Hippocampal Deformities in Schizophrenia Characterized by High Dimensional Brain Mapping
Abstract
OBJECTIVE: Abnormalities of hippocampal structure have been reported in schizophrenia subjects. However, such abnormalities have been difficult to discriminate from normal neuroanatomical variation. High dimensional brain mapping, which utilizes probabilistic deformations of a neuroanatomical template, was used to characterize disease-related patterns of changes in hippocampal volume, shape, and asymmetry. METHOD: T1-weighted magnetic resonance scans were collected in 52 schizophrenia and 65 comparison subjects who were similar in age, gender, and parental socioeconomic status. The schizophrenia subjects were clinically stable at the time of assessment. RESULTS: Significant abnormalities of hippocampal shape and asymmetry (but not volume after total cerebral volume was included as a covariate) were found in the schizophrenia subjects. The pattern of shape abnormality suggested a neuroanatomical deformity of the head of the hippocampus, which contains neurons that project to the frontal cortex. The pattern of hippocampal asymmetry observed in the schizophrenia subjects suggested an exaggeration of the asymmetry pattern observed in the comparison subjects. No correlations were found between the magnitude of hippocampal shape and asymmetry abnormality and the severity of residual symptoms or duration of illness. CONCLUSIONS: Schizophrenia is associated with structural deformities of the hippocampus, which suggest a disturbance of the connections between the hippocampus and the frontal cortex. However, the magnitude of these deformities are not related to severity or duration of illness.
Studies that have used magnetic resonance (MR) imaging and manual methods to define neuroanatomical boundaries have reported decreases in the volume of the hippocampal formation in schizophrenia subjects relative to healthy comparison subjects (1–6). In some cases, volume reductions have been observed to be asymmetric (i.e., on the left side only) (3) or limited to schizophrenia subjects with more chronic disease (7) or of a specific gender (5, 7). Meta-analyses of such data suggest that the degree of the hippocampal volume reduction in schizophrenia is small (approximately 5%) (8, 9), especially in relationship to the magnitude of normal variation of hippocampal volumes among healthy individuals. Thus, it is not surprising that not all groups have detected hippocampal volume decreases (10, 11).
Computerized image analysis methods that employ a neuroanatomical atlas or template are being increasingly used to quantify both normal and abnormal neuroanatomical variation (12, 13). Such methods have been recently used to analyze patterns of cortical gyri, which may be more variable in schizophrenia subjects than in healthy comparison subjects (14). These methods, which we call high dimensional brain mapping, represent the typical structure of using a template and variations in the conformation of the typical structure among individuals by applying probabilistic transformations to the template (13, 15, 16). Disease-related abnormalities of neuroanatomy are distinguished from normal variation by determining the principal dimensions of shape variation within populations and then using these dimensions in statistical analyses to discriminate subjects with and without neuropsychiatric illness (17, 18).
The proper organization of neurons within the hippocampal formation determines its highly characteristic shape and function (19). Thus, neurodevelopmental aberrations in schizophrenia that might alter the organization of hippocampal neurons (20, 21) could also be expected to alter its shape and function. We previously used high dimensional brain mapping to detect and quantify abnormalities of hippocampal shape in 15 pairs of schizophrenia and comparison subjects (17). In the present study, we replicate and extend these findings in a larger, nonoverlapping group of schizophrenia and healthy comparison subjects.
Method
Subjects
Table 1 summarizes the general characteristics of the 52 schizophrenia and 65 comparison subjects. All subjects were diagnosed according to DSM-IV on the basis of consensus between a research psychiatrist who conducted a semistructured interview and a trained research assistant who used the Structured Clinical Interview for DSM-IV Axis I Disorders (22). The healthy comparison subjects had never been mentally ill nor had they any first-degree relatives with a psychotic disorder. No subject had an unstable medical or a neurologic disorder or a head injury with loss of consciousness, nor did any meet DSM-IV criteria for substance abuse or dependence during the 3 months preceding the study. Handedness was evaluated in all subjects (23), and both groups had equivalent numbers of left-handed subjects (schizophrenia subjects: 11.5%, N=6; comparison subjects: 4.6%, N=3).
All but three schizophrenia subjects had been treated with antipsychotic drugs (risperidone [N=17], olanzapine [N=12], haloperidol [N=7], blinded medication that could have been risperidone or haloperidol [N=7], clozapine [N=1], thiothixene [N=1], and fluphenazine [N=1]). Three subjects were treated with antipsychotic combinations (i.e., two with fluphenazine/quetiapine and one with risperidone/haloperidol). All schizophrenia subjects were in the residual phase of illness and met predetermined criteria for clinical stability (i.e., their symptoms had remained unchanged for at least 2 weeks [24]). Eighteen of the schizophrenia subjects were also receiving anticholinergic drugs (benztropine mesylate [N=17] or trihexyphenidyl [N=1]).
The severity of residual psychopathology was assessed in the schizophrenia subjects by using the Scale for the Assessment of Positive Symptoms (SAPS) (25) and the Scale for the Assessment of Negative Symptoms (SANS) (26). Scores for three dimensions of psychopathology (i.e., negative symptoms, psychosis, and thought disorganization) were derived from a principal-components analysis of SAPS and SANS items for each schizophrenia subject to assess correlations between the severity of psychopathology and the neuromorphometric variables. The factor structure obtained yielded item weightings for the schizophrenia subjects that were nearly identical to weightings previously reported in a larger group of schizophrenia subjects by Andreasen et al. (27). In addition, neuropsychological tests of memory and general intelligence (Wechsler Memory Scale, immediate and delayed memory; Benton Visual Retention Test, immediate and delayed memory; and WAIS-III verbal and performance IQ) were administered to 43 of the schizophrenia subjects and 59 of the healthy comparison subjects.
After complete description of the study to the subjects, written informed consent was obtained.
Image Collection and Preparation
MR scans were collected by using a turbo-fast low-angle shot sequence (TR=20, TE=5.4, flip angle=30°, number of acquisitions=1, matrix=256×256, scanning time=13.5 minutes) that acquired three-dimensional datasets with 1 mm × 1 mm × 1 mm isotropic voxels across the entire cranium (28). Raw MR data were reformatted by using Analyze software (Rochester, Minn.), including compression of signed 16-bit MR datasets to unsigned 8-bit MR datasets to maximize tissue contrast. Landmarks were placed in all scans at the external boundaries of the brain, at the points where the anterior and posterior commissures intersect the midsagittal plane, and at preselected points on the surface of the hippocampus along its anterior-posterior axis (29).
High Dimensional Brain Mapping
An MR scan collected from an additional healthy comparison subject was used to construct the neuroanatomical template. The hippocampus was manually outlined in this scan by using predetermined neuroanatomical guidelines (29). Transformation of the template onto the target MR scans occurred in a two-step process (29). First, it was coarsely aligned to each target scan by using the previously placed landmarks, and then a probabilistic, large-deformation transformation was applied to it (13–15). During this transformation, the movement and deformation of template voxels were constrained by assigning them the physical properties of a fluid. The reliability of this process, including landmark placement and both steps of the template transformation, is equivalent or superior to manual outlining by experts for defining the neuroanatomical boundaries of the hippocampus (17, 29).
To quantify hippocampal shape and volume, the hippocampal surface within the template was first defined by superimposing a triangulated graph onto the template hippocampus; this surface was then carried along as the template was transformed to match each of the target scans. Left and right hippocampal volumes in each of the target scans were estimated by calculating the volumes enclosed by the transformed surfaces. Fields of vectors were also derived from the displacements of the triangulated graph points during the transformations. To compare hippocampal shapes between subject groups, a pooled within-group covariance matrix was computed from these transformation vectors, which was then reduced by using singular value composition to identify the major dimensions of shape variation within the population of subjects (i.e., eigenvectors) (13, 15).
To examine the asymmetry of hippocampal shapes, asymmetry vectors were generated by reflecting hippocampal surface points from one hemisphere across the mid-sagittal plane onto the opposite hemisphere in each subject. The mid-sagittal plane was defined as the plane that yielded the least sum of squared errors during this reflection. A covariance matrix was computed from these transformation vectors, and the principal dimensions of hippocampal asymmetry (i.e., eigenvectors) were determined (16).
Total cerebral volumes were derived by using elastic-based transformations of the template scan (18) so that comparisons of hippocampal volumes and shapes could be performed with total cerebral volume as a covariate.
Statistical Analyses
Hippocampal volumes were compared by using two-way, repeated-measures analysis of variance, with diagnostic group as a factor and hemisphere as a repeated factor. To compare hippocampal shapes, the first 20 eigenvectors derived from the transformation vector field covariance matrix were selected a priori as adequately representing hippocampal shape variation, and a logistic regression model that yielded maximal discrimination between the groups was generated. Log-likelihood ratio values were calculated for each subject according to this model, and the statistical significance of the group difference was assessed by using Wilks’s lambda. For the comparison of patterns of hippocampal asymmetry between groups, a similar number of eigenvectors derived from the asymmetry vector field were selected, and another logistic model generated. Log-likelihood ratios were then calculated from this model, and the significance of the group difference was again tested by using Wilks’s lambda.
To determine whether the logistic regression model used to compare hippocampal shapes depended upon the subjects used to generate it, 10 different subsets of 12 subjects each (about 10% of the total sample) were selected at random from the entire subject sample and then excluded while alternate logistic regression models to discriminate the schizophrenia and comparison subjects were generated. As a further test of the shape comparison model, we also determined whether the coefficients associated with the eigenvectors obtained in our prior study of schizophrenia (17) could be applied to the comparison of subject groups in this study.
To visualize the physical pattern of hippocampal deformity represented by the eigenvector solutions that yielded significant between-group differences, we reconstructed maps of the composite hippocampal surface in the schizophrenia and comparison subjects by using the selected eigenvectors. The patterns of these deformities were then compared to surface displacement maps comparing the groups at every point on the graphical surface of the left and right hippocampi. These displacements were calculated at each surface point as the difference between the means of the group vectors in magnitude.
Results
Hippocampal Volume
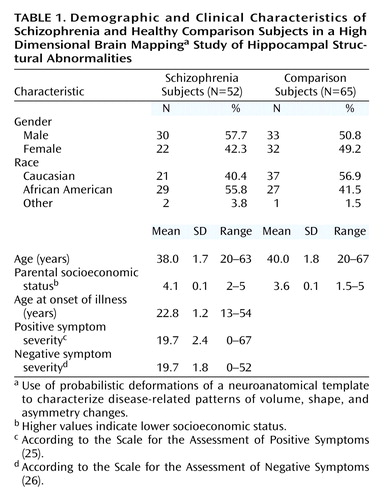
The mean hippocampal volume for the schizophrenia subjects was 2254 mm3 (SD=332) on the left and 2732 mm3 (SD=426) on the right, and the mean hippocampal volume for the comparison subjects was 2436 mm3 (SD=363) on the left and 2945 mm3 (SD=433) on the right (Figure 1). Hippocampal volumes discriminated the schizophrenia and comparison subjects (F=7.87, df=1, 115, p=0.006); however, this group difference became insignificant when total cerebral volume was included as a covariate (F=2.49, df=1, 114, p=0.12).
The analysis of hippocampal volumes showed a significant effect of hemisphere (F=623, df=1, 115, p<0.0001), but there was no group-by-hemisphere interaction (F=0.61, df=1, 115, p=0.43). An analysis of left/right volume ratios showed that schizophrenia and comparison subjects had similar degrees of volumetric asymmetry (comparison subjects: t=19.4, df=64, p<0.0001, effect size=2.2; schizophrenia patients: t=15.8, df=51, p<0.0001, effect size=2.4). Including total cerebral volume and gender as covariates in these analyses produced similar results, and exclusion of left-handed subjects did not alter the results.
Hippocampal Shape
Eigenvectors 1, 5, 7, 14, and 15 were selected a priori in the logistic regression model (χ2=27.7, df=5), and log-likelihood ratio values derived from the linear combination of these eigenvectors indicated a significant group difference (Figure 1). Similar results were found when total cerebral volume and gender were included as covariates and left-handed subjects were excluded.
To test whether this result was dependent on the particular subjects used to generate the eigenvectors, 10 comparisons were run after excluding in turn approximately 10% of the subjects in both groups, regenerating the logistic regression models, and then using them to categorize the excluded subjects. The overall mean rate of correct categorization was 68.3% (SD=6.5%), which was significantly higher than the 50% correct categorization rate obtainable by chance (z=2.79, p<0.006). Moreover, in all 10 trials, the selected eigenvectors were highly similar to those selected when the entire population was used (i.e., eigenvectors 1, 5, 14 in two trials; eigenvectors 1, 7, 14 in three trials; and eigenvectors 1, 14, 15 in five trials). As a test of the validity of the shape analysis, coefficients associated with the eigenvectors generated in our prior study of the hippocampus in schizophrenia (17) were used to compute log-likelihood ratio values for the present subjects. This analysis again yielded a categorization correction rate higher than the one obtainable by chance (87 out of 117 subjects [74%] were correctly identified).
Visual inspection of the pattern of hippocampal shape deformity in the schizophrenia subjects (Figure 2) suggested a loss of volume in the head of the hippocampus. Moreover, this pattern of deformity was similar regardless of whether the surface deformation maps were generated by using reconstructions from the eigenvector analysis or from maps of point-by-point displacements.
Hippocampal Asymmetry
Visual inspection of the pattern of hippocampal asymmetry suggested a highly similar pattern in both groups of subjects (Figure 3), the left hippocampus having a less prominent lateral surface and exaggerated bending along its longitudinal axis compared with the right. This pattern was similar whether the asymmetry maps in each group were generated by using asymmetry vector field eigenvectors 5 and 13 (see reference 16 for method of selecting eigenvectors) or point-by-point displacement maps.
For the between-group comparison of hippocampal asymmetry, eigenvectors 2, 11, and 12 were selected in the logistic regression model (χ2=15.2, df=3), and log-likelihood ratio values derived from the linear combination of these eigenvectors showed a significant group effect (F=5.63, df=3, 113, p<0.002; effect size=0.77). Visual inspection of the difference in asymmetry patterns between groups suggested an exaggeration of the normal pattern of asymmetry in the schizophrenia subjects (Figure 3).
Clinical Relationships
To investigate the clinical implications of hippocampal shape and asymmetry in the schizophrenia subjects, we estimated correlations among neuromorphometric variables (i.e., left and right hippocampal volumes and log-likelihood ratio values generated by shape and asymmetry comparisons) and variables reflecting the severity of residual psychopathology and other clinical characteristics. Small correlations in the unpredicted direction were found between thought disorganization and the log-likelihood ratio values derived from both hippocampal shape (r=0.25, df=50, p=0.07) and asymmetry (r=0.30, df=50, p=0.03) analyses. No other significant relationships were observed.
Significant group differences were found in performance on the selected cognitive tests of memory and general intelligence. In all cases, the schizophrenia subjects displayed evidence of substantial impairment relative to the comparison subjects as shown by scores on the Wechsler Memory Scale (immediate memory score: mean=80.3 [SD=19.8] versus 108.3 [SD=17.8], respectively [t=7.4, df=115, p<0.0001]; delayed memory score: mean=82.3 [SD=19.8] versus 108.2 [SD=17.7] [t=6.9, df=115, p<0.0001]) and the Benton Visual Retention Test (immediate memory score: mean=8.3 [SD=2.7] versus 9.6 [SD=1.0] [t=3.0, df=115, p=0.004]; delayed memory score: 5.3 [SD=2.4] versus 7.5 [SD=1.5] [t=5.1, df=115, p<0.0001]). Verbal IQ as assessed with the WAIS-III was significantly lower in the schizophrenia patients (mean=86.2, SD=19.8) than in the comparison subjects (mean=107.4, SD=15.4) (t=5.9, df=115, p<0.0001) as was performance IQ (mean=84.6 [SD=15.3] versus 108.5 [SD=15.8], respectively) (t=7.8, df=115, p<0.0001). No differences were found between schizophrenia subjects that were or were not receiving anticholinergic drugs, but in schizophrenia subjects only a significant correlation was observed between left hippocampal volumes and verbal IQ scores (r=0.30, df=50, p=0.04).
A significant correlation was found between hippocampal shape log-likelihood scores and performance IQ (r=0.37, df=63, p=0.003) in the healthy comparison subjects. Although they did not reach statistical significance, similar correlations were also found between left larger hippocampal volume and both verbal IQ (r=0.24, df=63, p=0.06) and performance IQ (r=0.24, df=63, p=0.06).
Discussion
The major finding of this study is that the quantification of hippocampal shape provided critical information for the discrimination of schizophrenia and healthy comparison subjects. Approximately three-quarters of both groups of subjects were correctly classified when this neuroanatomical measure was used. The robustness of this technique for categorizing the subjects was demonstrated by the results of repetitively generated logistic regression models, in which subsets of subjects were excluded before generating the models, as well as by successfully applying eigenvector coefficients generated in our prior study of the hippocampus to the subjects in the present study. These results suggest that the eigenvector solutions used to discriminate schizophrenia and comparison subjects are stable and applicable across different populations of subjects.
Visualization of the pattern of hippocampal deformity found in the schizophrenia subjects suggested a bilateral deformity of the head of the hippocampus. This observation is highly similar to the pattern of hippocampal deformity found in our prior study (17) and is consistent with the results of volumetric studies of the hippocampus that suggest greater volume losses in the anterior hippocampus (30). While neuropathological studies of the hippocampus have not reported the distribution of cellular abnormalities along the anterior-to-posterior axis of the hippocampus, some studies suggest greater cellular volume loss and disorganization within certain subfields (i.e., CA3/4 and the subiculum) (31). The pattern of hippocampal deformity observed in this study may also have important implications for understanding patterns of abnormal neural connectivity in schizophrenia. Hippocampal CA1 neurons that project to the medial prefrontal cortex are predominantly found in the head of the hippocampus (32, 33). Therefore, a shape deformity in this area provides support for hypotheses that schizophrenia involves a disturbance of the connections between the hippocampus and prefrontal structures (34).
An abnormality of hippocampal asymmetry was also identified in the schizophrenia subjects. In all subjects, the surface of the left hippocampus appeared to be depressed inward compared with the right hippocampus, especially on its lateral aspect. Schizophrenia subjects appeared to show an exaggeration of this pattern. Prior studies of the hippocampus in schizophrenia have suggested the presence of a more pronounced left-sided neuroanatomical abnormality (3). However, a loss of normal asymmetry has also been reported in brain structures such as the planum temporale (35). Such findings support hypotheses that schizophrenia may occur because of a defect in the developmental processes that determine hemispheric asymmetries (36).
The results of this study are also consistent with the results of previous meta-analytic studies of hippocampal volume in schizophrenia (8, 9, 30), insofar as small decreases in hippocampal volume were observed in the schizophrenia subjects; however, these were not significant after considering group differences in total cerebral volume.
Few relationships were found between neuroanatomical measures, including the degree of hippocampal shape deformity and clinical variables. While one-quarter of the schizophrenia subjects were not correctly classified according to hippocampal shape deformity, the lack of a correlation between this deformity and clinical characteristics in all schizophrenia subjects suggests that the incorrectly classified subjects were not clinically distinctive. Our failure to detect more substantial relationships between neuromorphometric and clinical features may be due to the fact that the subjects in this study had been treated with antipsychotic drugs. However, there were still measurable symptoms, and we were able to replicate the published factor structure of the SAPS and SANS (27). The absence of a correlation between neuromorphometric variables and duration of illness may suggest that hippocampal shape abnormalities in the schizophrenia subjects were not the consequence of chronic disease. In preliminary support of the possibility that such abnormalities may be related to the genetic risk of acquiring schizophrenia, a similar pattern of hippocampal deformity has been found in the well siblings of schizophrenia subjects (37).
We were surprised by the lack of relationships between neuroanatomical and cognitive variables in the schizophrenia subjects. Schizophrenia subjects display deficits on a wide range of cognitive tests, including those related to memory (38), and some (39, 40), but not all (41), investigators have reported relationships between brain structure volumes in schizophrenia and the severity of cognitive deficits. In our schizophrenia subjects, we observed only small correlations between verbal IQ and hippocampal volumes, and similar relationships were observed in the healthy comparison subjects.
The ability to obtain important information about neuropsychiatric diseases such as schizophrenia by examining the shapes of neuroanatomical structures supports claims that neuroanatomical shapes may reflect the integrity of neural connections. Van Essen (42) proposed a general hypothesis suggesting that the physical properties of neuropil combined with patterns of neural connectivity may determine the shape of specific brain structures. Moreover, development of appropriate cortical-cortical and subcortical-cortical connections is critical for successful brain organization (43) and produces specific patterns of neuroanatomical shapes (44). Postmortem studies of the hippocampus and other medial temporal lobe structures suggest that schizophrenia may be associated with abnormalities in the pattern of neural connectivity (20, 21, 31, 45, 46).
 |
Received Jan. 10, 2002; revision received May 30, 2002; accepted June 6, 2002. From the Department of Psychiatry, the Department of Anatomy and Neurobiology, and the Division of Biostatistics, Washington University School of Medicine; the Metropolitan St. Louis Psychiatric Center, St. Louis; and the Department of Biomedical Engineering, Johns Hopkins University, Baltimore. Address reprint requests to Dr. Csernansky, Department of Psychiatry (Box 8134), Washington University School of Medicine, 660 S. Euclid Ave., St. Louis, MO 63110; [email protected] (e-mail). Supported by NIMH grant MH-56584 and the Silvio Conte Center at Washington University School of Medicine (MH-62130).
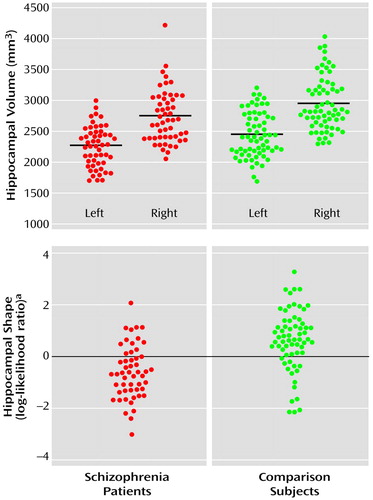
Figure 1. Hippocampal Volume and Shape in Subjects With Schizophrenia (N=52) and Healthy Comparison Subjects (N=65)
aLog-likelihood ratio values derived from the linear combination of eigenvectors 1, 5, 7, 14, and 15, which yielded the maximal between-group discrimination in a logistic regression model (39 [75.0%] of the schizophrenia and 50 [76.9%] of the healthy comparison subjects were correctly classified). Comparison of the log-likelihood ratio values revealed a significant difference between groups (F=6.90, df=5, 111, p<0.0001; effect size=1.1).
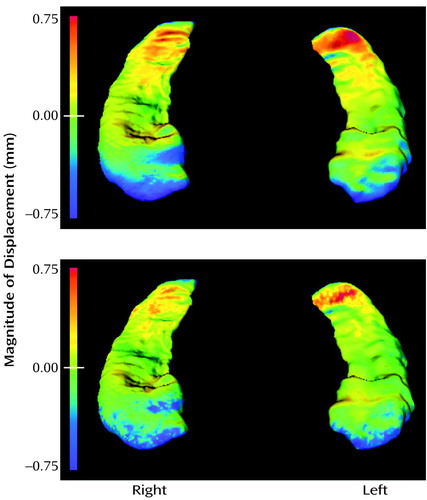
Figure 2. Surface Mapsa Depicting Patterns of Shape Deformities in Subjects With Schizophrenia (N=52) Relative to Healthy Comparison Subjects (N=65)
aThe top map shows between-group hippocampal shape differences derived from the eigenvector analysis. On the bottom, a point-by-point displacement map compares the two groups. Blue-to-purple shading denotes areas in which schizophrenia subjects show an inward deformity relative to the comparison subjects. In both maps, the hippocampus is viewed from above and toward the head of the structure.
Figure 3. Surface Mapsa Depicting Hippocampal Asymmetry in Subjects With Schizophrenia (N=52) and Healthy Comparison Subjects (N=65)
aThe top row of panels show reconstructions of hippocampal asymmetry differences derived from the eigenvector analysis. In the bottom row of panels, point-by-point displacement maps show the same asymmetry differences. The between-group comparison panels show right-to-left hippocampal surface displacements between schizophrenia and comparison subjects. In both cases, the left hippocampus is overlaid onto the right. Areas in which the left hippocampus is smaller than the right are noted in blue-to-purple shading.
1. DeLisi LE, Dauphinais ID, Gershon ES: Perinatal complications and reduced size of brain limbic structures in familial schizophrenia. Schizophr Bull 1988; 14:185-191Crossref, Medline, Google Scholar
2. Suddath RL, Casanova MF, Goldberg TE, Daniel DG, Kelsoe JR Jr, Weinberger DR: Temporal lobe pathology in schizophrenia: a quantitative magnetic resonance imaging study. Am J Psychiatry 1989; 146:464-472Link, Google Scholar
3. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia. N Engl J Med 1992; 327:604-612Crossref, Medline, Google Scholar
4. Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F: Brain morphology and schizophrenia: a magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry 1992; 49:921-926Crossref, Medline, Google Scholar
5. Bogerts B, Lieberman JA, Ashtari M, Bilder RM, Degreef G, Lerner G, Johns C, Masiar S: Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol Psychiatry 1993; 33:236-246Crossref, Medline, Google Scholar
6. Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, Arnold SE, Bilker WB, Gur RC: Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry 2000; 57:769-775Crossref, Medline, Google Scholar
7. Razi K, Greene KP, Sakuma M, Ge S, Kushner M, DeLisi LE: Reduction of the parahippocampal gyrus and the hippocampus in patients with chronic schizophrenia. Br J Psychiatry 1999; 175:388-389Medline, Google Scholar
8. Nelson MD, Saykin AJ, Flashman LA, Riordan HJ: Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry 1998; 55:433-440Crossref, Medline, Google Scholar
9. Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET: Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 2000; 157:16-25Link, Google Scholar
10. Swayze VW II, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC: Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry 1992; 31:221-240Crossref, Medline, Google Scholar
11. Zipursky RB, Marsh L, Lim KO, DeMent S, Shear PK, Sullivan EV, Murphy GM, Csernansky JG, Pfefferbaum A: Volumetric MRI assessment of temporal lobe structures in schizophrenia. Biol Psychiatry 1994; 35:501-516Crossref, Medline, Google Scholar
12. Thompson PM, MacDonald D, Mega MS, Holmes CJ, Evans AC, Toga AW: Detection and mapping of abnormal brain structure with a probabilistic atlas of cortical surfaces. J Comput Assist Tomogr 1997; 21:567-581Crossref, Medline, Google Scholar
13. Grenander U, Miller MI: Computational anatomy: an emerging discipline. Q Applied Mathematics 1998; 4:617-694Google Scholar
14. Narr KL, Thompson PM, Sharma T, Moussai J, Zoumalan C, Rayman J, Toga AW: Three-dimensional mapping of gyral shape and cortical surface asymmetries in schizophrenia: gender effects. Am J Psychiatry 2001; 158:244-255Link, Google Scholar
15. Miller MI, Banerjee A, Christensen G, Joshi S, Khaneja N, Grenander U, Matejic L: Statistical methods in computational anatomy. Stat Methods Med Res 1997; 6:267-299Crossref, Medline, Google Scholar
16. Wang L, Joshi SC, Miller MI, Grenander U, Csernansky JG: Statistical analysis of hippocampal asymmetry. Neuroimage 2001; 14:531-545Crossref, Medline, Google Scholar
17. Csernansky JG, Joshi S, Wang L, Haller J, Gado M, Miller JP, Grenander U, Miller MI: Hippocampal morphometry in schizophrenia via high dimensional brain mapping. Proc Natl Acad Sci USA 1998; 95:11406-11411Crossref, Medline, Google Scholar
18. Csernansky JG, Wang L, Joshi S, Miller JP, Gado M, Kido D, McKeel D, Morris JC, Miller MI: Early DAT is distinguished from aging by high dimensional mapping of the hippocampus. Neurology 2000; 55:1636-1643Crossref, Medline, Google Scholar
19. Wyss JM, van Groen R: Development of the hippocampus, in The Hippocampus—New Vistas: Neurology and Neurobiology, vol 52. Edited by Chan-Palay V, Kohler C. New York, Alan R Liss, 1989, pp 1-16Google Scholar
20. Conrad AJ, Abebe T, Austin R, Forsythe S, Scheibel AB: Hippocampal pyramidal cell disarray in schizophrenia as a bilateral phenomenon. Arch Gen Psychiatry 1991; 48:413-417Crossref, Medline, Google Scholar
21. Akbarian S, Vinuela A, Kim JJ, Potkin SG, Bunney WE Jr, Jones EG: Distorted distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Arch Gen Psychiatry 1993; 50:178-187Crossref, Medline, Google Scholar
22. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
23. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97-113Crossref, Medline, Google Scholar
24. Rastogi-Cruz D, Csernansky JG: Clinical rating scales, in Adult Psychiatry. Edited by Guze SB. St Louis, Mosby, 1997, pp 45-51Google Scholar
25. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
26. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
27. Andreasen NC, Arndt S, Alliger R, Miller D, Flaum M: Symptoms of schizophrenia: methods, meanings, and mechanisms. Arch Gen Psychiatry 1995; 52:341-351Crossref, Medline, Google Scholar
28. Venkatesan R, Haacke EM: Role of high resolution in magnetic resonance (MR) imaging: applications of MR angiography, intracranial T1-weighted imaging, and image interpolation. Int J Imaging Systems and Technology 1997; 8:529-543Crossref, Google Scholar
29. Haller JW, Banerjee A, Christensen GE, Gado M, Joshi S, Miller MI, Sheline YI, Vannier MW, Csernansky JG: Three-dimensional hippocampal MR morphometry by high-dimensional transformation of a neuroanatomic atlas. Radiology 1997; 202:504-510Crossref, Medline, Google Scholar
30. Heckers S: Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 2001; 11:520-528Crossref, Medline, Google Scholar
31. Harrison PJ, Eastwood SL: Neuropathological studies of synaptic connectivity in the hippocampal formation in schizophrenia. Hippocampus 2001; 11:508-519Crossref, Medline, Google Scholar
32. Barbas H, Blatt GJ: Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus 1995; 5:511-533Crossref, Medline, Google Scholar
33. Carmichael ST, Price JL: Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 1995; 363:615-641Crossref, Medline, Google Scholar
34. Weinberger DR, Berman KF, Suddath R, Torrey EF: Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry 1992; 149:890-897Link, Google Scholar
35. Sommer I, Ramsey N, Kahn R, Aleman A, Bouma A: Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta-analysis. Br J Psychiatry 2001; 178:344-351Crossref, Medline, Google Scholar
36. Crow TJ, Ball J, Bloom SR, Brown R, Bruton CJ, Colter N, Frith CD, Johnstone EC, Owens DGC, Roberts GW: Schizophrenia as an anomaly of development of cerebral asymmetry. Arch Gen Psychiatry 1989; 46:1145-1150Crossref, Medline, Google Scholar
37. Tepest R, Wang L, Falkai P, Csernansky JG: Shape analysis of the hippocampal formation in schizophrenics and their healthy siblings. Neuroimage 2001; 13:S265Google Scholar
38. Saykin A, Shtasel D, Gur RE, Kester D, Mozley L, Stafiniak P, Gur RC: Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry 1994; 51:124-131Crossref, Medline, Google Scholar
39. Nestor PG, Shenton ME, McCarley RW, Haimson J, Smith RS, O’Donnell B, Kimble M, Kikinis R, Jolesz FA: Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. Am J Psychiatry 1993; 150:1849-1855Link, Google Scholar
40. Seidman LJ, Yurgelun-Todd D, Kremen WS, Woods BT, Goldstein JM, Faraone SV, Tsuang MT: Relationship of prefrontal and temporal lobe MRI measures to neuropsychological performance in chronic schizophrenia. Biol Psychiatry 1994; 35:235-246Crossref, Medline, Google Scholar
41. Torres IJ, Flashman LA, O’Leary DS, Swayze V II, Andreasen NC: Lack of an association between delayed memory and hippocampal and temporal lobe size in patients with schizophrenia and healthy controls. Biol Psychiatry 1997; 42:1087-1096Crossref, Medline, Google Scholar
42. Van Essen D: Pulling strings to build a better brain: a tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 1997; 385:313-318Crossref, Medline, Google Scholar
43. Goldman-Rakic PS: Development of cortical circuitry and cognitive function. Child Dev 1987; 58:601-622Crossref, Medline, Google Scholar
44. Thompson PM, Schwartz G, Lin RT, Kan AA, Toga AW: Three-dimensional statistical analysis of sulcal variability in the human brain. J Neurosci 1996; 16:4261-4274Crossref, Medline, Google Scholar
45. Zaidel DW, Esiri MM, Harrison PJ: The hippocampus in schizophrenia: lateralized increased in neuronal density and altered cytoarchitectural asymmetry. Psychol Med 1997; 27:703-713Crossref, Medline, Google Scholar
46. Benes FM: Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol Psychiatry 1999; 46:589-599Crossref, Medline, Google Scholar



