Longitudinal MRI Study of Hippocampal Volume in Trauma Survivors With PTSD
Abstract
OBJECTIVE: The authors prospectively explored whether a reduction in the volume of the hippocampus occurs in recent trauma survivors who develop posttraumatic stress disorder (PTSD). METHOD: Thirty-seven survivors of traumatic events were assessed within a week of the traumatic event and 6 months later. The assessment included magnetic resonance imaging of the brain (including 124 coronal slices of 1.5-mm thickness), psychometric testing, and structured clinical interviews. The Clinician-Administered PTSD Scale conferred PTSD diagnoses at 6 months. RESULTS: Ten subjects (27%) had PTSD at 6 months. The subjects with PTSD did not differ from those without PTSD in hippocampal volume (right or left) at 1 week or 6 months. There was no reduction in hippocampal volume in the PTSD subjects between 1 week and 6 months. CONCLUSIONS: Smaller hippocampal volume is not a necessary risk factor for developing PTSD and does not occur within 6 months of expressing the disorder. This brain abnormality might occur in individuals with chronic or complicated PTSD.
Four magnetic resonance imaging (MRI) studies have shown that chronic posttraumatic stress disorder (PTSD) is associated with lower than normal hippocampal volume (1–4). In the first two studies, the reported differences in hippocampal volumes between PTSD and comparison subjects ranged from 5% for the left hippocampus alone (4) to over 22% bilaterally (2); the two other studies showed an 8% smaller right hippocampus (1) and 12% smaller left hippocampus (3).
The occurrence of lower hippocampal volume in human subjects with PTSD is buttressed by animal studies (e.g., references 5, 6) that show measurable hippocampal atrophy after prolonged exposure to stress. Accordingly, distressed survivors of traumatic events may progressively develop hippocampal atrophy. However, a recent study (7) failed to show low hippocampal volume in chronically maltreated children with PTSD. Furthermore, in a study of alcoholic women (8), the presence of PTSD did not add to the effect of alcohol consumption on hippocampal volume.
The MRI studies in which smaller hippocampi were found used a cross-sectional design and evaluated individuals with chronic PTSD. Consequently, they could not determine the origin of this brain abnormality. Low hippocampal volume may either precede the traumatic event, thereby increasing the likelihood of developing PTSD (a “vulnerability” hypothesis), or develop, along with PTSD, in the aftermath of traumatic events (an “acquisition” hypothesis). Smaller hippocampal volume may also be a risk factor for developing a particularly chronic and unremitting form of PTSD.
Prospective studies, starting before the traumatic event, can determine the origin of the smaller hippocampal volume in PTSD, but studying subjects before a traumatic event is daunting. A reliable approximation of subjects’ neuroanatomy before the traumatic event can nevertheless be obtained by performing MRI scans of the brain within few days of the traumatic event (the relevant animal literature will be discussed). Such a longitudinal design also reduces the influence of potentially confounding variables by using each subject as his or her own control. We present the results of a longitudinal study of hippocampal volume in PTSD in which images were obtained 1 week and 6 months after a traumatic event.
Method
Subjects
Forty-four adult survivors of traumatic events who met the DSM-IV PTSD criteria A.1 and A.2 were recruited into the study after their admission to a general hospital emergency room. Subjects were not included in the study if during the traumatic event they suffered head injury or physical injury necessitating hospital admission or major surgery. In addition, subjects with a lifetime history of neurological disorder, psychotic disorder, PTSD, or substance abuse were not included. Subjects were excluded from the study if during the study’s 6-month follow-up period they experienced another traumatic event or used illicit drugs or excessive (i.e., daily) amounts of alcohol. The subjects received a comprehensive description of the study and provided written informed consent.
Instruments
During the first assessment session the subjects completed the Spielberger State-Trait Anxiety Inventory, state version (9), Horowitz’s Impact of Event Scale—Revised (10), and the Structured Clinical Interview for DSM-IV (11). The last instrument served to identify current and lifetime disorders. After the interview, each subject had an MRI scan of the brain. A second evaluation took place 6 months later and included the previous psychometric measures, the Mississippi Scale for Combat-Related PTSD (12, 13), and the Clinician-Administered PTSD Scale (14). The Clinician-Administered PTSD Scale conferred PTSD diagnoses and severity at 6 months. Lifetime exposure to traumatic events was assessed by the Trauma History Questionnaire (15).
MRI Acquisition and Measurement
MR images were acquired on a 2-T Elscint GYREX “Prestige” MRI system. The intracranial cavity was measured by using TE=30 and 80 msec, TR=3000 msec, field of view=24 cm, acquisition matrix=56×256, and 192 phase-encoding steps, resulting in two double-echo images at 54 different levels with 3-mm slice thickness (contiguous slices). We used 124 coronal slices of 1.5-mm thickness (TE=5 msec, TR=35 msec, 45° angle, field of view=24 cm, acquisition matrix=256×256, 192 phase-encoding steps) to evaluate the volume of the hippocampus.
An independent rater, trained and supervised by one of us (M.E.S.) at the Department of Radiology of Brigham and Women’s Hospital, performed volumetric analyses according to a previously published procedure (2, 16). This rater was blind to the subjects’ diagnostic status and to the time of MR image acquisition (1 week or 6 months). Two other trained raters independently performed volumetric analyses on 12 randomly selected cases. Interrater reliability was evaluated by intraclass correlation, which yielded R=0.89 for the hippocampus and R=0.88 for the amygdala.
Procedure
Within 48 hours of subjects’ admission to the general hospital emergency room, the research psychologist (D.B.) contacted each subject and confirmed 1) the occurrence of an event involving direct threat of death or injury to self or others (DSM-IV PTSD criterion A.1.) and 2) the presence of an intense response involving fear, helplessness, or horror (DSM-IV PTSD criterion A.2). Individuals who met these criteria and whose ages were between 18 and 65 years were invited to participate in the study. Thirty-nine (89%) of 44 consenting subjects completed the study, and 37 (95%) of those had valid MRI scans at both time points.
Results
Ten subjects (27%) (seven women and three men) met the PTSD diagnostic criteria at 6 months, and 27 subjects (12 women and 15 men) did not. The groups did not differ significantly in gender distribution (Fisher’s exact test, p=0.26). The PTSD and non-PTSD subjects had similar mean ages—33.7 (SD=8.9) and 29.8 (SD=10.1) years, respectively—and similar mean education levels—13.4 (SD=2.0) and 12.4 (SD=3.8) years in school, respectively.
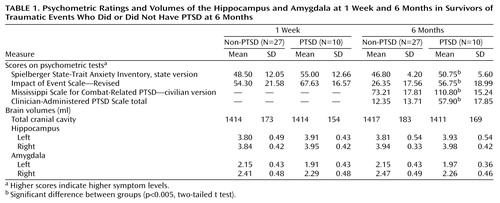
Table 1 presents the mean volumes of the hippocampus, amygdala, and total cranial cavity at 1 week and 6 months, along with concurrent psychometric test scores and total scores on the Clinician-Administered PTSD Scale. At 1 week the study groups had similar scores on the State-Trait Anxiety Inventory, state version (t=1.52, df=35, p<0.15), and Impact of Event Scale (t=1.41, df=35, p<0.13). At 6 months the PTSD group had significantly higher scores on the State-Trait Anxiety Inventory, state version (t=3.07, df=35, p<0.005), Impact of Event Scale (t=4.14, df=35, p<0.001), Mississippi Scale for Combat-Related PTSD (t=5.94, df=35, p<0.001), and total Clinician-Administered PTSD Scale (t=6.89, df=35, p<0.0001). There was no statistically significant correlation between hippocampal volume (left or right, 1 week and 6 months) and concurrent PTSD symptoms.
Three-way analysis of variance (ANOVA) using diagnosis (PTSD versus non-PTSD) as a grouping factor, time (1 week and 6 months) and side (left versus right) as within-subject factors, and volume of the hippocampus as the dependent variable revealed a nonsignificant main effect of diagnosis (F=1.26, df=1, 35, p=0.27), nonsignificant within-subject effects of time and side, and no significant diagnosis-by-time, diagnosis-by-side, time-by-side, or diagnosis-by-side-by-time interactions (F<1 in all cases). Three-way ANOVAs using the volume of the amygdala as the dependent variable revealed no significant main effect of diagnosis (F=2.08, df=1, 35, p<0.16), a significant effect of side (F=11.58, df=1, 35, p<0.02), a nonsignificant effect of time (F<1), and no significant interactions (F<1 except for the diagnosis-by-side-by-time interaction: F=3.78, F=1, 35, p<0.06). Similar results were obtained when relative volumes (absolute volume divided by total cranial cavity) were used and when female subjects were analyzed separately.
Discussion
The results of this study did not show an initial difference in hippocampal volume between trauma survivors who developed PTSD and those who did not. Smaller hippocampal volume, therefore, was not a necessary risk factor for developing PTSD in this group of subjects. In addition, individuals who developed PTSD did not show a progressive reduction of the hippocampus between 1 week and 6 months.
These results are in apparent contrast to those of the aforementioned MRI studies of PTSD (1–4). This discrepancy can be explained in several ways. First, structural damage to the hippocampus may result from exposure to prolonged traumatization, such as in child abuse or a 1-year war experience in Vietnam. Second, it is possible that more than 6 months of expressing PTSD are required to produce discernible reduction of the hippocampus. Third, the previous results could have been due to substance abuse or alcohol consumption, not properly accounted for by retrospective questionnaires. Finally, a smaller hippocampus may confer a specific vulnerability to highly chronic PTSD. Prolonged PTSD in Vietnam veterans has, in fact, been associated with other related findings, such as lower education level and lower premilitary IQ (see, for instance, reference 17). The current study’s groups did not differ significantly in level of education.
Another explanation may be linked to the age at which the traumatic event occurred. Subcortical gray matter and limbic structures (septal area, hippocampus, and amygdala) show an increase in volume until the third decade of life (18). In two of the four previous MRI studies (3, 4) the subjects were traumatized as children. The two others (1, 2) concerned Vietnam veterans, most of whom would have served in Vietnam between the ages of 18 and 21. All four studies, therefore, examined adults whose traumatic events occurred during a period of normal development of subcortical structures. The subjects in the current study, in contrast, were older, and none was younger than 20 at the time of the traumatic event. The negative results in the study of children with PTSD by De Bellis et al. (7) might also be explained by the fact that the MRI scans of those subjects were taken when the subjects were very young, that is, in the midst of hippocampal maturation. This could have masked the full effect of the psychological insult, which may not manifest itself before adulthood.
The size of the current study group, the severity of PTSD at 6 months, and the fact that the first MRI images were acquired 1 week after the traumatic event call for a discussion of potential methodological confounds. First, a type II error might have occurred such that the current results do not exclude a true reduction of hippocampal volume in PTSD. The smallest significant reduction previously reported was 5% (4). Given the current study’s finding of larger hippocampi in PTSD (3% at 1 week), the probability of having missed a hypothetical reduction of 5% in hippocampal volume between 1 week and 6 months is, for the left hippocampus, less than 4% (one-tailed t=1.77, df=35) and, for the right hippocampus, less than 3% (one-tailed t=2.01, df=35).
Second, the current study group may not have had a severe form of PTSD at 6 months. Indeed, scores of patients with chronic PTSD on the Clinician-Administered PTSD Scale are usually higher. However, the mean total score of the current PTSD group (57.90) resembles scores reported for 218 trauma survivors 4 months after the traumatic events (total score=54.6) (19) and for PTSD patients 6 months after road traffic accidents (total score=60.1) (20). Moreover, a similar mean score (58.0) was associated with a smaller hippocampus in the previously mentioned study of survivors of child abuse (4). The mean score on the Mississippi Scale for Combat-Related PTSD of the current PTSD group (110.80) is similar to the scores obtained in most studies of PTSD.
Third, a reduction of hippocampal volume could have occurred between the traumatic event and the 1-week assessment, such that the initial MRI scan may not represent pretrauma anatomy. Acute stress has, in fact, a rapid (21, 22) and reversible (23, 24) effect on hippocampal cells. The data supporting this effect, however, are mainly histological and come from studies of nonprimates. However, Fuchs and Flugge (25) have shown that measurable reduction in hippocampal volume does not occur before 21 days of continuous exposure to stress. Furthermore, in this study, a hypothetical acute stress-induced reduction in hippocampal volume should have equally affected the PTSD and non-PTSD subjects (as both groups reported substantial and similar distress at 1 week). Hence, the likelihood of having missed an acute and early reduction of the hippocampus among PTSD patients in this study is minimal.
Finally, the results of this study are in line with those from published neuropsychological studies (e.g., references 26, 27), in which hippocampal functions were not impaired in PTSD following recent traumatic events, whereas frontal lobe tasks, such as attention and set shifting, were impaired. Accordingly, the hippocampus may not be the primary dysfunctional area of the brain in PTSD following recent trauma. Moreover, reduced hippocampal volume has also been found in other prolonged mental disorders, such as depression (26), alcohol abuse (8, 28), and schizophrenia (e.g., reference 29), and hippocampal reduction in primates has been observed after prolonged social stress (6). Hippocampal atrophy, therefore, may be a common outcome of several pathogenic processes that share the element of protracted distress.
 |
Received Feb. 23, 2000; revisions received Oct. 12 and Dec. 6, 2000, and Jan. 24, 2001; accepted Feb. 7, 2001. From the Department of Psychiatry, Hadassah University Hospital; the Department of Radiology, Brigham and Women’s Hospital, Boston; and the Department of Psychiatry, Harvard Medical School, Boston. Address reprint requests to Dr. Shalev, Department of Psychiatry, Hadassah University Hospital, P.O. Box 12000, Jerusalem 91120, Israel; ashalev@ cc.huji.ac.il (e-mail). Supported by grant MH-50379 from NIMH.
1. Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB: MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995; 152:973-981Link, Google Scholar
2. Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, Orr SP, Kikinis R, Jolesz FA, McCarley RW, Pitman RK: Magnetic resonance imaging study of hippocampal volume in chronic combat-related posttraumatic stress disorder. Biol Psychiatry 1996; 40:1091-1099Google Scholar
3. Bremner JD, Randall PR, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS: MRI-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: a preliminary report. Biol Psychiatry 1997; 41:23-32Crossref, Medline, Google Scholar
4. Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B: Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 1997; 27:951-959Crossref, Medline, Google Scholar
5. Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM: Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci 1989; 9:1705-1711Google Scholar
6. Magarinos AM, McEwen BS, Flügge G, Fuchs E: Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci 1996; 16:3534-3540Google Scholar
7. De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND: AE Bennett Research Award: developmental traumatology: part II: brain development. Biol Psychiatry 1999; 45:1271-1284Google Scholar
8. Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW: Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry 1999; 56:356-363Crossref, Medline, Google Scholar
9. Spielberger CD, Gorsuch RL, Lushene RD: STAI Manual. Palo Alto, Calif, Consulting Psychologists Press, 1970Google Scholar
10. Weiss DS, Marmar CR: The Impact of Event Scale—Revised, in Assessing Psychological Trauma and PTSD. Edited by Wilson JP, Keane TM. New York, Guilford, 1997, pp 399-411Google Scholar
11. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-IV (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
12. Keane TM, Caddell JM, Taylor KL: Mississippi Scale for Combat-Related Posttraumatic Stress Disorder: three studies in reliability and validity. J Consult Clin Psychol 1988; 56:85-90Crossref, Medline, Google Scholar
13. Vreven DL, Gudanowski DM, King LA, King DW: The civilian version of the Mississippi PTSD scale: a psychometric evaluation. J Trauma Stress 1995; 8:91-110Crossref, Medline, Google Scholar
14. Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM: The development of a clinician-administered PTSD scale. J Trauma Stress 1995; 8:75-90Crossref, Medline, Google Scholar
15. Green BL: Trauma History Questionnaire, in Measurement of Stress, Trauma and Adaptation. Edited by Stamm B. Lutherville, Md, Sidran Press, 1996, pp 366-368Google Scholar
16. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604-612Crossref, Medline, Google Scholar
17. Macklin ML, Metzger LJ, Litz B, McNally RJ, Lasko NB, Orr SP, Pitman RK: Lower precombat intelligence is a risk factor for posttraumatic stress disorder. J Consult Clin Psychol 1998; 66:323-326Crossref, Medline, Google Scholar
18. Jernigan TL, Sowell ER: Magnetic resonance imaging studies of the developing brain, in Neurodevelopment and Adult Psychopathology. Edited by Keshavan MS, Murray RM. Cambridge, UK, Cambridge University Press, 1997, pp 63-70Google Scholar
19. Shalev AY, Freedman S, Peri T, Brandes D, Sahar T, Orr SP, Pitman RK: Prospective study of posttraumatic stress disorder and depression following trauma. Am J Psychiatry 1998; 155:630-637Link, Google Scholar
20. Blanchard EB, Hickling EJ, Mitnick N, Taylor AE, Loos WR, Buckley TC: The impact of severity of physical injury and perception of life threat in the development of post-traumatic stress disorder in motor vehicle accident victims. Behav Res Ther 1995; 33:529-534Crossref, Medline, Google Scholar
21. McEwen BS: Stress and hippocampal plasticity. Annu Rev Neurosci 1999; 22:105-122Crossref, Medline, Google Scholar
22. McEwen BS: Stress and the aging hippocampus. Front Neuroendocrinol 1999; 20:49-70Crossref, Medline, Google Scholar
23. Luine V, Villegas M, Martinez C, McEwen BS: Repeated stress causes reversible impairments of spatial memory performance. Brain Res 1994; 639:167-170Crossref, Medline, Google Scholar
24. McEwen BS, Magarinos AM: Stress effects on morphology and function of the hippocampus. Ann NY Acad Sci 1997; 821:271-284Crossref, Medline, Google Scholar
25. Fuchs E, Flugge G: Stress glucocorticoids and structural plasticity of the hippocampus. Neurosci Biobehav Rev 1998; 23:295-300Crossref, Medline, Google Scholar
26. Uddo M, Vasterling JJ, Brailey K, Sutker PB: Memory and attention in combat-related posttraumatic stress disorder (PTSD). J Psychopathol Behav Assess 1993; 15:43-52Crossref, Google Scholar
27. Vasterling JJ, Brailey K, Constans JI, Sutker PB: Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology 1998; 12:125-133Crossref, Medline, Google Scholar
28. Steffens DC, Byrum CE, McQuoid DR, Greenberg DL, Payne ME, Blitchington TF, MacFall JR, Krishnan KR: Hippocampal volume in geriatric depression. Biol Psychiatry 2000; 15:301-309Crossref, Google Scholar
29. Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, Arnold SE, Bilker WB, Gur RC: Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry 2000; 57:769-775Crossref, Medline, Google Scholar



