Double-Blind, Placebo-Controlled Comparison of Imipramine and Paroxetine in the Treatment of Bipolar Depression
Abstract
OBJECTIVE: This study compared the efficacy and safety of paroxetine and imipramine with that of placebo in the treatment of bipolar depression in adult outpatients stabilized on a regimen of lithium. METHOD: In a double-blind, placebo-controlled study, 117 outpatients with DSM-III-R bipolar disorder, depressive phase, were randomly assigned to treatment with paroxetine (N=35), imipramine (N=39), or placebo (N=43) for 10 weeks. In addition to lithium monotherapy, patients may have received either carbamazepine or valproate in combination with lithium for control of manic symptoms. Patients were stratified on the basis of trough serum lithium levels determined at the screening visit (high: >0.8 meq/liter; low: ≤0.8 meq/liter). Primary efficacy was assessed by change from baseline in scores on the Hamilton Rating Scale for Depression and the Clinical Global Impression illness severity scale. RESULTS: Differences in overall efficacy among the three groups were not statistically significant. For patients with high serum lithium levels, antidepressant response at endpoint also did not significantly differ from placebo. However, both paroxetine and imipramine were superior to placebo for patients with low serum lithium levels. Compared to imipramine, paroxetine resulted in a lower incidence of adverse events, most notably emergence of manic symptoms. CONCLUSIONS: Antidepressants may not be useful adjunctive therapy for bipolar depressed patients with high serum lithium levels. However, antidepressant therapy may be beneficial for patients who cannot tolerate high serum lithium levels or who have symptoms that are refractory to the antidepressant effects of lithium.
The treatment of bipolar depression represents a clinical challenge, and appropriate treatment strategies remain more anecdotal than data-based. There is an extensive literature guiding treatment of patients with unipolar depression, but treatment of bipolar depression has not been extensively studied, and effective treatments are not well-defined (1, 2). Lithium is considered standard mood-stabilizing therapy for bipolar disorder (3–6). However, up to 50% of patients effectively maintained with lithium therapy may be unresponsive to its antidepressant effects (4, 7). When lithium monotherapy is not effective in managing depression or if patients are unable to tolerate the side effects of high serum lithium levels, patients with bipolar disorder may require combination therapy with antidepressants (3).
Monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants, bupropion, and the selective serotonin reuptake inhibitors (SSRIs) have been evaluated for treatment of the depressive component of bipolar disorder (2, 3, 6, 8, 9). In a comparison of imipramine and tranylcypromine in depressed patients with bipolar disorder, the rate of response (defined as an endpoint Clinical Global Impression [CGI] score ≥ 2 or 3) to imipramine was 48%, and the response rate for tranylcypromine exceeded 80% (10). However, Prien et al. (11) noted that combined imipramine/lithium therapy offered no advantage over lithium alone in the treatment of bipolar depression. Additional clinical trials have demonstrated that the tricyclic antidepressants have a rate of response (defined as ≥50% improvement in score from baseline on the Hamilton Rating Scale for Depression) between 50% and 70% in the treatment of bipolar depression (10, 12–14). Bupropion was as effective as desipramine in one double-blind comparative study (14). Only a limited number of trials have evaluated the effectiveness of SSRIs in the treatment of bipolar depression (13, 15, 16). Cohn et al. (13) reported that bipolar patients treated with fluoxetine had a significantly greater response rate than those treated with imipramine. In a 6-week, double-blind comparison of paroxetine and amitriptyline in lithium-stabilized patients with breakthrough major depression, Bauer et al. (15) reported significantly greater responses (as determined by Hamilton depression scale and CGI severity of illness scores) in paroxetine-treated patients.
Although MAOIs appear to be effective in treating bipolar depression, safety issues and dietary restrictions often limit their use in general clinical practice. Therapy with tricyclic antidepressants is also associated with a high incidence of adverse events, and many patients are unable to tolerate the anticholinergic side effects (13). Both tricyclic antidepressants and MAOIs have a low therapeutic index, which is a major concern in patients with bipolar disorder because of their high rate of suicide attempts. The SSRIs have a lower incidence of adverse events, particularly anticholinergic and cardiac effects (17, 18). Thus, the safety profile of SSRIs may offer an advantage over tricyclic antidepressants and MAOIs and may increase patient compliance.
The potential for the so-called switch into mania is another risk that must be considered when initiating antidepressant therapy in patients with bipolar depression (19–22). In one analysis, induction of mania occurred in 3.7% of 242 bipolar patients treated with SSRIs and in 11.2% of 125 patients treated with tricyclic antidepressants (20). Bupropion was reported as having induced mania in one small, open-label series (23). However, other investigators have reported that, when added to lithium, thyroxine, or anticonvulsant regimens, bupropion is not associated with mania in rapidly cycling patients (24) and is less likely to induce mania than desipramine (14).
In order to address some of the unresolved issues regarding the treatment of bipolar depression, we compared the efficacy and safety of paroxetine and imipramine with that of placebo in the treatment of bipolar depression in adult outpatients stabilized with lithium therapy.
Method
Study Design
This was a multicenter, double-blind, randomized, placebo-controlled study that was conducted to assess the efficacy and safety of paroxetine and imipramine in combination with lithium therapy in the treatment of bipolar depression. Outpatients with bipolar disorder who were currently in a major depressive episode were enrolled. A 1-week, single-blind, placebo period was used to screen potential patients for inclusion in the study.
Diagnostic procedures included conducting a DSM-III-R multiaxial evaluation, physical examination, psychiatric and medical history, routine laboratory analyses, and pregnancy test; performing an ECG and vital signs assessment; measurement of serum trough lithium level and body weight; and administration of the 21-item version of the Hamilton depression scale (25) and the CGI severity of illness scale. Following the psychiatric and medical screening examination, eligible patients were stratified into two groups on the basis of whether their trough serum lithium level at the screening visit was low (≤0.8 meq/liter) or high (>0.8 meq/liter) and then were randomly assigned to one of the three treatment groups.
Patients randomly assigned to paroxetine treatment received 20 mg/day for the first 3 weeks; thereafter, dose increases of 10 mg/day were permitted every 7 days up to a maximum dose of 50 mg/day. Patients receiving imipramine began at a dose of 50 mg/day with a forced titration to 150 mg/day at the rate of 50 mg every 7 days over the first 3 weeks of the study. After this titration period, imipramine dose increases of 50 mg/day were permitted every 7 days up to a maximum dose of 300 mg/day. Dose reduction was permitted once if necessary for adverse events; retitration to the original dose level was allowed if the adverse event remitted. Following the 10-week treatment phase, patients were gradually tapered off all study medications.
The study was approved by the institutional review board at each of the 19 participating centers, and each patient provided written informed consent before entry into the study.
Patient Selection
All patients enrolled in the study fulfilled DSM-III-R criteria for bipolar disorder and scored ≥15 on the 21-item version of the Hamilton depression scale at both the screening and baseline evaluations. The total Hamilton depression scale score could not have decreased by more than 25% between the screening and baseline evaluations. Eligible patients experienced at least one previous episode of mania or major depression within the past 5 years and had been maintained on a regimen of lithium alone or in combination with sodium valproate or carbamazepine for at least 7 weeks before the screening visit. Serum lithium levels were between 0.5 and 1.2 meq/liter (0.4 meq/liter for patients intolerant to lithium) for at least 6 weeks before the screening evaluation. Serum lithium concentrations were measured 1 week after initiation of study medication and remained within prior defined levels for all eligible patients. Lithium dose adjustments were not allowed unless serum levels deviated beyond the 0.5–1.2 meq/liter range (0.4 meq/liter for lithium intolerance), in which case doses were adjusted to maintain levels within the permitted range. Patients were at least 18 years old.
Patients who met DSM-III-R criteria for bipolar disorder but who were not currently depressed were excluded, as were patients who required therapy with both sodium valproate and carbamazepine or those who had been diagnosed with an axis I disorder other than bipolar disorder as the primary disorder within 6 months of the screening, including dysthymia and bipolar II disorder. Patients who were rapid cyclers (four or more manic/hypomanic or depressive episodes within 12 months of the baseline evaluation), who had experienced recent manic/hypomanic episodes within 4 weeks of baseline, or who were prone to spontaneous remission (depressive episodes of no more than 8 weeks’ duration) were excluded. Additional exclusion criteria were any serious medical disorder or condition, such as cardiovascular disease or history of narrow-angle glaucoma, that would preclude the administration of tricyclic antidepressant therapy; concomitant therapy with other psychotropic drugs, not including chloral hydrate; and concomitant therapy with warfarin, cardiac glycosides, phenytoin, cimetidine, type 1C antiarrhythmic agents, quinidine, sulfonylurea derivatives, or tryptophan. Patients who met the DSM-III-R criteria for substance abuse within 3 months of the study or the criteria for substance dependence within 6 months of the study were ineligible. Patients who were judged by the investigator to be at serious suicidal or homicidal risk were also excluded from the study.
Assessment
During the 10-week study period, patients were assessed for both efficacy and adverse events at baseline and at weeks 1–6, 8, and 10. Laboratory evaluations and an ECG were performed at the screening visit and at weeks 4 and 10. Baseline laboratory evaluations were only performed if abnormal values were noted at the screening visit.
Primary efficacy parameters were mean change from baseline in the total score on the first 17 items of the Hamilton depression scale and mean change from baseline in score on the CGI severity of illness scale. Clinical response parameters included the proportion of patients achieving Hamilton depression scale scores ≤7 and the proportion of patients with CGI global improvement scores ≤2. These parameters are clinically accepted as indicative of therapeutic response.
Safety evaluations were based on routine adverse event monitoring, vital sign assessments, and a hypomania/mania assessment based on DSM-III-R criteria. Patients were asked a nonleading question at each postbaseline assessment, such as “Do you feel differently in any way since starting this treatment?” Positive responses were investigated and documented on the case report form. These evaluations, as well as body weight determinations, were evaluated at each visit. The effect of paroxetine and imipramine on serum lithium concentrations was monitored by obtaining blood samples at weeks 2, 4, 6, and 10. Adverse events were elicited by asking the patient nonleading questions.
Data Analysis
Data are presented from the intent-to-treat population. The endpoint data set was the primary time point of interest and was determined for each patient from the last available observation while receiving treatment. The group was stratified on the basis of serum lithium level at the screening examination (high: >0.8 meq/liter, low: ≤0.8 meq/liter). Lithium stratification criteria were determined a priori. The proportion of patients achieving dichotomous response was analyzed by the Cochran-Mantel-Haenszel test adjusting for lithium stratification or by Fisher’s exact test. The chi-square test was used for analyses within lithium strata. Change from baseline score, defined as score minus baseline score, of efficacy scales was assessed by parametric analysis of variance. The study was designed to enroll 35 patients per arm, which would allow 70% power to detect a 5-point difference on the Hamilton depression scale score (SD=8.5) between treatment groups.
The primary comparison of interest was between the paroxetine and placebo treatment groups regardless of lithium stratification. Because all other statistical comparisons were considered to be secondary, no adjustments for multiple comparisons were made. Therefore, the achievement of statistical significance for the primary efficacy variables at endpoint (i.e., changes from baseline in scores on the Hamilton depression scale and CGI severity of illness scale) was set at p≤0.05.
The general linear model procedure of SAS (Cary, N.C.) was used to perform the analysis with a model that included effects for treatment and lithium strata for scores on the Hamilton depression scale (first 17 items) and CGI severity of illness scale. Analyses of all other efficacy variables were performed with a model that included only an effect for treatment. Additional analyses were performed within lithium strata that included only the treatment effect. Type III sums of squares were used. The analyses were designed to include an investigator effect; however, 14 of the 19 investigational sites had fewer than eight total patients. Thus, no analyses with an investigator effect were performed. The treatment-by-lithium strata interaction was found to be nonsignificant and was not included in the model. Because only a small number of patients experienced manic and hypomanic episodes, these episodes were not analyzed.
All statistical tests were two-tailed. Tests of hypothesis of interactions were made at the 10% significance level, and all other tests were made at the 5% significance level. Data are presented as means and standard deviations. The CONTRAST statement from the general linear model procedure of SAS was used for treatment group comparisons. Interaction assessments were conducted as per protocol. However, significant interactions were not found and therefore not presented.
Results
Demographic and Clinical Characteristics
A total of 117 outpatients were enrolled by 19 centers: 35 patients (mean age=42.5 years, range=24–66) were randomly assigned to the paroxetine group, 39 (mean age=41.9 years, range=21–71) received imipramine, and 43 (mean age=40.4 years, range=21–66) were given placebo. The paroxetine, imipramine, and placebo groups were similar in age, gender (54.3%, 59.0%, and 53.5%, respectively, were female), race (97.1%, 100.0%, and 90.7%, respectively, were Caucasian), and cardiac history. Concomitant medications were used by 82.9% (N=29) of the patients in the paroxetine treatment group, 76.9% (N=30) of the patients in the imipramine group, and 81.4% (N=35) of the patients in the placebo group. The prevalence of concomitant use of valproic acid was similar for the paroxetine (11.4%, N=4) and placebo (9.3%, N=4) groups and was much less for the imipramine group (2.6%, N=1); only one patient each from the paroxetine (2.9%) and imipramine (2.6%) groups received carbamazepine during the study. Because of the small number of patients receiving concomitant therapy with these agents, no influence on overall efficacy in the treatment groups could be determined.
Mean daily doses at study endpoint (i.e., the last available observation for each patient while receiving treatment) were 32.6 mg for paroxetine (range=20–50 mg) and 166.7 mg for imipramine (range=50–300 mg). At endpoint, five imipramine-treated patients were receiving doses lower than the minimum 150 mg/day required by the protocol.
Efficacy
Mean changes in score on the Hamilton depression scale and CGI severity of illness scale from baseline to endpoint for the paroxetine and imipramine groups were not significantly different than those of the placebo-treated group (Table 1). A high placebo response rate also occurred in the high serum lithium level group, with no statistical separation from placebo for either paroxetine or imipramine. However, among the low serum lithium level patients, paroxetine and imipramine were superior to placebo in terms of mean change from baseline in scores on the Hamilton depression scale and CGI severity of illness scale (Table 1).
Therapeutic response was defined as Hamilton depression scale score ≤7 or CGI global improvement score ≤2. For the total intent-to-treat population, there were no statistically significant differences in response rates among those receiving paroxetine, imipramine, or placebo (per Hamilton criterion: 45.5% [N=15], 38.9%, [N=14], and 34.9% [N=15], respectively; per CGI criterion: 54.5% [N=18], 58.3% [N=21]; and 46.5% [N=20]). Among the study completers, Hamilton depression scale scores ≤7 were achieved by 56.0% (N=14 of 25) of the paroxetine-treated patients, 47.8% (N=11 of 23) of the imipramine-treated patients, and 53.8% (N=14 of 26) of the placebo-treated patients. Similarly, CGI global improvement scores ≤2 were achieved by 68.0% (N=17) of the paroxetine-treated patients, 73.9% (N=17) of the imipramine-treated patients, and 69.2% (N=18) of the placebo-treated patients.
Among patients with high serum lithium levels, similar response rates were noted among those receiving paroxetine, imipramine, or placebo (per Hamilton criterion: 35.7% [N=5], 41.2%, [N=7], and 38.1% [N=8], respectively; per CGI criterion: 57.1% [N=8], 47.1% [N=8]; and 52.4% [N=11]). For those with low serum lithium levels, no statistically significant differences in response rates were seen among those receiving paroxetine, imipramine, or placebo (per Hamilton criterion: 52.6% [N=10], 36.8%, [N=7], and 31.8% [N=7], respectively; per CGI criterion: 52.6% [N=10], 68.4% [N=13]; and 40.9% [N=9]).
There were five patients whose endpoint imipramine dose did not reach 150 mg/day. Each of these patients withdrew from the study before reaching the 150-mg dose level at week 3 (two were receiving 50 mg/day, and three were receiving 100 mg/day). The duration of therapy for these patients ranged from 5 to 14 days. In four of these patients, the Hamilton depression scale score decreased 1 to 18 points; in one patient (who was receiving 50 mg/day), the Hamilton depression scale score increased 2 points.
Emergent Adverse Events
Treatment-emergent adverse events were determined by asking open-ended, nonleading questions. Tremor (40.0%, N=14), insomnia (37.1%, N=13), and somnolence (34.3%, N=12) were the most frequently reported effects of the paroxetine-treated patients. For the patients in the imipramine group, dry mouth (61.5%, N=24), tremor (38.5%, N=15), and headache (41.0%, N=16) were noted most commonly. In the placebo group, headache (39.5%, N=17), somnolence (25.6%, N=11), and insomnia (23.3%, N=10) were the most frequently occurring adverse events, with tremor occurring in 9.3% (N=4) of the patients. Patients treated with imipramine reported a higher incidence of abnormal ejaculation (18.8%) and impotence (25.0%) than did patients receiving paroxetine (0.0% and 6.3%, respectively) or placebo (5.0% and 0.0%, respectively).
Adverse events precipitated study discontinuation in one paroxetine patient (2.9%), 12 imipramine patients (30.8%), and five placebo patients (11.6%). Other reasons for withdrawal from the study included lack of efficacy (paroxetine: 2.9% [N=1]; imipramine: 2.6% [N=1]; placebo: 18.6% [N=8]), deviation from protocol (including noncompliance) (5.7% [N=2], 5.1% [N=2], and 2.3% [N=1], respectively), and subjects lost to follow-up (17.1% [N=6], 2.6% [N=1], and 4.7% [N=2]).
No serious adverse events were reported in the paroxetine group. Two patients in the imipramine group (5.1%) and four patients in the placebo group (9.3%) experienced serious adverse events. In the imipramine group, one patient was hospitalized for mania on study day 42, and another patient developed physical aggression with homicidal ideation and was withdrawn from the study on day 29. Of the four placebo-treated patients experiencing serious adverse events, two were hospitalized for manic episodes (not necessarily protocol-defined mania), one developed increased depression with paranoid hallucinations and delusions, and the fourth did not complete the taper phase and developed reemergence of depression. The adverse events associated with active treatment were consistent with the safety profiles for SSRIs and tricyclic antidepressants.
By definition, participating patients did not meet the DSM-III-R criteria for hypomania or mania at the screening or baseline examination. Endpoint analysis revealed that no patient treated with paroxetine experienced induction to mania. However, three patients (7.7%) treated with imipramine and one patient (2.3%) treated with placebo experienced treatment-emergent mania. Among the three patients treated with imipramine who experienced mania, two were from the low serum lithium level group. The placebo-treated patient who developed mania was in the low serum lithium level group.
Lithium concentrations remained within the therapeutic range for all patients treated with paroxetine or imipramine (Figure 1). There was no evidence that either paroxetine or imipramine influenced lithium pharmacokinetics. Weight gain was observed in three patients (7.7%) treated with imipramine and in three patients (7.0%) in the placebo group. Four patients treated with paroxetine experienced a change in weight: two (5.7%) gained weight, and two (5.7%) lost weight.
Discussion
To our knowledge, this is the largest study to evaluate an SSRI for the treatment of bipolar depression and the first controlled clinical trial to assess the efficacy and safety of paroxetine treatment for this disorder. For both the total patient population and among those with high serum lithium levels, neither paroxetine nor imipramine were distinguishable from placebo. However, in the endpoint analysis, patients with low serum lithium levels who were treated with paroxetine or imipramine demonstrated significant improvement compared to those treated with placebo.
Although our study was not designed to measure the antidepressant effects of lithium, when lithium stratification groups were compared, it could be inferred from the data that the antidepressant effects of lithium were more prominent in patients with high serum lithium levels. The antidepressant effects of high serum lithium levels are not surprising in view of the considerable literature suggesting an antidepressant effect of lithium in bipolar depression and to a lesser extent in unipolar depression and depression associated with schizoaffective disorder (6, 26–28).
Clinical response parameters for the endpoint analysis and the completer analysis were a Hamilton depression scale score ≤7 or a CGI global improvement scale score ≤2. Overall, in the clinical response analyses, we observed no statistically significant differences for the treatment groups. This lack of statistical difference may be associated with the relatively small patient population and the high placebo response rate in these lithium-treated patients. Tondo et al. (29) reported in an open study of 26 patients with bipolar disorder that fluoxetine was effective in treating depressive episodes. It is of interest that the mean serum lithium level for those patients was 0.57 meq/liter, well within the range of our low serum lithium level group.
Paroxetine was well-tolerated in these patients. Adverse events led to withdrawal from the study for one patient (2.9%) in the paroxetine group compared with 12 patients (30.8%) in the imipramine group. These findings are consistent with other reports of adverse events during SSRI therapy (12, 17, 30).
Resting tremor was noted by 40.0%, 38.5%, and 9.3% of the patients treated with paroxetine, imipramine, and placebo, respectively. Psychopharmacologically active drugs, including tricyclic antidepressants and SSRIs, may exacerbate existing lithium-related tremor (15, 31, 32). Although baseline tremor was not assessed, making it impossible to determine the causal relationship of paroxetine or imipramine, tremor was likely associated with lithium inasmuch as similar rates of tremor have been reported in patients with bipolar disorder maintained on a regimen of lithium alone (31–33). The high incidence of anticholinergic adverse reactions and tremor has also been reported in previous studies that evaluated imipramine alone and imipramine and lithium combination therapy (11, 12).
Imipramine-treated patients voluntarily reported a higher incidence of abnormal ejaculation (18.8%) and impotence (25.0%) than did paroxetine-treated patients. In clinical trials evaluating paroxetine for the treatment of unipolar depression, sexual dysfunction was reported in 6%–33% of patients (34, 35). In our study, the incidence of abnormal ejaculation and impotence was 0% and 6.3%, respectively, in paroxetine-treated patients.
There is considerable evidence supporting the association of antidepressants and the induction of mania and rapid cycling in patients with bipolar disorder (19–22, 36). Paroxetine did not precipitate a switch to mania in any patient, whereas the incidence of mania in imipramine-treated patients was 5.9% and 10.5% among the high and low serum lithium level groups, respectively. Among the total patient population, 7.7% of patients receiving imipramine and 2.3% of patients in the placebo group developed mania. It should be noted, however, that concomitant use of valproic acid was more common in the paroxetine and placebo groups than in the imipramine group. This is consistent with previous studies that also have shown a high propensity for imipramine to cause mania (10, 11, 37). In a review of other similar clinical trials (20), tricyclic antidepressants (11.2%) were much more likely to induce a switch to mania in patients with bipolar depression than were placebo (4.2%) or SSRIs (3.7%) (SSRIs versus tricyclic antidepressants, p<0.01).
In evaluating the effect of paroxetine and imipramine on serum lithium levels, lithium concentrations remained within the accepted therapeutic range throughout the course of the study. No treatment-emergent adverse events were attributed to lithium toxicity. These results are consistent with previous studies that evaluated the effects of concomitant imipramine (11, 37) and paroxetine (38) on lithium levels in patients with bipolar disorder. Thus, the lack of effect by paroxetine and imipramine on lithium toxicity minimizes additional safety concerns regarding the use of lithium with these agents.
Several limitations of our study must be considered. The high response rate in the placebo group and the small sample sizes may have limited our ability to detect statistical differences between treatment groups. All patients in the paroxetine group were taking therapeutic daily doses of paroxetine (20 to 50 mg), but five patients in the imipramine group (12.8%) were receiving daily doses of 50 mg or 100 mg, which are at the lower end of the therapeutic range for this antidepressant. Previous studies indicate that as many as one-half of patients receiving lithium may respond to the antidepressant effects of this agent (4, 7). Furthermore, carbamazepine may be useful in the treatment of refractory depression (39). Thus, all patients in this study were receiving medication (i.e., lithium and, in a small number of patients, carbamazepine or valproate) capable of improving scores on the depression efficacy scales. It is unlikely that the low rate of concomitant carbamazepine or valproate use in this study influenced overall outcome. However, it is noteworthy that an antidepressant effect was evident in patients in the total patient population analysis, as well as among those with high serum lithium levels. Yet in those patients receiving active drug who had low lithium serum levels, a pronounced therapeutic effect was demonstrated with imipramine and paroxetine, and significant differences from placebo were seen.
The results of this study indicate that patients with bipolar depression who maintain high serum lithium levels may not require additional antidepressant medications. However, patients with low serum lithium levels or those who cannot tolerate high serum lithium levels may benefit from augmentation therapy with either paroxetine or imipramine. These findings suggest the need for additional studies of antidepressant treatment of bipolar depression, particularly in patients stabilized on a regimen of lithium.
Acknowledgments
This study was conducted with the participation of the following collaborating investigators and sites: Jay D. Amsterdam, M.D. (University of Pennsylvania School of Medicine, Philadelphia); Bijan Bastani, M.D. (Portage Path Community Mental Health, Akron, Ohio); Charles L. Bowden, M.D. (University of Texas Health Science Center at San Antonio); Joseph Calabrese, M.D. (University Hospitals of Cleveland); David Dunner, M.D. (University of Washington Medical Center, Seattle); Robert Dupont, M.D. (Institute for Behavior and Health, Inc., Rockville, Md.); Dwight L. Evans, M.D. (University of Florida–Health Science Center, Gainesville); Jan Fawcett, M.D. (St. Luke’s Medical Center, Institute for Mental Well-Being, Chicago); David Goldstein, M.D. (Georgetown University Hospital, Washington, D.C.); Laszlo Gyulai, M.D. (University of Pennsylvania, Philadelphia); Robert M.A. Hirschfeld, M.D. (University of Texas Medical Branch, Galveston); Barbara Kennedy, M.D. (University of Louisville School of Medicine, Louisville, Ky.); R. Bruce Lydiard, Ph.D., M.D. (Medical University of South Carolina, Charleston); Susan McElroy, M.D. (University of Cincinnati Affiliated Hospitals, Cincinnati); Charles B. Nemeroff, M.D., Ph.D. (Emory University School of Medicine, Atlanta); Gary S. Sachs (Massachusetts General Hospital, Boston); Trisha Suppes, M.D. (University of Texas—Southwestern Medical Center, Dallas); Phebe Tucker, M.D. (The University of Oklahoma Health Sciences Center, Oklahoma City); Kenneth Weiss, M.D. (Delaware Valley Research Associates, Inc., King of Prussia, Pa.).
 |
Received Mar. 12, 1999; revisions received May 24 and Dec. 1, 2000; accepted Jan. 6, 2001. From the Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine. Address reprint requests to Dr. Nemeroff, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, 1639 Pierce Dr., Suite 4000, Atlanta, GA 30322. Supported by NIMH grant MH-51761 and a grant from GlaxoSmithKline.
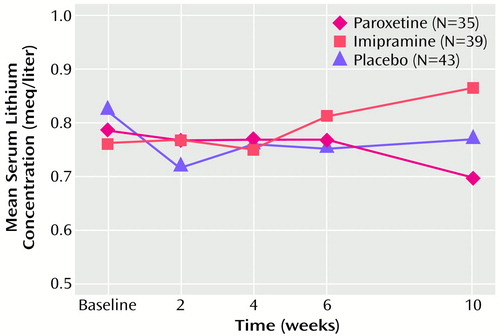
Figure 1. Mean Serum Lithium Concentrations of 117 Outpatients With Bipolar Depression Randomly Assigned to 10 Weeks of Treatment With Paroxetine, Imipramine, or Placebo
1. Kalin NH: Management of the depressive component of bipolar disorder. Depress Anxiety 1996/1997; 4:190–198Google Scholar
2. Zornberg GL, Pope HG: Treatment of depression in bipolar disorder: new directions for research. J Clin Psychopharmacol 1993; 13:397–408Crossref, Medline, Google Scholar
3. Gelenberg AJ, Hopkins HS: Report on efficacy of treatments for bipolar disorder. Psychopharmacol Bull 1993; 29:447–456Medline, Google Scholar
4. Sachs GS: Treatment-resistant bipolar depression. Psychiatr Clin North Am 1996; 19:215–236Crossref, Medline, Google Scholar
5. Schou M: Forty years of lithium treatment. Arch Gen Psychiatry 1997; 54:9–13Crossref, Medline, Google Scholar
6. Srisurapanont M, Yatham LN, Zis AP: Treatment of acute bipolar depression: a review of the literature. Can J Psychiatry 1995; 40:533–544Crossref, Medline, Google Scholar
7. Gershon S, Soares JC: Current therapeutic profile of lithium. Arch Gen Psychiatry 1997; 54:16–20Crossref, Medline, Google Scholar
8. Angst J, Stabl M: Efficacy of moclobemide in different patient groups: a meta-analysis of studies. Psychopharmacology (Berl) 1992; 106:S109–S113Google Scholar
9. Dantzler A, Salzman C: Treatment of bipolar depression. Psychiatr Serv 1995; 46:229–230Link, Google Scholar
10. Himmelhoch JM, Thase ME, Mallinger AG, Houck P: Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry 1991; 148:910–916Link, Google Scholar
11. Prien RF, Kupfer DJ, Mansky PA, Small JG, Tuason VB, Voss CB, Johnson WE: Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders. Arch Gen Psychiatry 1984; 41:1096–1104Google Scholar
12. Baumhackl U, Bizière K, Fischbach R, Geretsegger CH, Hebenstreit G, Radmayr E, Stabl M: Efficacy and tolerability of moclobemide compared with imipramine in depressive disorder (DSM-III): an Austrian double-blind, multicentre study. Br J Psychiatry 1989; 155(suppl 6):78–83Google Scholar
13. Cohn JB, Collins G, Ashbrook E, Wernicke JF: A comparison of fluoxetine, imipramine, and placebo in patients with bipolar depressive disorder. Int Clin Psychopharmacol 1989; 4:313–322Crossref, Medline, Google Scholar
14. Sachs GS, Lafer B, Stoll AL, Banov M, Thibault AB, Tohen M, Rosenbaum JF: A double-blind trial of bupropion versus desipramine for bipolar depression. J Clin Psychiatry 1994; 55:391–393Medline, Google Scholar
15. Bauer M, Zaninelli R, Muller-Oerlinghausen B, Meister W: Paroxetine and amitriptyline augmentation of lithium in the treatment of major depression: a double-blind study. J Clin Psychopharmacol 1999; 19:164–171Crossref, Medline, Google Scholar
16. Simpson SG, DePaulo JR: Fluoxetine treatment of bipolar II depression. J Clin Psychopharmacol 1991; 11:52–54Crossref, Medline, Google Scholar
17. Kasper S, Lepine JP, Mendlewicz J, Montgomery SA, Rush AJ Jr: Efficacy, safety, and indications for tricyclic and newer antidepressants. Depression 1994/1995; 2:127–137Google Scholar
18. Sheline YI, Freedland KE, Carney RM: How safe are serotonin reuptake inhibitors for depression in patients with coronary heart disease? Am J Med 1997; 102:54–59Google Scholar
19. Dilsaver SC, Swann AC: Mixed mania: apparent induction by a tricyclic antidepressant in five consecutively treated patients with bipolar depression. Biol Psychiatry 1995; 37:60–62Crossref, Medline, Google Scholar
20. Peet M: Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry 1994; 164:549–550Crossref, Medline, Google Scholar
21. Stoll AL, Mayer PV, Kolbrener M, Goldstein E, Suplit B, Lucier J, Cohen BM, Tohen M: Antidepressant-associated mania: a controlled comparison with spontaneous mania. Am J Psychiatry 1994; 151:1642–1645Google Scholar
22. Wehr TA, Goodwin FK: Rapid cycling in manic-depressives induced by tricyclic antidepressants. Arch Gen Psychiatry 1979; 36:555–559Crossref, Medline, Google Scholar
23. Fogelson DL, Bystritsky A, Pasnau R: Bupropion in the treatment of bipolar disorders: the same old story? J Clin Psychiatry 1992; 53:443–446Google Scholar
24. Haykal RF, Akiskal HS: Bupropion as a promising approach to rapid cycling bipolar II patients. J Clin Psychiatry 1990; 51:450–455Medline, Google Scholar
25. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
26. Greil W, Ludwig-Mayerhofer W, Erazo N, Engel RR, Czernik A, Giedke H, Muller-Oerlinghausen B, Osterheider M, Rudolf GA, Sauer H, Tegeler J, Wetterling T: Comparative efficacy of lithium and amitriptyline in the maintenance treatment of recurrent unipolar depression: a randomised study. J Affect Disord 1996; 40:179–190Crossref, Medline, Google Scholar
27. Greil W, Ludwig-Mayerhofer W, Erazo N, Engel RR, Czernik A, Giedke H, Muller-Oerlinghausen B, Osterheider M, Rudolf GA, Sauer H, Tegeler J, Wetterling T: Lithium vs carbamazepine in the maintenance treatment of schizoaffective disorder: a randomised study. Eur Arch Psychiatry Clin Neurosci 1997; 247:42–50Crossref, Medline, Google Scholar
28. Greil W, Ludwig-Mayerhofer W, Erazo N, Schochlin C, Schmidt S, Engel RR, Czernik A, Giedke H, Müller-Oerlinghausen B, Osterheider M, Rudolf GA, Sauer H, Tegeler J, Wetterling T: Lithium versus carbamazepine in the maintenance treatment of bipolar disorders: a randomised study. J Affect Disord 1997; 43:151–161Crossref, Medline, Google Scholar
29. Tondo L, Mannu P, Silvetti F, Altamura C: Fluoxetine augmentation in bipolar disorder patients on maintenance lithium treatment. Int J Psychiatry Clin Pract 1997; 1:203–206Crossref, Medline, Google Scholar
30. Cohn JB, Wilcox CS: Paroxetine in major depression: a double-blind trial with imipramine and placebo. J Clin Psychiatry 1992; 53(suppl):52–56Google Scholar
31. Gelenberg AJ, Jefferson JW: Lithium tremor. J Clin Psychiatry 1995; 56:283–287Medline, Google Scholar
32. Vestergaard P, Poulstrup I, Schou M: Prospective studies on a lithium cohort, III: tremor, weight gain, diarrhea, psychological complaints. Acta Psychiatr Scand 1988; 78:434–444Crossref, Medline, Google Scholar
33. Gelenberg AJ, Kane JM, Keller MB, Lavori P, Rosenbaum JF, Cole K, Lavelle J: Comparison of standard and low serum levels of lithium for maintenance treatment of bipolar disorder. N Engl J Med 1989; 321:1489–1493Google Scholar
34. Battegay R, Hager M, Rauchfleisch U: Double-blind comparative study of paroxetine and amitriptyline in depressed patients of a university psychiatric outpatient clinic (pilot study). Neuropsychobiology 1985; 13:31–37Crossref, Medline, Google Scholar
35. Dunbar GC, Cohn JB, Fabre LF, Feighner JP, Fieve RR, Mendels J, Shrivastava RK: A comparison of paroxetine, imipramine, and placebo in depressed out-patients. Br J Psychiatry 1991; 159:394–398Crossref, Medline, Google Scholar
36. Altshuler LL, Post RM, Leverich GS, Mikalauskas K, Rosoff A, Ackerman L: Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995; 152:1130–1138Google Scholar
37. Quitkin FM, Kane J, Rifkin A, Ramos-Lorenzi JR, Nayak DV: Prophylactic lithium carbonate with and without imipramine for bipolar 1 patients. Arch Gen Psychiatry 1981; 38:902–907Crossref, Medline, Google Scholar
38. Haenen J, De Bleeker E, Mertens C, Batholome F, Pardoen D, Stellamans G, Leyman S, Schotte G: An interaction study of paroxetine on lithium plasma levels in depressed patients stabilised on lithium therapy. Eur J Clin Res 1995; 7:161–167Google Scholar
39. Post RM, Uhde TW: Carbamazepine as a treatment for refractory depressive illness and rapid cycling manic-depressive illness, in Treating Resistant Depression. Edited by Zohar JH, Belmaker RH. New York, PMS, 1987, pp 175–235Google Scholar



