional treatments produce beneficial effects remain incompletely understood, convergent data from the pharmacology, neuroimaging, neuropsychology, and neurosurgery literature have been synthesized to produce integrated neurobiological models of OCD (12). Central to these models is the corticostriatal circuitry model of OCD. Dysfunction of this circuitry, which involves the orbitofrontal cortex, caudate nucleus, pallidum, and thalamus, as well as the anterior cingulate cortex, has been implicated in the pathophysiology of OCD (12, 13). A variety of different neurosurgical procedures including subcaudate tractotomy, limbic leukotomy, cingulotomy, and capsulotomy, have been used for the treatment of patients with intractable OCD. Each of these operations entails lesioning the corticostriatal circuitry at different locations.
Anterior cingulotomy targets the anterior cingulate cortex as well as the fibers of the cingulum. Anterior capsulotomy and subcaudate tractotomy interrupt the frontothalamic fibers at different sites. Lastly, limbic leukotomy, a multisite operation, combines the lesions of anterior cingulotomy with those of the frontothalamic projections (see the review by Cosgrove and Rauch [14]). Although making meaningful comparisons between the various procedures is difficult, the data support the idea that the different procedures have roughly comparable efficacy, but the issues of relative safety and side effect profile remain less clear (see the review by Jenike et al. [15]). The Massachusetts General Hospital Cingulotomy Assessment Committee has continued to offer cingulotomies, as opposed to other neurosurgical options, because of the large amount of experience supporting the efficacy and relative safety of the procedure.
Of the 44 patients in the current study, two reported adverse effects with enduring sequelae and one patient completed suicide. Specifically, one patient developed seizures that have responded to anticonvulsant medication, and one patient had a worsening of preexisting (secondary to prostate cancer) urinary incontinence. Numerous studies have estimated the risk of postoperative epilepsy at less than 1% (3, 16–19). Although two patients reported memory difficulties and one patient reported apathy and decreased energy after cingulotomy, these cognitive symptoms ultimately resolved. Most studies of cognitive function, which have used various psychometric tests both preoperatively and postoperatively, have shown no significant cognitive deficits after cingulotomy for psychiatric illness (16, 20, 21, unpublished 1986 study of S. Corkin and N. Hebben). In fact, in these reports patients are noted to exhibit improved cognitive function, perhaps because of symptom reduction. However, one study (22) described subtle attentional impairments after cingulotomy, and another (23) described mild alterations of intention and self-initiated action after cingulotomy. It is noteworthy that the patients in these studies underwent cingulotomy for chronic pain, not for OCD or major affective illness. It may be that cingulotomy for OCD or major affective illness is less likely to produce cognitive deficits precisely because anterior cingulate function is already compromised in association with these diseases (12, 24). In summary, although two subjects did experience persistent adverse effects after cingulotomy, most patients noted improvement in many areas of functioning as indicated by the Sickness Impact Profile.
Improvements in the clinical severity of OCD at most recent follow-up were strongly and significantly related to self-ratings of improvements in both depression and anxiety. Also, the average Beck Depression Inventory score markedly decreased after surgery. This finding raises the question of whether OCD improvement was primary or secondary to changes in either depression or anxiety. This question is not possible to resolve statistically with the data collected in this study. However, the vast majority of patients had a period of OCD while they were not depressed at some point in the course of their illness. Also, since most patients received ongoing pharmacotherapy after surgery, it is not possible to determine whether clinical improvements were due to cingulotomy per se, or rather to an interaction with medication that had not been effective presurgically. This caveat in interpreting our results also holds for patients having undergone behavior therapy postsurgically, when the follow-up evaluations were conducted.
We have sought to interpret our data conservatively and cautiously. Still, it is important to acknowledge the strengths and limitations of this study. The strengths include the careful diagnostic work-up at baseline, the documentation of adequate previous trials of medication and behavior therapy, and the prospective assessment of OCD and depressive symptoms.
The limitations of our study include the absence of a sham comparison group. However, operations (i.e., actual lesions) for which there is no likelihood or evidence of efficacy have historically been considered unethical (25). However, methods for conducting randomized, blinded clinical trials of neurosurgical treatment are emerging (26). Thus, although such procedures might well help answer questions of efficacy, a sham operation (i.e., burr holes only) is not currently feasible for cingulotomy. Also, the data generated from this study reflect results from anterior cingulotomies performed according to the specific parameters described in the Method section. Therefore these findings may not be generalizable to other neurosurgical treatments for OCD.
In conclusion, almost a third of the patients who had OCD that was unresponsive to an exhaustive array of medication and behavior treatments and who underwent cingulotomy for OCD were much improved at follow-up, according to very conservative criteria. If less stringent criteria had been applied, more than 45% of the patients improved. As with any treatment, the potential benefits must be weighed against the associated potential risks. Future studies might seek to identify reliable predictors of treatment response so that this risk/benefit ratio may be improved (27).
 |
 |
Received Oct. 17, 2000; revision received July 6, 2001; accepted Aug. 23, 2001. From the Departments of Psychiatry, Neurology, and Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston. Address reprint requests to Dr. Rauch, Psychiatric Neuroscience Program, Massachusetts General Hospital–East, CNY-9130, Bldg. 149, 13th St., Charlestown, MA 02129. The authors thank Valerie Giorgione, Ida Giriunas, R.N., Linda Leahy, Peter Manzo, and the late H. Thomas Ballantine, Jr., M.D., for their contributions to this study. Supported in part by the Massachusetts General Hospital Psychosurgery Fund.
1. Karno M, Golding JM, Sorenson SB, Burnam MA: The epidemiology of obsessive-compulsive disorder in five US communities. Arch Gen Psychiatry 1988; 45:1094-1099Crossref, Medline, Google Scholar
2. Robins LN, Helzer JE, Weissman MM, Orvaschel H, Gruenberg E, Burke JD Jr, Regier DA: Lifetime prevalence of specific psychiatric disorders in three sites. Arch Gen Psychiatry 1984; 41:949-958Crossref, Medline, Google Scholar
3. Jenike MA, Baer L, Ballantine T, Martuza RL, Tynes S, Giriunas I, Buttolph ML, Cassem NH: Cingulotomy for refractory obsessive-compulsive disorder: a long-term follow-up of 33 patients. Arch Gen Psychiatry 1991; 48: 548-555Google Scholar
4. Baer L, Rauch SL, Ballantine HT Jr, Martuza R, Cosgrove R, Cassem E, Giriunas I, Manzo PA, Dimino C, Jenike MA: Cingulotomy for intractable obsessive-compulsive disorder: prospective long-term follow-up of 18 patients. Arch Gen Psychiatry 1995; 52:384-392Crossref, Medline, Google Scholar
5. Spitzer RL, Williams JBW, Gibbon M, First MB: Users Guide for the Structured Clinical Interview for DSM-III-R (SCID). Washington, DC, American Psychiatric Press, 1990Google Scholar
6. Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS: The Yale-Brown Obsessive Compulsive Scale: I. development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006-1011Crossref, Medline, Google Scholar
7. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 41:561-571Crossref, Google Scholar
8. Bergner M, Bobbitt RA, Carter WB, Gilson BS: The Sickness Impact Profile: development and final revision of a health status measure. Med Care 1981; 19:787-805Crossref, Medline, Google Scholar
9. Rauch SL, Kim H, Makris N, Cosgrove GR, Cassem EH, Savage CR, Price BH, Nierenberg AA, Shera D, Baer L, Buchbinder B, Caviness VS Jr, Jenike MA, Kennedy DN: Volume reduction in the caudate nucleus following stereotactic placement of lesions in the anterior cingulate cortex in humans: a morphometric magnetic resonance imaging study. J Neurosurg 2000; 93:1019-1025.Crossref, Medline, Google Scholar
10. Jenike MA, Baer L, Greist JH: Clomipramine versus fluoxetine in obsessive-compulsive disorder: a retrospective comparison of side effects and efficacy. J Clin Psychopharmacol 1990; 10:122-124Crossref, Medline, Google Scholar
11. Jenike MA, Hyman S, Baer L, Holland A, Minichiello WE, Buttolph L, Summergrad P, Seymour R, Ricciardi J: A controlled trial of fluvoxamine in obsessive-compulsive disorder: implications for a serotonergic theory. Am J Psychiatry 1990; 147:1209-1215Link, Google Scholar
12. Rauch SL, Whalen PJ, Dougherty D, Jenike MA: Neurobiologic models of obsessive-compulsive disorder, in Obsessive-Compulsive Disorders: Practical Management. Edited by Jenike MA, Baer L, Minichiello WE. St. Louis, Mosby, 1998, pp 222-253Google Scholar
13. Saxena S, Brody AL, Schwartz JM, Baxter LR: Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Suppl 1998; (35):26-38Google Scholar
14. Cosgrove GR, Rauch SL: Psychosurgery. Neurosurg Clin N Am 1995; 6:167-176Crossref, Medline, Google Scholar
15. Jenike MA, Rauch SL, Baer L, Rasmussen SA: Neurosurgical treatment of obsessive-compulsive disorder, in Obsessive-Compulsive Disorders: Practical Management. Edited by Jenike MA, Baer L, Minichiello WE. St. Louis, Mosby, 1998, pp 592-610Google Scholar
16. Ballantine HT Jr: Neurosurgery for behavioral disorders, in Neurosurgery. Edited by Wilkins RH, Rengachary SS. New York, Elsevier/North Holland Biomedical Press, 1985, pp 2527-2537Google Scholar
17. Ballantine HT Jr, Bouckoms AJ, Thomas EK, Giriunas IE: Treatment of psychiatric illness by stereotactic cingulotomy. Biol Psychiatry 1987; 22:807-819Crossref, Medline, Google Scholar
18. Bingley T, Leksell L, Meyerson BA, Rylander G: Long-term results of stereotactic capsulotomy in chronic obsessive-compulsive neurosis, in Neurosurgical Treatment in Psychiatry. Edited by Sweet WH, Obrador S, Martin-Rodriquez JG. Baltimore, University Park Press, 1977, pp 287-299Google Scholar
19. Bingley T, Persson A: EEG studies on patients with chronic obsessive compulsive neurosis before and after psychosurgery (stereotaxic bilateral anterior capsulotomy). Electroencephalogr Clin Neurophysiol 1978; 44:691-696Crossref, Medline, Google Scholar
20. Corkin S, Twitchell TE, Sullivan EV: Safety and efficacy of cingulotomy for pain and psychiatric disorder, in Modern Concepts in Psychiatric Surgery. Edited by Hitchcock ER, Ballantine HT, Myerson BA. New York, Elsevier/North Holland, 1979, pp 253-272Google Scholar
21. Corkin S: A prospective study of cingulotomy, in The Psychosurgery Debate. Edited by Valenstein ES. San Francisco, WH Freeman & Co, 1980, p 164-204Google Scholar
22. Cohen RA, Kaplan RF, Moser DJ, Jenkins MA, Wilkinson H: Impairments of attention after cingulotomy. Neurology 1999; 53:819-824Crossref, Medline, Google Scholar
23. Cohen RA, Kaplan RF, Zuffante P, Moser DJ, Jenkins MA, Salloway S, Wilkinson H: Alteration of intention and self-initiated action associated with bilateral anterior cingulotomy. J Neuropsychiatry Clin Neurosci 1999; 11:444-453Crossref, Medline, Google Scholar
24. Ebert D, Ebmeier KP: The role of the cingulate gyrus in depression: from functional anatomy to neurochemistry. Biol Psychiatry 1996; 39:1044-1050Crossref, Medline, Google Scholar
25. Levine RJ: Ethics and Regulation of Clinical Research, Second Edition. New Haven, Yale Press, 1988Google Scholar
26. de Bie RM, de Haan RJ, Nijssen PC, Rutgers AW, Beute GN, Bosch DA, Haaxma R, Schmand B, Schuurman PR, Staal MJ, Speelman JD: Unilateral pallidotomy in Parkinson’s disease: a randomised, single blind, multicentre trial. Lancet 1999; 354:1665-1669Crossref, Medline, Google Scholar
27. Rauch SL, Dougherty DD, Cosgrove GR, Cassem EH, Alpert NM, Price BH, Nierenberg AA, Mayberg HS, Baer L, Jenike MA, Fischman AJ: Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for obsessive compulsive disorder. Biol Psychiatry 2001; 50:659-667Crossref, Medline, Google Scholar



