Clozapine, Olanzapine, Risperidone, and Haloperidol in the Treatment of Patients With Chronic Schizophrenia and Schizoaffective Disorder
Abstract
OBJECTIVE: The authors compared the efficacy and safety of three atypical antipsychotics (clozapine, olanzapine, and risperidone) with one another and with haloperidol in the treatment of patients with chronic schizophrenia or schizoaffective disorder. METHOD: In a double-blind trial, 157 inpatients with a history of suboptimal treatment response were randomly assigned to treatment with clozapine, olanzapine, risperidone, or haloperidol for 14 weeks (an 8-week escalation and fixed-dose period followed by a 6-week variable-dose period). RESULTS: Clozapine, risperidone, and olanzapine (but not haloperidol) resulted in statistically significant improvements in total score on the Positive and Negative Syndrome Scale. Improvements seen in total and negative symptom scores with clozapine and olanzapine were superior to haloperidol. The atypical drugs, particularly olanzapine and clozapine, were associated with weight gain. CONCLUSIONS: The effects of atypical antipsychotics in this population were statistically significant but clinically modest. The overall pattern of results suggests that clozapine and olanzapine have similar general antipsychotic efficacy and that risperidone may be somewhat less effective. Clozapine was the most effective treatment for negative symptoms. However, the differences among treatments were small.
Clozapine is the established treatment for patients with schizophrenia who do not respond adequately to other antipsychotic medications (1). The role of other atypical antipsychotics in this indication is less clear. Olanzapine was not more effective than chlorpromazine in an 8-week trial of very ill, chronically institutionalized patients with schizophrenia (2). However, another 6-week study that compared olanzapine to haloperidol in a large sample of less severely ill inpatients and outpatients with treatment-resistant schizophrenia found that olanzapine was superior to haloperidol (3).
Several studies have reported results of risperidone treatment in patients with refractory schizophrenia or in those who had achieved partial response. Risperidone and clozapine appeared to have similar efficacy in one 8-week trial (4). In another study, however, clozapine was used at higher doses, and it was superior to risperidone in its effects on positive symptoms (5). Clozapine appeared to have higher efficacy than risperidone in two open studies of patients with treatment-resistant schizophrenia (6, 7). In patients with refractory schizophrenia, risperidone was more effective than haloperidol after 4 weeks of treatment, but that superiority was no longer present after an additional 4 weeks (8).
Because of methodological differences, it is difficult to interpret the results of these studies and draw conclusions across them in terms of the comparative efficacy and safety of the atypical drugs with respect to conventional agents and each other. The inclusion criteria and definition of treatment resistance varied. Some patients had a history of treatment intolerance rather than treatment resistance (4, 8). The number and the definitions of treatment failures varied. Some studies employed prospective determination of failure (1, 2), whereas other studies relied on retrospective data. Thus, these studies are difficult to compare. The contrast between the results of two olanzapine studies (2, 3) may be explained by differences in the severity of illness in subjects selected (9). Our objective was to compare four antipsychotics in a single trial.
Method
Patient Group
Subjects were 18- to 60-year-old inpatients at four psychiatric state hospitals (two in New York and two in North Carolina). For inclusion in the study, patients were required to have a diagnosis of DSM-IV chronic schizophrenia or schizoaffective disorder and suboptimal response to previous treatment, which was defined by two criteria that needed to be present. The first criterion of suboptimal response was persistent positive symptoms (hallucinations, delusions, or marked thought disorder) after at least 6 contiguous weeks of treatment, presently or documented in the past, with one or more typical antipsychotics at doses ≥600 mg/day in chlorpromazine equivalents. The second criterion was a poor level of functioning over the past 2 years, defined by the lack of competitive employment or enrollment in an academic or vocational program and not having age-expected interpersonal relations with someone outside the biological family of origin with whom ongoing regular contacts were maintained. We are using the term “suboptimal response to treatment” instead of “treatment resistance” to highlight that our criteria are different from those used in the multicenter clozapine trial (1). In addition, patients were required to have a baseline total score ≥60 on the Positive and Negative Syndrome Scale (10).
Patients were excluded from the study if they had a history of nonresponse to clozapine, risperidone, or olanzapine, defined as an unambiguous lack of improvement despite a contiguous adequate trial of risperidone or olanzapine for at least 6 weeks, or clozapine for at least 14 weeks. The longer clozapine trial duration was required because Meltzer et al. (11) observed that less than 50% of patients who improve with clozapine reach that improvement within the first 6 weeks of treatment. Patients with a history of clozapine, olanzapine, risperidone, or haloperidol intolerance as well as those who received a depot antipsychotic within 30 days before randomization were also excluded.
Treatments
During a baseline screening period of 1 to 2 weeks, patients’ prestudy antipsychotic medications were adjusted as needed so that the daily dose at the end of the screening period did not exceed 750 mg/day in chlorpromazine equivalents. Other concomitant medications such as mood stabilizers and antidepressants were gradually tapered and discontinued before the patients received study medication. After completing baseline assessments, patients were randomly assigned to one of the four treatment arms: clozapine, olanzapine, risperidone, or haloperidol. Trial antipsychotics were administered double-blind; all patients had weekly blood tests. Throughout the trial, each patient received five tablets twice daily; all tablets looked alike. Psychiatrists blind to treatment group assignment could change the doses by prescribing various “levels” of medication (detailed explanation of the procedures available on request). The 14-week trial consisted of an 8-week escalation and fixed-dose period and a 6-week variable-dose period.
The project was originally designed to compare clozapine, risperidone, and haloperidol and was initiated as a three-arm study funded by the National Institute of Mental Health (NIMH) in June 1996. Olanzapine became commercially available in October 1996 and became widely used. We therefore decided to add an olanzapine arm to our study. NIMH agreed, but supplemental funding was not immediately available. Therefore, with the approval of NIMH we requested supplemental funding from Eli Lilly and Company to support this expansion of the project.
The olanzapine arm was added in November 1997 and required a modified randomization procedure. Because of its late introduction, subjects were more frequently randomly assigned to olanzapine than to other treatments in order to ultimately acquire similar numbers of subjects for each of the four treatment arms. Although this is a frequently used procedure in clinical trials, it entails the potential for a bias that could be manifested as a cohort effect. However, blind conditions were never compromised, since all tablets looked the same and subjects continued to be assigned to the original three treatments at rates that were unknown to the study personnel and the subjects.
During the first 8 weeks of the study, the prestudy antipsychotic was gradually discontinued while the doses of olanzapine, risperidone, and haloperidol were escalated to their target levels (20, 8, and 20 mg/day, respectively) at which they remained fixed until the end of the first study period. We endeavored to reach the target level of clozapine, 500 mg/day, on day 24; then the dose remained fixed until the end of the first study period. These dosing schedules were adjusted depending on the patient’s clinical status, including side effects. Mean dose levels (mg/day) achieved during this first period of the study (last observation carried forward) were 401.6 (SD=160.4) for clozapine, 19.6 (SD=2.1) for olanzapine, 7.9 (SD=2.1) for risperidone, and 18.9 (SD=3.1) for haloperidol.
During the last 6 weeks of the study, antipsychotic dose was allowed to vary within the following ranges: clozapine, 200–800 mg/day; olanzapine, 10–40 mg/day; risperidone, 4–16 mg/day; and haloperidol, 10–30 mg/day. In general, doses were gradually increased if adequate improvement was not achieved. Side effects could preclude dose escalation and could lead to dose reductions. Psychiatrists blind to treatment group assignment prescribed all dose changes. Mean dose levels (mg/day) achieved during this second period (last observation carried forward) were 526.6 (SD=140.3) for clozapine, 30.4 (SD=6.6) for olanzapine, 11.6 (SD=3.2) for risperidone, and 25.7 (SD=5.7) for haloperidol.
Throughout the study, all patients were receiving (double-blind) either benztropine or benztropine placebo or a combination of both. Benztropine (4 mg/day) was administered prophylactically to all patients receiving haloperidol. Patients assigned to atypical antipsychotics were initially receiving only benztropine placebo, but if the patient’s psychiatrist (who was unaware of the patient’s antipsychotic assignment) determined clinically that the patient should be treated for extrapyramidal side effects, a prescription could be written for “benztropine supplements” that would result in real benztropine gradually replacing benztropine placebo (up to 6 mg/day). An analogous arrangement for “supplements” was available to raise the dose of benztropine from 4 to 6 mg/day for emerging extrapyramidal symptoms in patients assigned to haloperidol. Propranolol was allowed for the treatment of akathisia.
Lorazepam, diphenhydramine hydrochloride, or chloral hydrate were prescribed open-label (by psychiatrists who were blind to antipsychotic treatment assignment) as needed for the treatment of agitation and insomnia in the dose ranges recommended by the manufacturers. No other adjunctive psychotropic medications (e.g., mood stabilizers and antidepressants) were allowed.
Assessments
Raters blind to treatment group performed all clinical research assessments. The Positive and Negative Syndrome Scale (10) was the principal measure of efficacy. It was administered at baseline and then weekly during the first month of the study and every other week thereafter. Every effort was made to ensure that the same raters provided all ratings in each patient. Two independent raters performed assessments at baseline, week 8, and week 14 (or endpoint); the average of these two ratings was included for the analyses of efficacy, together with the single-rater ratings from the other time points. (These paired ratings were also used for the assessment of interrater reliability.) One of the paired raters did the other ratings. One of the Positive and Negative Syndrome Scale’s original contributors (J.-P.L.) trained the raters (12). The interrater reliability, estimated by intraclass correlation coefficient (ICC), of the paired ratings for the Positive and Negative Syndrome Scale total score at the four sites was high (ICC=0.93–0.98).
Extrapyramidal side effects were assessed by trained raters using the Extrapyramidal Symptom Rating Scale (13). The time schedule for the paired and single-rater Extrapyramidal Symptom Rating Scale assessments was the same as for the Positive and Negative Syndrome Scale. Interrater reliability for the Extrapyramidal Symptom Rating Scale total score for raters at the four sites was high (ICC=0.86–0.91).
Statistical Analyses
The total score on the Positive and Negative Syndrome Scale was adopted as the primary measure of efficacy. We planned two approaches to analyze all available data from the entire cohort: 1) the traditional analysis of covariance (ANCOVA) for determining change over time, with baseline severity as a covariate; and 2) random regression hierarchical linear modeling (14–16).
Preliminary data analyses revealed that subjects in our four treatment groups displayed a statistically significant difference in symptom severity at baseline. Since hierarchical linear modeling—in contrast to the traditional ANCOVA approach—allows for heterogeneity among treatment groups, the hierarchical linear modeling analysis was adopted as the primary statistical approach for our study.
The principal goal of the hierarchical linear modeling analysis is to estimate and test trajectories that describe individual patterns of change of some characteristics (e.g., symptoms) over time. The variation in change of a characteristic over time is described at each of two levels in the hierarchical linear modeling model. At level 1, the characteristic is described as varying within a subject over time as a person-specific change trajectory (plus an error). At level 2, the person-level change trajectories are viewed as systematically varying across subjects. The random-effect component of the hierarchical linear modeling analysis provides information about the systematic individual differences in change trajectories over time. The fixed-effect component of the hierarchical linear modeling analysis provides information about the mean change trajectory for a group of subjects.
In our hierarchical linear modeling analysis, all repeated assessments of symptom severity over time (i.e., total score on the Positive and Negative Syndrome Scale for all time points in all subjects) served as the dependent variable. The symptom change trajectory in each person was represented by two parameters: the trajectory’s initial value (intercept) and its slope. The two independent factors were treatment group and time. Treatment group served as the between-subject factor. Time (in weeks) from baseline was used as the within-subject factor. Interaction between treatment group and time was included in the model. In order to account for a site-to-site variation in terms of baseline severity and change over time, an unstructured covariance matrix with heterogeneity among participating centers was specified in the hierarchical linear modeling analysis. The purpose of this provision was to assure that changes of clinical variables over time were not confounded by intersite variability.
The analysis had two principal objectives. First, we assessed whether a significant change over time occurred in any of the four treatment groups (i.e., the slope of the symptom trajectory significantly differed from zero; this analysis is analogous to the traditional test of pre-post difference). Second, we tested whether there was a slope difference among the four groups in symptom change trajectories over time. This analysis is analogous to the traditional test of interaction between time and treatment effects. The time effect and the slope difference (interaction) effect were tested by the F statistic.
Measures of Efficacy
Similar to others (2), we adopted the 0.05 alpha level for the tests involving the Positive and Negative Syndrome Scale total score, identified a priori as the primary measure of efficacy. If a significant effect was detected, post hoc analyses were performed to examine the direction of changes (time effect) or the differences in change over time among the treatment groups (interaction effect). Post hoc analyses were based on linear functions (contrast variables) of parameter estimates obtained from the overall hierarchical linear modeling analysis. The four antipsychotics were compared with each other in six pairwise tests.
Hierarchical linear modeling analyses analogous to those described for the primary measure were performed for our secondary measures (the Positive and Negative Syndrome Scale subscales and the Extrapyramidal Symptom Rating Scale). The analyses for the secondary measures were considered exploratory. Our objective was to investigate, for each measure, the time effect of each of the four medications and to determine the difference in time effects for all six pairs of medications. The alpha levels were corrected for test multiplicity. For the Positive and Negative Syndrome Scale subscales, there were 12 tests of the time effect (three subscales, four drugs). This resulted in a corrected alpha of 0.0042 (0.05/12). The comparisons of medication effects that used secondary measures (the three Positive and Negative Syndrome Scale subscales) were also corrected for test multiplicity. There were 18 tests (three subscales and six pairwise comparisons of the four medications for each subscale). Thus the corrected alpha level is 0.0028 (0.05/18).
An analogous correction for test multiplicity was implemented for extrapyramidal symptom ratings. There were four tests of time effects (one for each drug; corrected alpha=0.0125 [0.05/4] and six pairwise comparisons (corrected alpha=0.0083 [0.05/6]).
A nonparametric survival analysis with Kaplan-Meier estimates was used to test whether the four treatment groups differed in terms of time to attrition (survival) in the study. Adjunctive medication use was investigated by chi-square analyses (categorical variables) and by analyses of variance (continuous variables).
Results
A total of 167 subjects were randomly assigned to a treatment group, but 10 of them terminated before receiving study medication. Thus, the study is based on data from 157 subjects randomly assigned to treatment with clozapine (N=40), olanzapine (N=39), risperidone (N=41), or haloperidol (N=37). Their diagnosis was schizophrenia (86.0%, N=135) or schizoaffective disorder (14.0%, N=22). There were 133 male subjects (84.7%). The mean age was 40.8 years (SD=9.2), mean duration of illness was 19.5 years (SD=8.4), and the mean number of hospitalizations was 10.5 (SD=8.3). There were no statistically significant differences among treatment arms on any demographic variable. After complete description of the study to the subjects, written informed consent was obtained.
The 14-week study was completed by 91 (58.0%) of the 157 subjects. The Kaplan-Meier estimates of the probability of completing the study are displayed in Figure 1. The differences in the attrition rates among treatments were not statistically significant (log rank test: χ2=1.52, df=3, p=0.68). The most frequent reason for premature discontinuation (N=22) was consent withdrawal (five patients receiving clozapine, four receiving olanzapine, eight receiving risperidone, and five receiving haloperidol). Clinical deterioration caused premature termination in 14 patients (two receiving clozapine, four receiving olanzapine, two receiving risperidone, and six receiving haloperidol). Six patients were discharged and could not be followed up (three receiving risperidone, and one each from the other three treatment arms). Hematological problems led to discontinuation in three patients receiving clozapine; seizures caused premature termination in four patients (two receiving clozapine, two receiving risperidone). The remaining 17 premature discontinuations occurred for administrative reasons (one receiving clozapine, two receiving olanzapine, four receiving risperidone, and three receiving haloperidol), intercurrent illnesses (three receiving clozapine, one receiving olanzapine, and one receiving haloperidol), or protocol violations (one receiving clozapine, one receiving olanzapine).
Antipsychotic Efficacy
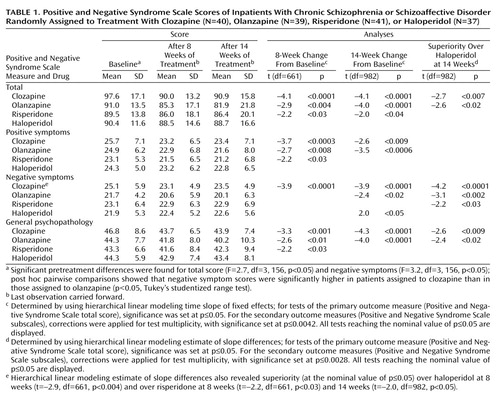
The Positive and Negative Syndrome Scale mean scores and standard deviations of the last observations carried forward are in Table 1 (data for each time point are available on request).
Analyses of variance showed statistically significant differences among the treatment groups at baseline for the total scores and for negative symptoms. The total scores at baseline were higher in the clozapine group than in the other groups, but post hoc pairwise comparisons (six separate Tukey studentized range tests comparing treatment groups with each other) indicated no statistically significant differences between treatment groups for the total score. However, post hoc pairwise comparisons showed that baseline negative symptom scores in the clozapine group were significantly higher than in the olanzapine group (Table 1).
The hierarchical linear modeling analysis of the data for the 14-week trial indicated a statistically significant change over time for each of the Positive and Negative Syndrome Scale measures (total score: F=23.9, df=1, 153, p<0.0001; positive symptoms: F=21.6, df=1, 153, p<0.0001; negative symptoms: F=6.3, df=1, 153, p<0.02; general psychopathology: F=23.7, df=1, 153, p<0.0001). The time effects were similar after the first 8 weeks of the trial (available on request).
Hierarchical linear modeling tests for fixed effects were used as the first step in the direct comparison of treatments. For the primary measure of efficacy (total score on the Positive and Negative Syndrome Scale for the entire 14-week trial [or endpoint]), there was a statistically significant interaction between medication and time. This interaction indicated a general difference in the efficacy among the four medications. To interpret this general difference, we did six pairwise post hoc tests of specific differences between treatments. Analogous analysis was performed for the 8-week period (results available on request).
Positive and Negative Syndrome Scale data at 14 weeks indicated statistically significant improvements in total score for the three atypical antipsychotics, improvements in negative symptoms and general psychopathology for clozapine, and improvements in positive symptoms and general psychopathology for olanzapine.
Clozapine and olanzapine were superior to haloperidol in terms of improvement on total score and negative symptoms. Haloperidol had no statistically significant effects on any Positive and Negative Syndrome Scale measure.
The data at 8 weeks (Table 1) exhibit the last observations carried forward from the period of week 1 to week 8; observations beyond week 8 were censored. These 8-week data, which show trends similar to the entire 14-week trial, are displayed because that time point marks the end of the fixed-dose part of the study. Furthermore, this display facilitates comparisons with studies ending at 8 weeks (8) or earlier. These ancillary results were corrected for test multiplicity in a way analogous to the results for the entire 14-week study. Symptom improvements were clinically modest. A standard measure for effect size (Cohen’s d [17]) was adopted to describe improvement produced by each of the four drugs in statistical terms. The effect size index for each group was expressed as the ratio of the mean absolute improvement to the standard deviation within group. The effect sizes of the improvement in total score on the Positive and Negative Syndrome Scale at 14 weeks for clozapine, olanzapine, risperidone, and haloperidol were 0.33, 0.51, 0.18, and 0.11, respectively. As expected in a study group selected for suboptimal response to treatment with typical antipsychotics, haloperidol had no beneficial effects.
Since the olanzapine arm was added after the study had run for approximately 15 months, we considered the possibility of a cohort effect confounding our data. To explore this, we classified the patients in the clozapine, risperidone, and haloperidol arms (N=118) into two cohorts: those started before (N=68) and after (N=50) the introduction of the olanzapine treatment arm. The difference in Positive and Negative Syndrome Scale total score between baseline and endpoint was tested. An ANOVA showed no cohort effect (F=0.22, df=1, 117, p=0.64) or a cohort-by-medication interaction (F=0.51, df=1, 116, p=0.60). For subjects who began the study before and after the introduction of the olanzapine arm, the mean baseline severity (Positive and Negative Syndrome Scale total score) was 91.4 (SD=13.8) and 94.1 (SD=15.9), respectively. The mean overall improvement over the entire 14-week trial was 2.7 (SD=19.6) for those who started the study before olanzapine was added and 5.5 (SD=15.6) for those who started after. To explore this difference, we computed the improvements separately for each drug. A cohort effect would be expected to affect equally all three medication groups. Mean improvements for those who started the study before and after the introduction of the olanzapine arm, respectively, were 6.48 (SD=22.00) and 7.05 (SD=18.69) for those given clozapine, –0.03 (SD=20.07) and 7.92 (SD=12.48) for those given risperidone, and 1.68 (SD=15.78) and 1.62 (SD=15.51) for those given haloperidol. Thus, although the cohort-by-medication interaction was not significant, we see that the slightly better overall effect for those who started the study after olanzapine was introduced was largely attributable to patients in one medication group (risperidone). Similar results were obtained for the positive symptoms, negative symptoms, and general psychopathology subscales. Thus, although we cannot prove that there was no overall cohort effect, these and other computations (available on request) failed to detect it.
Since negative symptoms may be in part secondary to extrapyramidal side effects (18), we repeated the hierarchical linear modeling analyses of negative symptoms with the Extrapyramidal Symptom Rating Scale total score as a covariate. The results of the analyses were not substantially affected by this covariate. Thus, the differences in treatment effects on negative symptoms were not mediated by those extrapyramidal side effects that are measured by the Extrapyramidal Symptom Rating Scale.
For clinical reasons, clozapine dose was escalated more slowly than the doses of the other medications. It was therefore possible that the aforementioned analyses did not adequately assess the effect of clozapine; some of the last observations in the clozapine group were perhaps obtained while the patients had not yet reached their full therapeutic doses. To explore this issue, we repeated the efficacy analyses for the subset of patients who completed the first 4 weeks of the study. (Thus, this was not a set of last observations carried forward). There were 33 patients receiving clozapine, 33 receiving olanzapine, 35 receiving risperidone, and 32 receiving haloperidol. At 4 weeks, the average daily dose of clozapine in these 33 patients was 453 mg (SD=66.6). The results of the hierarchical linear modeling analysis of these data indicated a statistically significant change over time for the main efficacy measure (overall time effect for Positive and Negative Syndrome Scale total score: F=26.2, df=1, 129, p<0.0001). The interaction between medication and time reached statistical significance (F=3.32, df=3, 963, p<0.02). The tests of fixed effects of time for each group were significant for clozapine (t=–4.3, df=963, p<0.0001), olanzapine (t=–4.2, df=963, p<0.0001), and risperidone (t=–2.0, df=963, p<0.04). Post hoc pairwise comparisons revealed superiority of clozapine over haloperidol (t=–2.8, df=963, p<0.006) and of olanzapine over haloperidol (t=–2.6, df=963, p<0.01). Thus, the results in this subset of patients who completed at least 4 weeks were essentially the same as those reported for the entire set at 14 weeks. Analogous analyses were performed for the positive, negative, and general psychopathology subscales of the Positive and Negative Syndrome Scale; these analyses essentially again replicated the results obtained in the complete set at 14 weeks. Thus, these analyses provided no support for the notion that the slower escalation rate of clozapine confounded the principal results.
Extrapyramidal Symptoms
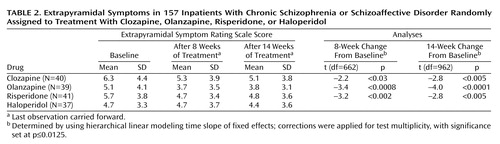
The hierarchical linear modeling analysis of fixed effects for extrapyramidal symptoms showed a significant time effect for both the 8-week (F=22.8, df=1, 152, p=0.0001) and 14-week data (F=24.1, df=1, 152, p=0.0001) but no significant interaction between time and medication. Decreases in scores on the Extrapyramidal Symptom Rating Scale occurred with each of the atypical antipsychotics (Table 2). No significant results were detected for the total Extrapyramidal Symptom Rating Scale score, dyskinesia, or akathisia. Similar to the data in Table 1, the tests are corrected for multiplicity.
Benztropine was administered prophylactically to all patients assigned to haloperidol, but for patients receiving the three atypical antipsychotics it was prescribed only if needed. The prescribers were blind as to the antipsychotic treatment assignment; benztropine usage could thus be employed as a variable reflecting the prescribers’ perception of the severity of the extrapyramidal side effects. We determined the number of patients assigned to atypical antipsychotics receiving benztropine after the first 2 weeks of the study. (The first 2 weeks were excluded in order to minimize carryover effects from prestudy treatments.)
The 8-week data indicate that benztropine was prescribed for 7.5% (N=3 of 40) of the patients receiving clozapine, 5.1% (N=2 of 39) of those receiving olanzapine, and 29.3% (N=12 of 41) of those receiving risperidone (χ2=11.8, df=2, p=0.003). The data for the entire 14 weeks were similar: benztropine was prescribed for 12.5% (N=5) of the patients receiving clozapine, 12.8% (N=5) of those receiving olanzapine, and 31.7% (N=13) of those receiving risperidone (χ2=6.32, df=2, p=0.04). Thus, benztropine prescriptions were significantly more frequent among patients assigned to risperidone, reflecting higher perceived frequency or severity of extrapyramidal side effects in the risperidone group.
Other Adverse Events
Agranulocytosis occurred in one patient receiving clozapine; there was full recovery. Two additional clozapine patients, two risperidone patients, and one haloperidol patient developed neutropenia. Four patients receiving clozapine developed seizures. Hypertensive episodes occurred in two clozapine patients.
The average weight gains (in kg) were as follows: clozapine=4.2, olanzapine=5.4, risperidone=2.3, and haloperidol=0.2. Details are reported elsewhere (19).
Agitation and insomnia were treated as needed with lorazepam, chloral hydrate, or diphenhydramine. The usage differences between treatment arms were not statistically significant.
Discussion
Clozapine and olanzapine were therapeutically superior to haloperidol on our principal measure of efficacy (Positive and Negative Syndrome Scale total score). This superiority was not mediated by the extrapyramidal symptoms assessed with the Extrapyramidal Symptom Rating Scale. Clozapine, olanzapine, and risperidone (but not haloperidol) showed significant reductions in total score on the Positive and Negative Syndrome Scale.
The relative lack of efficacy of haloperidol was expected in view of the subject selection criteria. Patients who failed to respond to any typical antipsychotic were eligible. Thus, we included patients who had failed to respond to haloperidol. However, those who showed a clear failure to respond to clozapine, olanzapine, or risperidone were excluded. This selection bias, shared with many other studies, would be expected to result in data that tend to show superior efficacy of atypical antipsychotics.
The pattern of weight gains we observed with atypical antipsychotics is in agreement with other published data (20). The observation of neutropenia in patients receiving risperidone and haloperidol highlights the fact that clozapine is not the only antipsychotic that causes this side effect (21).
We used high doses of all medications because we did not want to risk the possibility of undertreatment in this severely ill population selected for suboptimal previous treatment response. During the first 8 weeks of the study, our target dose of risperidone was 8 mg/day; this was the modal daily dose for adult inpatients in New York State hospitals at the time when our study was designed. The modal dose of 7.5 mg/day of risperidone is used clinically for long-term hospitalized adult psychiatric patients (22) and for patients with treatment-resistant schizophrenia (7). In a study that assigned patients to 1, 4, 8, 12, or 16 mg/day of risperidone, the optimal doses were 4 and 8 mg/day; 8 mg/day was slightly more effective than 4 mg/day (23). Another randomized double-blind study of chronic schizophrenia that compared two dose regimens of risperidone—4 and 8 mg/day—with placebo demonstrated optimal effects with the 8 mg/day dose (24). The average dose of risperidone was 8.95 mg/day in a study of patients with treatment-resistant schizophrenia (6). Thus, our target fixed dose of risperidone in period 1 was close to the doses used by many clinicians and investigators in similar patient populations.
However, other authors have used the dose of 6 mg/day for patients with refractory schizophrenia (8), and this dose is considered clinically optimal in many populations. We are not aware of any efficacy study comparing the risperidone doses of 6 and 8 mg/day. Plasma levels with these two dosage schedules overlap almost completely (25).
During the variable-dose period of the study (weeks 9–14), the dose increases of risperidone, clozapine, and haloperidol did not result in any beneficial effects. The Positive and Negative Syndrome Scale scores at the end of 8 and 14 weeks were almost identical for these medications. However, there seemed to be some additional improvement with the elevation of olanzapine dose, and this dose increase could explain the difference between our results and those of a prior study (2), which had failed to find a difference and had used a lower dose of olanzapine. The dose of risperidone during the second period of the study was probably too high; this represents a limitation of the study.
On the basis of these results, we conclude that atypical antipsychotics are more effective than haloperidol in chronic patients with a history of suboptimal response to treatment. However, the effects were modest and their clinical significance limited. Regarding general antipsychotic efficacy, the results (including effect sizes) suggest that clozapine and olanzapine are similar while risperidone appeared somewhat less effective. Similarity of clozapine and olanzapine effects in patients with treatment-resistant schizophrenia was recently demonstrated in a large study (26).
Clozapine was the most effective treatment for negative symptoms. However, the differences among treatments were small. This study cannot answer the question of whether patients who do not respond to risperidone or olanzapine would eventually respond to clozapine; there is evidence suggesting that this is a possibility (27). Our results indicate the benefits of atypical antipsychotic drugs and also their limitations, particularly when the drugs are used as monotherapeutic agents in patients with chronic and refractory illness.
 |
 |
Received Oct. 20, 2000; revisions received Feb. 23 and May 24, 2001; accepted Aug. 24, 2001. From the Nathan S. Kline Institute for Psychiatric Research; Dorothea Dix Hospital, Raleigh, N.C.; Manhattan Psychiatric Center, New York; and John Umstead Hospital, Butner, N.C. Address reprint requests to Dr. Volavka, Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Rd., Orangeburg, NY 10982; [email protected] (e-mail). Supported by grants from NIMH (MH-53550), the UNC-Mental Health and Neuroscience Clinical Research Center (MH-33127), and the Foundation of Hope, Raleigh, N.C. Janssen Pharmaceutica Research Foundation, Eli Lilly and Company, Novartis Pharmaceuticals Corporation, and Merck and Co., Inc., provided the medications. Eli Lilly and Company contributed supplemental funding that covered approximately 18% of the total cost of the study. However, overall experimental design, data acquisition, statistical analyses, and interpretation of the results were implemented with no input from any of the pharmaceutical companies. The authors thank Linda Kline, R.N., M.S., C.S., the chief coordinator of the project.
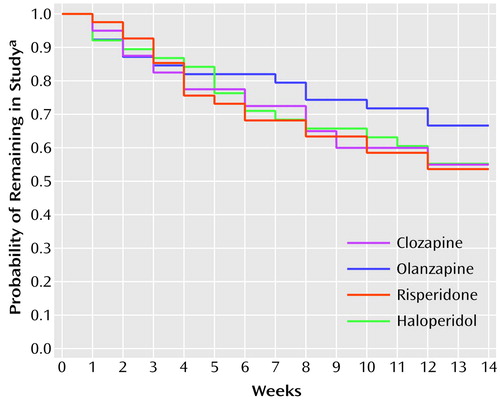
Figure 1. Attrition Among 157 Inpatients With Chronic Schizophrenia or Schizoaffective Disorder Randomly Assigned to Treatment With Clozapine (N=40), Olanzapine (N=39), Risperidone (N=41), or Haloperidol (N=37)
aDetermined by a nonparametric survival analysis with Kaplan-Meier estimates.
1. Kane J, Honigfeld G, Singer J, Meltzer H (Clozaril Collaborative Study Group): Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789-796Crossref, Medline, Google Scholar
2. Conley RR, Tamminga CA, Bartko JJ, Richardson C, Peszke M, Lingle J, Hegerty J, Love R, Gounaris C, Zaremba S: Olanzapine compared with chlorpromazine in treatment-resistant schizophrenia. Am J Psychiatry 1998; 155:914-920Link, Google Scholar
3. Breier A, Hamilton SH: Comparative efficacy of olanzapine and haloperidol for patients with treatment-resistant schizophrenia. Biol Psychiatry 1999; 45:403-411Crossref, Medline, Google Scholar
4. Bondolfi G, Dufour H, Patris M, May JP, Billeter U, Eap CB, Baumann P (Risperidone Study Group): Risperidone versus clozapine in treatment-resistant chronic schizophrenia: a randomized double-blind study. Am J Psychiatry 1998; 155:499-504Link, Google Scholar
5. Breier AF, Malhotra AK, Su TP, Pinals DA, Elman I, Adler CM, Lafargue RT, Clifton A, Pickar D: Clozapine and risperidone in chronic schizophrenia: effects on symptoms, parkinsonian side effects, and neuroendocrine response. Am J Psychiatry 1999; 156:294-298Abstract, Google Scholar
6. Lindenmayer J-P, Iskander A, Park M, Apergi F-S, Czobor P, Smith R, Allen D: Clinical and neurocognitive effects of clozapine and risperidone in treatment refractory schizophrenics: a prospective study. J Clin Psychiatry 1998; 59:521-527Crossref, Medline, Google Scholar
7. Sharif ZA, Raza A, Ratakonda SS: Comparative efficacy of risperidone and clozapine in the treatment of patients with refractory schizophrenia or schizoaffective disorder: a retrospective analysis. J Clin Psychiatry 2000; 61:498-504Crossref, Medline, Google Scholar
8. Wirshing DA, Marshall BD Jr, Green MF, Mintz J, Marder SR, Wirshing WC: Risperidone in treatment-refractory schizophrenia. Am J Psychiatry 1999; 156:1374-1379Abstract, Google Scholar
9. Marder SR: Newer antipsychotics in treatment-resistant schizophrenia (letter). Biol Psychiatry 1999; 45:383-384Crossref, Medline, Google Scholar
10. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261-276Crossref, Medline, Google Scholar
11. Meltzer HY, Bastani B, Kwon KY, Ramirez LF, Burnett S, Sharpe J: A prospective study of clozapine in treatment-resistant schizophrenic patients, I: preliminary report. Psychopharmacology (Berl) 1989; 99(suppl):S68-S72Google Scholar
12. Kay SR, Opler LA, Lindenmayer JP: Reliability and validity of the Positive and Negative Syndrome Scale for schizophrenics. Psychiatry Res 1988; 23:99-110Crossref, Medline, Google Scholar
13. Chouinard G, Ross-Chouinard A, Annable L, Jones B: Extrapyramidal Symptom Rating Scale (abstract). Can J Neurol Sci 1980; 7:233Google Scholar
14. Lindley DV, Smith AFM: Bayes estimates for the linear model. J Royal Statistical Society Series B 1972; 34:1-41Google Scholar
15. Bryk AS, Raudenbush SW: Hierarchical Linear Models: Applications and Data Analysis Methods. Newbury Park, Calif, Sage Publications, 1992Google Scholar
16. Gibbons RD, Hedeker D, Waternaux C, Davis JM: Random regression models: a comprehensive approach to the analysis of longitudinal psychiatric data. Psychopharmacol Bull 1988; 24:438-443Medline, Google Scholar
17. Cohen J: Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar
18. Carpenter WT Jr, Heinrichs DW, Alphs LD: Treatment of negative symptoms. Schizophr Bull 1985; 11:440-452Crossref, Medline, Google Scholar
19. Czobor P, Volavka J, Sheitman B, Lindenmayer J-P, Citrome L, McEvoy J, Cooper TB, Chakos M, Liberman JA: Antipsychotic-induced weight gain and therapeutic response: a differential association. J Clin Psychopharmacol (in press)Google Scholar
20. Wirshing DA, Wirshing WC, Kysar L, Berisford MA, Goldstein D, Pashdag J, Mintz J, Marder SR: Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry 1999; 60:358-363Crossref, Medline, Google Scholar
21. Dernovsek Z, Tavcar R: Risperidone-induced leucopenia and neutropenia. Br J Psychiatry 1997; 171:393-394Crossref, Medline, Google Scholar
22. Chengappa KNR, Sheth S, Brar JS, Parepally H, Marcus S, Gopalani A, Palmer A, Baker RW, Schooler NR: Risperidone use at a state hospital: a clinical audit 2 years after the first wave of risperidone prescriptions. J Clin Psychiatry 1999; 60:373-378Crossref, Medline, Google Scholar
23. Peuskens J: Risperidone in the treatment of patients with chronic schizophrenia: a multi-national, multi-centre, double-blind, parallel-group study versus haloperidol. Br J Psychiatry 1995; 166:712-726Crossref, Medline, Google Scholar
24. Physicians’ Desk Reference, 54th ed. Montvale, NJ, Medical Economics Co, 2001, p 1580Google Scholar
25. Aravagiri M, Marder SR, Wirshing D, Wirshing WC: Plasma concentrations of risperidone and its 9-hydroxy metabolite and their relationship to dose in schizophrenic patients: simultaneous determination by a high performance liquid chromatography with electrochemical detection. Pharmacopsychiatry 1998; 31:102-109Crossref, Medline, Google Scholar
26. Tollefson GD, Birkett MA, Kiesler GM, Wood AJ: Double-blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biol Psychiatry 2001; 49:52-63Crossref, Medline, Google Scholar
27. Conley RR, Tamminga CA, Kelly DL, Richardson CM: Treatment-resistant schizophrenic patients respond to clozapine after olanzapine non-response. Biol Psychiatry 1999; 46:73-77Crossref, Medline, Google Scholar



