Perceptual Asymmetries in Schizophrenia: Subtype Differences in Left Hemisphere Dominance for Dichotic Fused Words
Abstract
OBJECTIVE: Dichotic listening techniques have been used to study hemispheric dominance for language in schizophrenia. The authors’ goal was to compare subjects with paranoid and undifferentiated subtypes of schizophrenia. METHOD: The Fused Rhymed Words Test was used to compare perceptual asymmetries in 16 patients with paranoid schizophrenia, 28 patients with undifferentiated schizophrenia, and 29 healthy comparison subjects. RESULTS: Patients with paranoid schizophrenia had the largest left hemisphere advantage and patients with undifferentiated schizophrenia had the smallest. The asymmetry of healthy subjects was intermediate. Hemisphere advantage varied as a function of gender only in the patients with undifferentiated schizophrenia. CONCLUSIONS: The findings support the hypotheses that undifferentiated schizophrenia is associated with underactivation of left hemisphere resources for verbal processing and that paranoid schizophrenia is characterized by preserved left hemisphere processing.
Dichotic listening tests, in which two different words or syllables are simultaneously presented to the two ears, provide a noninvasive method for assessing lateralized temporal lobe function. Several studies have revealed a smaller right ear (left hemisphere) advantage for patients with schizophrenia compared with normal subjects (1–3). This is not a universal finding, however (4). One explanation may be that laterality differs as a function of illness subtype. Given evidence that verbal abilities are more intact in paranoid than nonparanoid schizophrenia (5), patients with paranoid schizophrenia might be expected to exhibit less evidence of left hemisphere deficits on dichotic tests. Two early dichotic studies did find larger right ear (left hemisphere) advantages in paranoid than in nonparanoid schizophrenia (6, 7). However, these studies used a dichotic recall test that is confounded by response bias and memory decay.
The current study examined patterns of laterality as a function of illness subtype by comparing patients with paranoid and undifferentiated schizophrenia. We used the Fused Rhymed Words Test (8, 9), a well-validated dichotic listening measure that is free from response bias and memory effects. On the basis of previous studies comparing patients with paranoid and nonparanoid schizophrenia, we hypothesized that patients with paranoid schizophrenia would exhibit a larger right ear (left hemisphere) advantage than patients with undifferentiated schizophrenia. The Positive and Negative Syndrome Scale (10) and the Brief Psychiatric Rating Scale (BPRS) (11) were also used to examine the relation between perceptual asymmetry alterations and symptom severity.
Method
Subjects
Inpatients were recruited from the Mental Health Clinical Research Center in Schizophrenia Studies at the New York State Psychiatric Institute and at Creedmoor Psychiatric Center in New York City. All procedures were fully explained, and written informed consent was obtained from each subject. Patients in both facilities underwent identical diagnostic assessments conducted by the same group of raters, coordinated by the Diagnosis and Assessment Core of the Mental Health Clinical Research Center in Schizophrenia Studies.
Sixteen patients met DSM-IV criteria for paranoid schizophrenia, and 28 met criteria for undifferentiated schizophrenia. All were fluent in English, and all were right-handed, as assessed by the Edinburgh Inventory (12). An audiometric examination excluded subjects with a hearing loss greater than 30 dB in either ear or a difference between ears greater than 10 dB at 500, 1000, and 2000 Hz. Patients were also excluded if they had a history of neurological insult or illness, current substance abuse, or a history of substance abuse that obscured their diagnosis. Healthy, right-handed comparison subjects were recruited by advertisement and screened by using the Structured Clinical Interview for DSM-IV (13). They were excluded if past or present axis I disorders, substance abuse, organic brain impairment, or hearing loss was present.
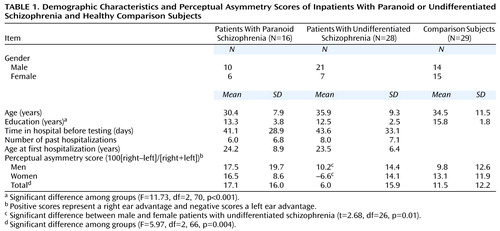
Table 1 presents demographic data for the three groups. The subject groups did not differ in mean age or gender but differed in mean years of education. The comparison subjects had a higher education level than the patients with paranoid (t=–3.02, df=43, p<0.01) and undifferentiated (t=–5.64, df=55, p<0.001) schizophrenia. The two patient groups, however, did not differ significantly in education, and there was no correlation between perceptual asymmetry scores and education (r=–0.03, df=71, p=0.80). There was also no difference between the patient groups in the number of days of hospitalization before testing, number of past hospitalizations, or age at first hospitalization.
The two patient groups were similar in medication status: five patients with paranoid and seven with undifferentiated schizophrenia were receiving no medication. Seven patients with paranoid and seven with undifferentiated schizophrenia were receiving atypical neuroleptics, and four patients with paranoid and 14 with undifferentiated schizophrenia were receiving typical neuroleptics. Patients who were receiving no medication (N=12), patients receiving atypical medications (N=14), and patients receiving typical medications (N=18) did not differ significantly in right ear advantages for dichotic words (F=0.47, df=2, 41, p=0.63).
Measurements
Diagnosis
Research diagnoses were made according to DSM-IV criteria by using a structured diagnostic interview (the Diagnostic Interview for Genetic Studies [14]) as well as a review of patient records. All Diagnostic Interview for Genetic Studies ratings were followed by consensus diagnosis by the rater, the patient’s physician, and a senior member of the Diagnosis and Assessment Core, all of whom were blind to the dichotic listening data. BPRS and Positive and Negative Syndrome Scale ratings were available for all but one patient with paranoid and seven with undifferentiated schizophrenia. Ratings were based on information gathered from a semistructured interview as well as review of recent hospital records. Raters were blind to the dichotic data and were required to achieve adequately high interrater reliability (intraclass correlations greater than 0.85) before being permitted to evaluate patients. An index of overall symptom severity was derived by totaling scores for 18 Positive and Negative Syndrome Scale items, corresponding to the 18-item BPRS.
Fused Rhymed Words Test
Two single-syllable rhyming words (e.g., pig/dig) were presented simultaneously to the two ears. The words fused to form a single percept, so that the subject heard only one word. The subject was instructed to circle this word, which appeared on a prepared answer sheet with a list of four rhyming words for each trial. A total of 120 fused word pairs were presented to each subject (divided into four 30-item blocks). Words were presented through a matched pair of TDH-49 headphones at a level of 75 dB (SPL) while the subject was seated in a sound-attenuated booth. The orientation of the headphones was switched after the first and third blocks.
Statistical Analysis
The number of right- and left-ear words correctly reported was used to compute an asymmetry score: 100(right–left)/(right+left). Positive scores represent a right ear advantage and negative scores a left ear advantage. Errors, in which the subject chooses a word that was not presented to either ear, were infrequent and do not enter into the equation. Asymmetry scores for paranoid, undifferentiated, and comparison groups were evaluated by using a three-by-two analysis of covariance with group and gender as factors and education entered as a covariate. Post hoc comparisons among groups were performed with Bonferroni correction. T tests were used to evaluate group differences in Positive and Negative Syndrome Scale and BPRS ratings, and the relations of asymmetry scores to these measures were examined with Pearson correlation coefficients.
Results
Table 1 gives the perceptual asymmetry scores for each group. There was a significant difference in right ear advantages among groups. Patients with paranoid schizophrenia had the largest and those with undifferentiated schizophrenia the smallest right ear advantage. Healthy comparison subjects had intermediate ear asymmetry. Post hoc analyses indicated a significant difference between the paranoid and undifferentiated groups (t=3.29, df=42, p<0.01) and between the undifferentiated and comparison groups (t=2.53, df=55, p<0.05) but not between the paranoid and comparison groups (t=0.90, df=43, n.s.). Education was not a significant covariate of asymmetry (F=0.80, df=1, 66, p=0.37), and the main effect for gender was not significant (F=1.47, df=1, 66, p=0.23). There was a significant group-by-gender interaction (F=3.33, df=2, 66, p=0.04). The source of this interaction was a significant difference in perceptual asymmetry between men and women in the undifferentiated group (Table 1), but not in the other groups. Women with undifferentiated schizophrenia showed a small left ear advantage, and men with undifferentiated schizophrenia showed a right ear advantage, which was smaller than the right ear advantage of patients with paranoid schizophrenia but not that of comparison subjects.
Patients with undifferentiated schizophrenia were rated as more symptomatic (mean=38.3, SD=8.8) than those with paranoid schizophrenia (mean=32.3, SD=6.1) on the BPRS (t=–2.28, df=34, p=0.03). However, BPRS ratings did not correlate significantly with asymmetry scores (r=0.10, df=34, p=0.56). Patients with undifferentiated schizophrenia also had greater conceptual disorganization (mean=3.3, SD=2.1) than patients with paranoid schizophrenia (mean=1.4, SD=0.8) (t=–3.71, df=33, p=0.001). Positive and Negative Syndrome Scale negative and positive symptoms tended to be greater in patients with undifferentiated schizophrenia, but these differences did not reach statistical significance. The Positive and Negative Syndrome Scale scores for negative symptoms, positive symptoms, conceptual disorganization, and hallucinatory behavior were not significantly correlated with asymmetry scores.
Discussion
The findings support the hypothesis that patients with paranoid schizophrenia have a greater left hemisphere advantage for language processing than those with undifferentiated schizophrenia. The patients with the undifferentiated subtype showed a smaller left hemisphere advantage for dichotic words than healthy comparison subjects, but those with the paranoid subtype did not. These findings are in accord with previous studies demonstrating reduced left hemisphere advantage in schizophrenia (1–3) and further specify the relation of diagnostic subtype to this anomalous brain lateralization.
These results are consistent with previous dichotic recall studies that compared patients with paranoid and nonparanoid schizophrenia (6, 7). Differences in dichotic listening and cognitive function between these subtypes led to hypotheses that paranoid schizophrenia is characterized by left hemisphere overactivation and that nonparanoid schizophrenia is associated with reduced left hemisphere processing or right hemisphere overactivation (7, 15, 16). Although the patients with nonparanoid schizophrenia in the current study did show a smaller left hemisphere advantage than those with paranoid schizophrenia and healthy subjects, patients with paranoid schizophrenia did not differ significantly from healthy subjects. Thus, our findings provide only partial support for the above hypotheses, in that there was no evidence for left hemisphere overactivation in patients with paranoid schizophrenia.
Neuroimaging and neuropsychological studies provide further support for the hypothesis that left temporal underactivation is involved in undifferentiated (nonparanoid) schizophrenia but not paranoid schizophrenia. Using proton magnetic resonance spectroscopy, Fukuzako and colleagues (17) found that the metabolite ratios in the left medial temporal lobe of subjects with undifferentiated and disorganized schizophrenia were smaller than those of subjects with paranoid schizophrenia. Furthermore, neuropsychological studies (5) have found relatively preserved cognitive functioning in paranoid compared with nonparanoid schizophrenia, particularly on tests thought to reflect left hemisphere processing of verbal material. Other studies (18), however, have not found significant paranoid/nonparanoid differences.
An unexpected finding emerged when we controlled for gender in the statistical analysis. Dichotic listening asymmetry varied as a function of gender in the undifferentiated group, but not in the paranoid or healthy comparison groups. Male patients with undifferentiated schizophrenia showed a right ear advantage, whereas female patients showed a slight left ear advantage. It is important to note, however, that the number of women in the subgroups was quite small. More research will be necessary before making any conclusions concerning this finding.
The current finding of greater conceptual disorganization and reduced left hemisphere advantage in patients with undifferentiated schizophrenia is consistent with Magaro and Chamrad’s notion (19) that nonparanoid schizophrenia compromises the ability to process information in a serial, sequential manner, particularly on tests requiring left hemisphere involvement. Conceptual disorganization may reflect problems with sequencing ideas, words, and associations into coherent discourse, perhaps originating from underactivation of left-hemisphere resources in individuals with undifferentiated schizophrenia.
The results of the current study also support the view that paranoid and undifferentiated subtypes represent distinct taxonomic entities. Although subtypes of schizophrenia have a rich tradition within clinical psychiatry, subtype differences are frequently overlooked in neurophysiological studies of schizophrenia. In the current study, combining paranoid and nonparanoid subgroups into one group would have masked important differences between patient and healthy comparison groups. Further research using more direct electrophysiological and neuroimaging techniques is needed to clarify the nature of anomalous asymmetries in schizophrenia subtypes.
 |
Presented in part at the annual meeting of the Society for Research in Psychopathology, Montreal, Nov. 18–21, 1999. Received Nov. 13, 2000; revision received April 17, 2001; accepted May 3, 2001. From the New York State Psychiatric Institute and College of Physicians & Surgeons, Columbia University, New York; the University of Massachusetts Boston; and the Brockton VA Medical Center, Harvard Medical School, Brockton, Mass. Address reprint requests to Ms. Friedman, Department of Psychology, University of Massachusetts Boston, 100 Morrissey Blvd., Boston, MA 02125; [email protected] (e-mail). Supported in part by NIMH project grant MH-50715 (Dr. Bruder) and Mental Health Clinical Research Center grant MH-59342 (Dr. Gorman).
1. Wexler BE, Giller EL Jr, Southwick S: Cerebral laterality, symptoms, and diagnosis in psychotic patients. Biol Psychiatry 1991; 29:103-116Crossref, Medline, Google Scholar
2. Green MF, Hugdahl K, Mitchell S: Dichotic listening during auditory hallucinations in patients with schizophrenia. Am J Psychiatry 1994; 151:357-362Link, Google Scholar
3. Bruder G, Rabinowicz E, Towey J, Brown A, Kaufmann CA, Amador X, Malaspina D, Gorman JM: Smaller right ear (left hemisphere) advantage for dichotic fused words in patients with schizophrenia. Am J Psychiatry 1995; 152:932-935Link, Google Scholar
4. Wale J, Carr V: Dichotic listening asymmetries and psychotic symptoms in schizophrenia: a preliminary report. Psychiatry Res 1988; 25:31-39Crossref, Medline, Google Scholar
5. Seltzer J, Conrad C, Cassens G: Neuropsychological profiles in schizophrenia: paranoid versus undifferentiated distinctions. Schizophr Res 1997; 23:131-138Crossref, Medline, Google Scholar
6. Gruzelier J, Hammond NV: Lateralized deficits and drug influences on the dichotic listening of schizophrenic patients. Biol Psychiatry 1980; 15:759-779Medline, Google Scholar
7. Nachshon I: Hemispheric dysfunctioning in schizophrenia. J Nerv Ment Dis 1980; 168:241-242Crossref, Medline, Google Scholar
8. Wexler BE, Halwes T: Increasing the power of dichotic methods: the Fused Rhymed Words Test. Neuropsychologia 1983; 21:59-66Crossref, Medline, Google Scholar
9. Zatorre RJ: Perceptual asymmetry on the dichotic fused words test and cerebral speech lateralization determined by the carotid sodium Amytal test. Neuropsychologia 1989; 27:1207-1219Google Scholar
10. Kay SR, Opler LA, Fishbein A: Positive and Negative Syndrome Scale (PANSS) Rating Manual. Toronto, Multihealth System, 1992Google Scholar
11. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799-812Crossref, Google Scholar
12. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97-113Crossref, Medline, Google Scholar
13. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1998Google Scholar
14. Nurnberger JI Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T (NIMH Genetics Initiative): Diagnostic Interview for Genetic Studies: rationale, unique features, and training. Arch Gen Psychiatry 1994; 51:849-859Crossref, Medline, Google Scholar
15. Gruzelier J: Hemispheric imbalances masquerading as paranoid and nonparanoid syndromes? Schizophr Bull 1981; 7:662-673Crossref, Medline, Google Scholar
16. Magaro PA: The paranoid and the schizophrenic: the case for distinct cognitive style. Schizophr Bull 1981; 7:632-661Crossref, Medline, Google Scholar
17. Fukuzako H, Kodama S, Fukuzako T, Yamada K, Doi W, Sato D, Takigawa M: Subtype-associated metabolite differences in the temporal lobe in schizophrenia detected by proton magnetic resonance spectroscopy. Psychiatry Res 1999; 92:45-56Crossref, Medline, Google Scholar
18. Zalewski C, Johnson-Selfridge MT, Ohriner S, Zarrella K, Seltzer JC: A review of neuropsychological differences between paranoid and nonparanoid schizophrenia patients. Schizophr Bull 1998; 24:127-145Crossref, Medline, Google Scholar
19. Magaro PA, Chamrad DL: Information processing and lateralization in schizophrenia. Biol Psychiatry 1983; 18:29-44Medline, Google Scholar



