Ventricular and Periventricular Structural Volumes in First- Versus Multiple-Episode Bipolar Disorder
Abstract
OBJECTIVE: Ventriculomegaly has been reported in bipolar disorder, although whether it occurs at illness onset or progresses during the course of the disorder is unknown. In addition, it is unknown whether ventriculomegaly in bipolar disorder reflects acquired volume loss or underdevelopment of periventricular structures. METHOD: Magnetic resonance imaging was used to measure the volumes of the lateral and third ventricles and periventricular structures (caudate, putamen, thalamus, hippocampus). Patients with DSM-IV bipolar disorder, 18 who were having a first episode and 17 with multiple episodes, were compared with 32 healthy subjects. RESULTS: The lateral ventricles were significantly larger in the patients with multiple-episode bipolar disorder than in the first-episode patients or the healthy subjects, even after periventricular and total cerebral volumes were taken into account. Having larger lateral ventricles was associated with a higher number of prior manic episodes. The multiple-episode patients had a smaller total cerebral volume than the healthy subjects but not the first-episode patients. The putamen was significantly larger in the first-episode patients (and nearly so in the multiple-episode patients) than in the healthy subjects, although there was no difference between patient groups. CONCLUSIONS: Lateral ventriculomegaly was greater in bipolar disorder patients who had had repeated manic episodes, but it does not appear to be secondary to small critical periventricular structures. A larger than normal striatum, which has been reported in previous studies, was observed in first-episode patients. These results support the importance of prospectively studying neuroanatomic changes in bipolar disorder.
Abnormally large brain ventricles have been reported frequently, albeit inconsistently, in bipolar disorder (1, 2). Inconsistencies among studies may reflect differences in clinical characteristics of patient groups (1, 2). Specifically, groups with less chronic or severe illness may be less likely to have ventriculomegaly. For example, first-episode or adolescent patients have only somewhat larger ventricles than healthy subjects (3, 4). In addition, Hauser et al. (5) found lateral ventriculomegaly in bipolar disorder type I but not bipolar disorder type II, suggesting that the severity of the manic phase, which differentiates these subtypes, may be associated with ventriculomegaly. Therefore, lateral ventriculomegaly might progress with repeated affective episodes and greater illness severity in bipolar disorder.
Evidence for an association between illness duration and ventriculomegaly in patients with severe mental illness can be drawn from studies of chronic schizophrenia, in which progressive ventricular enlargement has been reported (6, 7). Ventricular enlargement in schizophrenia appears to be more pronounced than in affective disorders (8); this difference may reflect the differences in course severity between these illnesses. Again, these observations suggest that ventricular enlargement may occur as a consequence of the severity or chronicity of major mental illness.
The most direct approach to determining whether ventricular enlargement develops progressively in bipolar disorder would be to recruit first-episode patients and examine ventricular measurements longitudinally. The feasibility of such a study is uncertain in the absence of studies that estimate the rate at which this hypothesized progression occurs. An alternative cross-sectional approach is to compare ventricular volumes in first-episode patients to volumes in those who have experienced repeated affective episodes. Significantly larger ventricles in multiple- than first-episode patients would support the hypothesis that ventricular size progresses during the course of bipolar disorder, particularly if enlargement is associated with course severity variables (e.g., number of affective episodes). In contrast, no differences in ventricular volumes between first- and multiple-episode bipolar patients would imply that progressive ventricular enlargement is unlikely.
The neuropathologic meaning of large ventricles is unclear but may reflect small volumes of periventricular brain regions. Several periventricular structures (e.g., striatum, thalamus, hippocampus) appear to modulate mood and cognition and so may be relevant to the expression of affective symptoms. Studies of patients with unipolar depression suggest associations between greater lateral ventricular volume and basal ganglia abnormalities (2). To our knowledge, these associations have not been directly examined in bipolar disorder.
With these considerations in mind, we used magnetic resonance imaging (MRI) to compare lateral and third ventricular volumes in first- and multiple-episode bipolar patients. We predicted that the multiple-episode patients would have larger ventricles than the first-episode patients and that the volumes would be associated with course of illness. In addition, we studied differences among groups in periventricular structures, to determine associations with ventricular volumes. The periventricular structures measured included the thalamus, which is adjacent to the lateral and third ventricles; the hippocampus, which abuts the temporal horn of the lateral ventricle; and the striatum (i.e., caudate and putamen), which bounds the main body of the lateral ventricles.
Method
Subjects
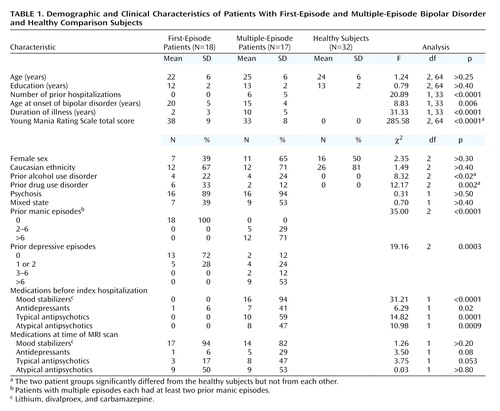
Patients 16–35 years old who met the DSM-IV criteria for bipolar disorder, type I, with a manic or mixed current episode, were recruited from consecutive admissions to the University of Cincinnati Hospital and the Cincinnati Children’s Hospital Medical Center. All patients were hospitalized and had a score of at least 15 on the Young Mania Rating Scale (9) at the time of the MRI scan. Eighteen patients met the criteria for the “first-episode group,” defined as having 1) no prior manic episodes, 2) no prior psychiatric hospitalizations, and 3) no prior treatment with mood-stabilizer or antipsychotic medications. Seventeen patients met the criteria for the “multiple-episode group,” defined as having had 1) two or more prior manic episodes, 2) one or more prior psychiatric hospitalizations, and 3) prior antipsychotic or mood-stabilizer treatment. We recruited 32 healthy subjects, also 16–35 years old, from the hospitals’ catchment areas. The healthy subjects had no axis I DSM-IV psychiatric disorder and no first-degree relatives with a known history of psychiatric disorders. Patients or healthy subjects were excluded for 1) any substance use disorder within 3 months of the scan, 2) medical or neurological illness that might influence brain structure, 3) contraindications to receiving an MRI, 4) a positive pregnancy test in women, or 5) mental retardation or a documented IQ of less than 70. Each subject (or, if under 18, the subject’s legal guardian) provided written informed consent after the study procedures were explained. All subjects were deemed competent to consent (or assent if under 18 years old) for the study by the investigators and their primary clinicians. This study was approved by the University of Cincinnati and Children’s Hospital Medical Center investigational review boards. None of these subjects was included in other imaging publications from this research group (3, 10, 11).
Both the patients and healthy subjects were evaluated by using the Structured Clinical Interview for DSM-IV Axis I, Patient Version (SCID) (12), administered by investigators (M.P.D, C.M.A., S.M.S.) extensively trained in the use of this instrument and with excellent established interrater reliability (kappa>0.90) (13). The clinical variables extracted from the SCID included age at illness onset (i.e., the age of the first affective episode), numbers of prior manic and depressive episodes, current affective state (i.e., manic or mixed), history of prior substance use disorders, and number of previous hospitalizations. Ratings on the Young Mania Rating Scale were obtained at the time of the MRI scan by trained raters (M.P.D, C.M.A., M.E.Z.); the interrater reliability for total scores was measured with the intraclass correlation coefficient (ICC) and was judged to be good (ICC>0.80) (13). Finally, current and prior use of antipsychotics, antidepressants, and mood stabilizers was reviewed with each subject and recorded. All patients were receiving medications at the time of the MRI scan (Table 1). Treatment was prescribed by inpatient clinicians who were not study personnel. The first-episode patients had been receiving medication for only a few days as part of the current hospitalization (by definition), so it is unlikely that these medications influenced neuroanatomy.
Image Acquisition
MRI scans were obtained with a 1.5-T GE Signa system by using three-dimensional inversion-recovery-prepped fast spoiled gradient recall acquisition with axial-plane graphic prescription from a sagittal localizer (TR=15 msec, TE=5 msec, TI=300 msec, flip angle=20°) in order to obtain whole-brain T1-weighted images with an in-plane field of view of 24 cm2 and a 256×256-pixel matrix. The third (z) dimension of acquisition was defined so that 1.5-mm-thick axial slices covered the entire brain (approximately 124 image slices per subject). The data matrix was then interpolated to a 256×256×256-pixel matrix on reconstruction to provide an approximately 1-mm isotropic image data set.
Image Analysis
Image analysis proceeded as described in previous reports (10, 11). Briefly, the image data were analyzed by using BrainImage, version 3.4.11 (A. Reiss et al., 1995). Before measurements of the specific regions of interest, all image sets were reformatted into the same three-dimensional space. Specifically, the sagittal plane was defined as the plane bisecting the left and right cerebral hemispheres in both axial and coronal views in which the posterior commissure was most clearly visualized and the cerebral aqueduct was most patent. An orthogonal axial plane was then defined as the slice that intersected both the anterior and posterior commissures at the midline. Finally, the coronal plane was defined as the plane orthogonal to the axial and sagittal planes. One exception to this orientation was for measurements of the hippocampus, in which the axial plane was defined as the orthogonal (to the sagittal plane) slice that was parallel to the left hippocampal long axis (11, 14). The measurements were performed by trained raters (M.P.D., M.E.Z., G.E.G., N.P.M., J.R.) with established high interrater reliability (ICC>0.90), who were blind to subject identity and group. Our procedures for establishing reliability involve extensive training with experienced raters (10, 11). The boundaries of the regions of interest were previously published (10, 11), and so we describe them only briefly here.
The total cerebrum was measured coronally, beginning at the most anterior point of the prefrontal cortex and ending at the most posterior point of the occipital cortex. Measurements were made every fifth slice and included both gray and white matter but not cerebrospinal fluid. The brainstem and cerebellum were excluded from this measurement.
The lateral ventricles were measured in every coronal slice in which they were visible, including the temporal horns, by segmenting CSF from brain tissue. The left and right lateral ventricles were measured separately.
The third ventricle was measured coronally in every slice in which it was visible, beginning posteriorly at the point where the superior colliculus merges with the cerebrum (inferior to the thalamus) and proceeding anteriorly to the optic chiasm.
The striatum included separate measurements of the caudate and putamen. The head and body of the caudate were traced in each slice until the tail turned inferolaterally. The putamen was traced in its entirety. It was bounded laterally and anteriorly by the external capsule and separated from the globus pallidus by the lateral medullary lamina.
The thalamus was manually traced in its entirety in the coronal plane. The medial boundary was the lateral ventricle, and the lateral boundary was the posterior limb of the internal capsule (11).
The hippocampus was separated from the amygdala by identifying the alveus (11, 14) and measured posteriorly to the coronal slice in which the right and left superior colliculi were jointly visualized.
Statistical Analysis
All analyses were performed by using the Statistical Analysis System for Windows, version 8 (SAS Institute, Cary, N.C., 2000). Total cerebral volumes were compared by using analysis of variance. A multivariate analysis of variance (MANOVA) tested the primary hypothesis that the multiple-episode patients would have significantly larger ventricles than the other two groups. The dependent variables were right and left lateral ventricular and third ventricular volumes. The independent variable was group (i.e., first-episode patient, multiple-episode patient, healthy subject). To identify specific group differences after omnibus analysis, Tukey’s honestly significant difference test was performed. Periventricular structures in the three groups were compared by using MANOVA in which the volumes of the caudate, putamen, thalamus, and hippocampus were the dependent variables. Right- and left-sided periventricular measurements were combined to reduce the number of dependent variables, since no significant laterality differences among groups were observed in preliminary comparisons.
A two-step analysis identified clinical correlates of lateral ventricular volumes. For this analysis, left and right lateral ventricular volumes were combined (since no specific laterality differences were observed, as noted subsequently). First, we performed simple correlations between lateral ventricular volumes and clinical variables: duration of illness, age at illness onset, number of prior manic episodes, number of prior depressive episodes, total Young Mania Rating Scale score, history of alcohol or drug use disorder, affective state (mixed or manic), presence or absence of psychosis, and prior exposure to medications. Clinical variables that demonstrated associations with lateral ventricular measurements at p<0.10 were then entered into a multivariate regression controlled by group. From this regression, significant associations (p<0.05) between clinical variables and lateral ventricular measurements were identified. Ranked scores were used for ratings of prior manic and depressive episodes, since several subjects reported chronic symptoms, which made it difficult to distinguish specific episodes.
Results
The three groups were demographically and clinically well matched (Table 1). The differences between groups that we observed were expected from the group definitions (e.g., more hospitalizations in multiple-episode than first-episode patients). Because the groups were matched by age, the patients with multiple prior manic episodes had a significantly earlier illness onset than the first-episode patients. Age at onset was not significantly associated with the volume of any region of interest (r<0.10, N=35, p>0.50). In addition, the patients had significantly more past alcohol and drug use disorders than the healthy subjects (although they did not differ from each other according to Fisher’s exact test; p>0.90 for alcohol and p>0.20 for drug use disorders). Neither of these variables was significantly correlated with the region-of-interest measurements (r<0.27, N=35, p>0.10).
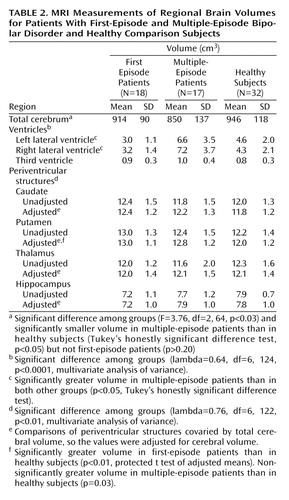
Group means for the region-of-interest measurements are listed in Table 2. The total volume of cerebral tissue significantly differed among groups, and the multiple-episode patients’ volumes were significantly smaller than those of the healthy subjects but not the first-episode patients. The cerebral volumes of the first-episode patients and healthy subjects did not significantly differ (p>0.60).
MANOVA of the ventricular volumes revealed significant group differences. Specifically, the multiple-episode patients had significantly larger right and left lateral ventricles than both the first-episode patients and healthy subjects (Table 2). The latter two groups did not significantly differ from each other. Laterality differences in these ventricular volumes were not observed among groups. To determine whether differences in total cerebral volume explained these group differences, we performed analysis of covariance (MANCOVA), covarying total cerebral volume. The results remained essentially unchanged (lambda=0.61, df=6, 122, p<0.0001), suggesting that the differences in ventricles were not secondary to differences in cerebral volume. In addition, removing subjects with any drug or alcohol use disorder did not appreciably change the results (lambda=0.69, df=6, 102, p<0.004). To further illustrate group differences, the distributions of the measurements of total (right and left combined) lateral ventricular volume, as the percentage of total cerebral tissue volume, are provided in Figure 1. All of the first-episode patients’ (and all but one of the healthy subjects’) measurements were less than the median (1.7%) and mean (1.7%) values of the multiple-episode patients. There was no significant difference among groups in the volume of the third ventricle (p>0.10 for all comparisons).
As in prior studies (4, 15), age was associated with lateral ventricular volume (r=0.27, N=67, p<0.03). However, the interaction between age and group was not significant (F=0.16, df=2, 61, p>0.20), suggesting no differential age effects on ventricular volume among groups.
Because total cerebral volume was significantly correlated with the volumes of the caudate (r=0.53, N=67, p<0.0001), putamen (r=0.53, N=67, p<0.0001), thalamus (r=0.54, N=67, p<0.0001), and hippocampus (r=0.36, N=67, p=0.003), cerebral volume was covaried in the comparisons of periventricular structures. The MANCOVA of periventricular structures identified significant group differences (lambda=0.74, df=8, 120, p<0.02). Protected t tests (based on adjusted means) were used to identify specific group differences, adjusting for multiple comparisons by defining alpha as <0.01. The first-episode patients had significantly greater putamen volumes than the healthy subjects (Table 2), although the difference between the multiple-episode patients and healthy subjects was also nearly significant. The patient groups did not significantly differ in putamen volumes (p>0.60). There were no significant differences among groups in caudate volume (p>0.10 for all comparisons) or thalamic volume (p>0.80 for all comparisons). The differences among groups in hippocampal volume did not meet the adjusted significance level, although nearly significant differences were observed in the comparisons of the first-episode patients with both the multiple-episode patients (p=0.05) and healthy subjects (p=0.05). Hippocampal volume did not differ between the multiple-episode patients and healthy subjects (p>0.70).
To determine whether group differences in lateral ventricular volume were explained by differences in periventricular volumes, MANCOVA of the ventricular volume was performed. After covarying for periventricular volumes, we found that the differences in lateral ventricular volume among groups remained essentially unchanged (lambda=0.60, df=6, 116, p<0.0001). To extend this evaluation further, we performed simple correlations to determine whether higher ventricular volumes were associated with lower periventricular volumes, or the converse (i.e., higher periventricular volumes associated with lower ventricular volumes). None of the ventricular measurements exhibited a significant inverse correlation with any of the periventricular measurements.
Simple correlations identified associations at the p<0.10 level between lateral ventricular measurements and several course variables, including duration of illness (r=0.42, N=35, p<0.02), number of prior manic episodes (r=0.69, N=35, p<0.0001), number of prior depressive episodes (r=0.41, N=35, p<0.02), and prior exposure to mood stabilizers (r=0.59, N=35, p<0.0002) and atypical antipsychotics (r=0.51, N=35, p<0.002). As planned, these variables were then entered into a multivariate regression so that we could look at the unique association for each clinical measure after accounting for the effects of the other measures. In this analysis, only the number of prior manic episodes was significantly associated with lateral ventricular volume (semipartial r2=0.19, N=35, p<0.02).
Discussion
To our knowledge, this is the first study to compare MRI ventricular and periventricular volumes in patients with first- and multiple-episode bipolar disorder. We also believe that it is one of only two studies comparing first- and multiple-episode patients on any neuroanatomic measure (10). Comparing these types of patients is one step toward clarifying whether morphometric changes occur during the course of bipolar illness. These results may guide subsequent prospective research into the progression of neuroanatomic changes in bipolar disorder.
The multiple-episode patients had significantly larger lateral ventricles than the first-episode patients and healthy subjects. One explanation of this difference is that patients with ventriculomegaly at illness onset have a worse illness course and, therefore, become overrepresented in multiple-episode groups. If this is correct, then there should be some first-episode patients with pronounced lateral ventriculomegaly. However, scatterplots of ventricular volumes from the patient groups showed that all of the first-episode patients had lateral ventricular volumes that were less than the median measurement of the multiple-episode group and that were similar to those of healthy subjects. Therefore, within the constraints of this cross-sectional study, there is no evidence that the ventriculomegaly observed in the multiple-episode group was present at the time of the first manic episode.
An alternative explanation is that ventricular size progresses during the course of bipolar disorder. This possibility is supported by the difference in distribution of ventricular volumes between the patient groups as well as associations between lateral ventricular volume and course variables, including duration of illness, numbers of prior manic and depressive episodes, and prior treatment. Of these, the number of prior manic episodes was the most strongly linked to ventricular volume and, when controlled for, diminished the associations with the other variables. Brambilla et al. (16) also reported a correlation between ventricular volume and prior number of affective episodes in bipolar disorder.
Previously, we found significantly smaller cerebellar vermal volume in multiple- than in first-episode bipolar patients (10). In that study, vermal volume was inversely associated with the number of prior depressive episodes. Brambilla et al. (16) also reported an inverse correlation between vermal size and the number of affective episodes in bipolar disorder. Coupled with the results of our current study, these findings suggest that neuroanatomic changes occur in bipolar disorder in response to repeated affective episodes.
In this study, multiple-episode patients had a mean lateral ventricular volume that was 122% greater than that of first-episode patients with an 8-year difference in duration of illness. If the ventriculomegaly of the multiple-episode group occurred as a steady, progressive increase in lateral ventricular volume during this period of 8 years, these results predict a change of 15% per year. However, changes associated with normal aging might account for some of these effects. Nonetheless, given these assumptions, prospectively studying 15–20 first-episode patients for 2–3 years would provide sufficient statistical power to detect progression in ventricular volumes (17). The results of this cross-sectional study, then, support the feasibility of a prospective study of MRI neuroanatomic changes in bipolar disorder.
The specific neurophysiologic mechanisms underlying ventriculomegaly remain elusive (1–8). Covarying for the volumes of periventricular structures and total cerebral volume did not affect the differences observed. Therefore, the greater ventricular volume does not appear to result from smaller volumes of these structures or generally smaller total cerebral tissue volume. Other studies have shown periventricular white matter lesions in bipolar disorder (18), and histopathological studies suggest low glial density in bipolar disorder (19). Therefore, ventriculomegaly could reflect a loss of periventricular white matter. Since we did not specifically measure this, we cannot address this possibility directly.
Differences in striatal volumes between patients and healthy subjects were observed in the current study and are consistent with previous findings (11, 20). Specifically, the first-episode patients had significantly larger putamen volumes than the healthy subjects (and the difference between the multiple-episode patients and healthy subjects was nearly significant), although the patient groups did not differ from each other. This observation suggests that, in contrast to lateral ventriculomegaly, enlargement of striatal structures is unlikely to be progressive. Studies of unaffected family members, particularly children, of bipolar probands are needed to determine whether striatal abnormalities exist before the onset of bipolar illness. If so, these results would suggest that an abnormally large striatum represents a neuroanatomic marker or vulnerability factor for bipolar disorder.
In schizophrenia, caudate enlargement has been associated with antipsychotic use over 18 months (21). However, the first-episode patients in our study had no antipsychotic exposure before the index hospitalization and, at most, only a few days of antipsychotic exposure before the MRI scan. Therefore, the larger putamen observed in these bipolar patients appears to be due to a mechanism different from that for patients with schizophrenia who are receiving antipsychotic medication.
To our knowledge, there have been no histopathological studies of the striatum in bipolar disorder to help interpret this finding. Functional imaging studies suggest that, at least during depression, metabolism and blood flow are decreased in the striatum of bipolar patients (1). In addition, studies using magnetic resonance spectroscopy have identified higher concentrations of choline in the striatum of patients with bipolar disorder than in healthy subjects (1). Together, these studies suggest that the striatum is structurally, functionally, and chemically abnormal in bipolar disorder.
The striatum is integrally involved in subcortical-cortical loops that connect prefrontal, thalamic, and medial temporal structures that constitute the neural networks thought to modulate human mood and cognitive states (22). The ventral striatum specifically projects to orbitofrontal and medial prefrontal cortical regions that are involved in the modulation of socially appropriate emotional behavior (23) and that may be dysfunctional in bipolar disorder (1). Therefore, a larger than normal striatum may represent an anatomic correlate to dysfunction within these important brain networks. Studies of bipolar patients that integrate imaging modalities (e.g., functional and structural) to examine this brain region are needed in order to clarify the clinical significance of this finding.
Several limitations of this study need to be considered when interpreting findings. First, only hospitalized, acutely manic patients were included, which limits comparisons with less severely ill populations, as well as other bipolar subgroups (e.g., bipolar disorder, type II). Most of these patients exhibited psychosis during the affective episode. Although psychosis was not associated with any region of interest, this may reflect the high rate, and low variability, of psychosis in these patients. Second, medication histories were reduced to dichotomous variables to simplify analyses and minimize the difficulties inherent in obtaining reliable treatment information from patients. This approach, although pragmatic, did not permit more detailed evaluation of potential medication effects. Third, there is no consensus for defining a “first-episode” bipolar population. As has been suggested, identifying a patient at the time of the first hospitalization, first treatment, or first manic episode provides a reliable index (13). In contrast, trying to identify the onset of illness on the basis of “prodromal” cyclothymic or hypomanic symptoms is limited by the difficulty of making these diagnoses retrospectively (13). We believe that the criteria used in this study are a logical application of these prior suggestions. Finally, the multiple-episode patients were significantly younger at illness onset than the first-episode patients. Although age at onset was not significantly associated with any region of interest, it is possible that patients with an earlier onset have a more severe phenotype of the illness and are therefore more likely to have structural brain abnormalities than patients with later onsets. In particular, the multiple-episode patients’ mean age at onset was during a time when major neuroplastic events occur (24). Therefore, the greater ventricular volume may represent a greater loss of cerebral tissue during this time period. Despite these limitations, our findings suggest that prospective studies of neuroanatomic changes during the course of bipolar disorder are feasible and might help clarify the functional neuroanatomy of this disorder.
 |
 |
Received Nov. 13, 2001; revision received May 20, 2002; accepted May 23, 2002. From the Bipolar and Psychotic Disorders Research Program, Department of Psychiatry, University of Cincinnati College of Medicine. Address reprint requests to Dr. Strakowski, Bipolar and Psychotic Disorders Research Program, Department of Psychiatry, University of Cincinnati College of Medicine, Cincinnati, OH 45267-0559; [email protected] (e-mail). Supported by a grant from the Stanley Medical Research Institute and by NIMH grant MH-58170.
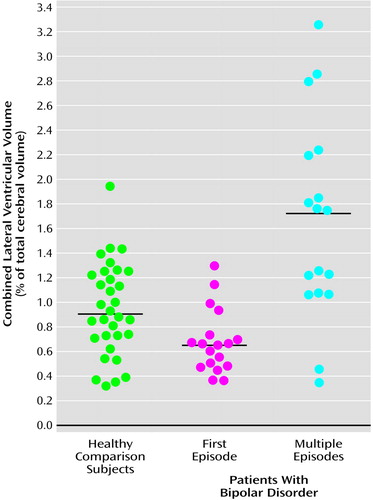
Figure 1. Individual Volumes of Combined Right and Left Lateral Ventricles, Relative to Total Cerebrum, in Patients With First-Episode (N=18) and Multiple-Episode (N=17) Bipolar Disorder and Healthy Comparison Subjects (N=32)a
aHorizontal lines indicate mean values.
1. Strakowski SM, DelBello MP, Adler C, Cecil KM, Sax KW: Neuroimaging in bipolar disorder. Bipolar Disord 2000; 2:148-164Crossref, Medline, Google Scholar
2. Strakowski SM, Adler CM, DelBello MP: Volumetric brain imaging in mood disorders. Bipolar Disord 2002; 4:1-9Crossref, Medline, Google Scholar
3. Strakowski SM, Wilson DR, Tohen M, Woods BT, Doublass AW, Stoll AL: Structural brain abnormalities in first-episode mania. Biol Psychiatry 1993; 33:602-609Crossref, Medline, Google Scholar
4. Botteron KN, Vannier MW, Geller B, Todd RD, Lee BCP: Preliminary study of magnetic resonance imaging characteristics in 8- to 16-year-olds with mania. J Am Acad Child Adol Psychiatry 1995; 34:742-749Crossref, Medline, Google Scholar
5. Hauser P, Matochik J, Altshuler LL, Denicoff KD, Conrad A, Li X, Post RM: MRI-based measurements of temporal lobe and ventricular structures in patients with bipolar I and bipolar II disorders. J Affect Disord 2000; 60:25-32Crossref, Medline, Google Scholar
6. DeLisi LE, Tew W, Xie S, Hoff AL, Sakuma M, Kushner M, Lee G, Shedlack K, Smith AM, Grimson R: A prospective follow-up study of brain morphology and cognition in first-episode schizophrenic patients: preliminary findings. Biol Psychiatry 1995; 38:349-360Crossref, Medline, Google Scholar
7. Davis KL, Buchsbaum MS, Shihabuddin L, Spiegel-Cohen J, Metzger M, Frecska E, Keefe RS, Powchik P: Ventricular enlargement in poor-outcome schizophrenia. Biol Psychiatry 1998; 43:783-793Crossref, Medline, Google Scholar
8. Elkis H, Friedman L, Wise A, Meltzer HY: Meta-analysis of studies of ventricular enlargement and cortical sulcal prominence in mood disorders: comparisons with controls or patients with schizophrenia. Arch Gen Psychiatry 1995; 52:735-746Crossref, Medline, Google Scholar
9. Young RC, Biggs JT, Ziegler VE, Meyer DA: A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry 1978; 133:429-435Crossref, Medline, Google Scholar
10. DelBello MP, Strakowski SM, Zimmerman ME, Sax KW, Hawkins JM: MRI analysis of the cerebellum in bipolar disorder. Neuropsychopharmacology 1999; 21:63-68Crossref, Medline, Google Scholar
11. Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER: Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry 1999; 56:254-260Crossref, Medline, Google Scholar
12. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
13. Strakowski SM, Williams JR, Sax KW, Fleck DE, DelBello MP, Bourne ML: Is impaired outcome following a first manic episode due to mood-incongruent psychosis? J Affect Disord 2000; 61:87-94Crossref, Medline, Google Scholar
14. Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, Wilkins J, Gerner R, Mintz J: An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry 2000; 48:147-162Crossref, Medline, Google Scholar
15. Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO: A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol 1994; 51:874-887Crossref, Medline, Google Scholar
16. Brambilla P, Harenski K, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC: MRI study of posterior fossa structures and brain ventricles in bipolar patients. J Psychiatr Res 2001; 35:313-322Crossref, Medline, Google Scholar
17. Stevens J: Applied Multivariate Statistics for the Social Sciences, 3rd ed. Mahwah, NJ, Lawrence Erlbaum Associates, 1996, pp 468-470Google Scholar
18. Altshuler LL, Curran JG, Hauser P, Mintz J, Denicoff K, Post R: T2 hyperintensities in bipolar disorder: magnetic resonance imaging comparison and literature meta-analysis. Am J Psychiatry 1995; 152:1139-1144Link, Google Scholar
19. Rajkowski G: Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry 2000; 48:766-777Crossref, Medline, Google Scholar
20. Aylward EH, Roberts-Twillie JV, Barta PE, Kumar AJ, Harris GJ, Geer M, Peyser CE, Pearlson GD: Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. Am J Psychiatry 1994; 151:687-693Link, Google Scholar
21. Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M: Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 1994; 151:1430-1436Link, Google Scholar
22. Haber SN, McFarland NR: The concept of the ventral striatum in nonhuman primates. Ann NY Acad Sci 1999; 877:33-48Crossref, Medline, Google Scholar
23. Cummings JL: Frontal-subcortical circuits and human behavior. Arch Neurol 1993; 50:873-880Crossref, Medline, Google Scholar
24. Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL: Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci USA 2001; 98:11650-11655Crossref, Medline, Google Scholar



