A Randomized Double-Blind Study of Risperidone and Olanzapine in the Treatment of Schizophrenia or Schizoaffective Disorder
Abstract
OBJECTIVE: The safety and efficacy of risperidone and olanzapine were compared in a double-blind trial that used doses widely accepted in clinical practice. METHOD: Subjects (N=377) who met DSM-IV criteria for schizophrenia or schizoaffective disorder were randomly assigned to receive 2–6 mg/day of risperidone (mean modal dose=4.8 mg/day) or 5–20 mg/day of olanzapine (mean modal dose=12.4 mg/day) for 8 weeks. RESULTS: The two study groups were similar at baseline except that the olanzapine group was slightly younger than the risperidone group. Seventy-five percent of the participants completed the trial, with no between-treatment differences in the proportion of dropouts. Similar proportions of the risperidone and olanzapine groups reported extrapyramidal symptoms (24% and 20%, respectively). Severity of extrapyramidal symptoms was low in both groups, with no between-group differences. Total Positive and Negative Syndrome Scale scores and scores on the five Positive and Negative Syndrome Scale factors were improved in both groups at week 8 (subjects who completed the study) and endpoint (all subjects, including dropouts). There were overall between-treatment differences in efficacy. Comparison of individual factors found no significant differences at endpoint; at week 8, however, improvements on Positive and Negative Syndrome Scale factors for positive symptoms and anxiety/depression were greater with risperidone than olanzapine. An increase in body weight of ≥7% was seen in 27% of olanzapine participants and 12% of risperidone participants. CONCLUSIONS: Both treatments were well tolerated and efficacious. The frequency and severity of extrapyramidal symptoms were similar in the two treatment groups. Greater reductions in severity of positive and affective symptoms were seen with risperidone than with olanzapine treatment among study completers. There was no measure on which olanzapine was superior. Greater weight gain was associated with olanzapine than with risperidone treatment.
Risperidone and olanzapine have been shown to be both well tolerated and efficacious in the treatment of psychotic disorders (1–6). Almost half of all new prescriptions for antipsychotics in the United States are for these two medications. Large separate trials performed to obtain regulatory approval for risperidone and olanzapine have compared these newer agents with haloperidol. The findings have suggested that both drugs may be superior to haloperidol in amelioration of negative symptoms and that risperidone is superior in amelioration of the positive symptoms of schizophrenia (3, 5). Overall, second-generation drugs do provide clear advantages in terms of fewer adverse effects, particularly drug-induced parkinsonism, akathisia, and tardive dyskinesia. Advantages in alleviating refractory symptoms, negative symptoms, depression, and suicidal behavior have been reported; however, much remains to be done methodologically in establishing the relative merits of specific drugs in these multiple domains of interest (6). Only now are actual head-to-head studies being conducted; these will contribute to establishing relative efficacy and safety in relevant study populations.
Risperidone and olanzapine have been compared in two prospective studies. Tran et al. (7) evaluated risperidone (4–12 mg/day; mean modal dose=7.2 mg/day) and olanzapine (10–20 mg/day; mean modal dose=17.2 mg/day) in a double-blind, 28-week study involving 339 inpatients with schizophrenia, schizophreniform disorder, or schizoaffective disorder. Both antipsychotics significantly reduced positive and negative symptoms of schizophrenia, with no significant between-group differences except on one of multiple measures of negative symptoms, where olanzapine was superior to risperidone. More olanzapine than risperidone participants were rated as treatment responders (defined as >40% reduction in scores on the Positive and Negative Syndrome Scale), and fewer reported adverse events. The Tran et al. study has been criticized on several statistical and methodologic grounds, including the use of one-tailed statistical tests to evaluate between-treatment differences, lack of correction for differences in baseline scores, failure to address issues of multiple comparisons, and use of doses of risperidone that were substantially higher than is common in current clinical practice (8–10).
In an open-label study by Ho et al. (11), 42 people with DSM-IV schizophrenia received risperidone (N=21) or olanzapine (N=21) for a few weeks of acute treatment and then for an additional 6 months. After an average of 4 weeks, improvements in negative, psychotic, and disorganized symptoms were noted with both risperidone and olanzapine. The only between-treatment difference was a higher score on the Barnes Akathisia Scale in the risperidone group. Further improvements in symptoms and quality-of-life scores were noted in both groups at 6 months (13 people in each group), with one substantial between-treatment difference: the improvement in psychotic symptoms was greater in the risperidone group.
We report the results of a double-blind, randomized trial comparing the safety and efficacy of risperidone and olanzapine at clinically common doses in 377 people with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder.
Method
Design
The trial was a multicenter, randomized, double-blind, parallel-group comparison of risperidone and olanzapine conducted at 41 sites in the United States. Two sites (30 subjects) were excluded from the analysis because of noncompliance with regulatory requirements. The quality of data from the remaining 39 sites was verified by thorough poststudy auditing of all 377 participant files.
After complete description of the study to the subjects, written informed consent was obtained. During the week before the subject’s assignment to one of the two treatment groups, all oral antipsychotic and anticholinergic medications currently taken by the subjects were gradually discontinued. Any depot antipsychotics were discontinued for at least one treatment cycle before the subject was assigned to a study group. The 377 participants were then randomly assigned to receive risperidone (2–6 mg/day) or olanzapine (5–20 mg/day) for 8 weeks.
Eligibility Criteria
Inclusion criteria included a DSM-IV diagnosis of schizophrenia or schizoaffective disorder, a baseline Positive and Negative Syndrome Scale (12) score of 60 to 120 (calculated by using 1–7 scoring), and age 18–64 years. Participants could be outpatients or inpatients hospitalized ≤4 weeks at the time of screening. Informed consent was obtained from either the participant or a legal guardian or representative.
Exclusion criteria included a DSM-IV axis I diagnosis other than schizophrenia or schizoaffective disorder, a DSM-IV diagnosis of substance abuse in the 3 months before selection, documented disease of the central nervous system, the use of disallowed concomitant therapy such as mood stabilizers or antidepressants, a history of treatment with clozapine for more than 4 consecutive weeks, or being known by the investigator to be sensitive or unresponsive to risperidone or olanzapine.
Dosing Regimen
Both drugs were given once daily according to the following regimen: days 1–2, 2 mg/day of risperidone or 10 mg/day of olanzapine; days 3–7, 2–4 mg/day of risperidone or 5–10 mg/day of olanzapine; days 8–14, 2–6 mg/day of risperidone or 5–15 mg/day of olanzapine; and days 15–56, 2–6 mg/day of risperidone or 5–20 mg/day of olanzapine.
Assessments
Participants were seen once a week. Participants’ reports of extrapyramidal symptoms were recorded at each study visit. Severity of extrapyramidal symptoms and psychopathology was evaluated with the Extrapyramidal Symptom Rating Scale (13) and the Positive and Negative Syndrome Scale, respectively, at baseline and at weeks 2, 4, 6, and 8 (or withdrawal). Positive and Negative Syndrome Scale total scores and scores on five Positive and Negative Syndrome Scale factors (3) were used to evaluate changes in symptom severity. The overall severity of illness was rated with the severity scale of the Clinical Global Impression (CGI) scale, and changes in severity were assessed with the CGI change scale. Clinical improvement was defined as a ≥20% reduction in total Positive and Negative Syndrome Scale scores.
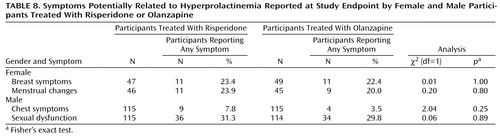
Adverse events were recorded weekly. Standard laboratory tests were performed at randomization and weeks at 2, 4, 6, and 8 (or withdrawal). Vital signs were recorded at screening, randomization, and weeks 1, 2, 3, 4, 6, and 8. An ECG was performed at screening and at week 8 (or withdrawal). Participants completed self-assessment questionnaires at baseline and week 8 (or withdrawal), including a questionnaire that inquired about symptoms potentially related to prolactin in three areas (menstrual changes, breast or chest symptoms, and male sexual function).
Statistical Analysis
The size of the study group was determined on the basis of the difference in expected rates of spontaneously reported extrapyramidal symptoms (obtained from medication package inserts). Statistical tests on all data were performed at the 5%, two-tailed significance level. The principal analysis of tolerability and efficacy measures was conducted on an intent-to-treat basis, meaning that data for all participants who were treated and who had at least one postbaseline assessment were included in the analysis. Between-group comparisons of continuous measures were analyzed with analysis of covariance with the following factors: investigator, baseline values (Positive and Negative Syndrome Scale and Extrapyramidal Symptom Rating Scale), and age. To address issues of multiple comparisons in efficacy, a single multivariate F test was used to compare treatment groups on 12 Positive and Negative Syndrome Scale tests (total Positive and Negative Syndrome Scale score and scores on the five Positive and Negative Syndrome Scale factors at week 8 and at endpoint) with the following factors: treatment, investigator, age, age squared, and baseline scores. Within-group differences were then evaluated with paired t tests. Results at week 8 (observed-case analysis) and endpoint (missing data estimated by carrying the last observation forward) were considered primary. Overall differences in categoric measures were examined by using the Cochran-Mantel-Haenszel chi-square test controlling for investigator and, if necessary, baseline stratum. The ratio of beneficial to adverse changes in observed laboratory test values was computed by dividing the reduction from an above-normal score at baseline to a normal score by the increase from a normal score at baseline to an above-normal score. Between-treatment risk ratios were computed as the previous ratio for the risperidone group divided by the value for the olanzapine group. Because maximal drug exposure and thus potential toxicity occurred among the participants who remained in the trial for 8 weeks, 8-week results are reported. In general, as would be expected with a shorter period of drug exposure, endpoint analyses produced ratios closer to 1.
Results
Participant Characteristics
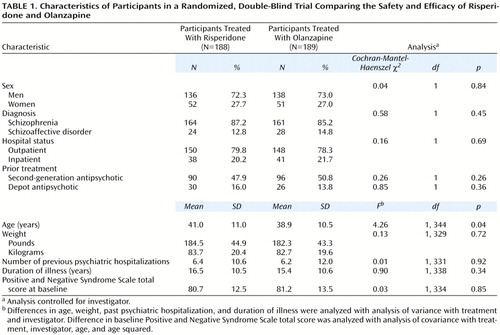
The participants’ characteristics are given in Table 1. Most participants were men (72.7%, N=274). The mean age of the group was 40.0 years (SD=10.8), 86.2% (N=325) had a diagnosis of schizophrenia (65.5% of those had the paranoid type, N=213), and 79.0% (N=298) were outpatients. The mean baseline Positive and Negative Syndrome Scale score of the group was 80.9 (SD=13.0). There were no significant differences in background characteristics between the two treatment groups, except that the participants who received olanzapine were slightly younger than those who received risperidone.
Participant Disposition
Similar proportions of the participants in the two treatment groups completed the study (71.8% [N=135] in the risperidone group and 77.2% [N=146] in the olanzapine group) (Cochran-Mantel-Haenszel χ2=1.55, df=1, p=0.21) or discontinued because of an adverse event (11.7% [N=22] and 9.0% [N=17]) (Cochran-Mantel-Haenszel χ2=0.82, df=1, p=0.37). The mean duration of treatment was 45.8 days (SD=18.8) in the risperidone group and 48.9 days (SD=16.5) in the olanzapine group.
Medication Doses
The mean modal doses received by the participants during the trial were 4.8 mg/day (SD=1.2) of risperidone and 12.4 mg/day (SD=4.6) of olanzapine. The endpoint mean doses were 4.7 mg/day (SD=1.4) of risperidone and 13.1 mg/day (SD=5.1) of olanzapine; endpoint median doses were 4 mg/day and 10 mg/day, respectively. Distribution of the modal daily doses was as follows: 2 mg of risperidone was received by 6.9% of the risperidone participants (N=13), 4 mg by 47.3% (N=89), and 6 mg by 45.7% (N=86); 5 mg of olanzapine was received by 10.1% of the olanzapine participants (N=19), 10 mg by 51.3% (N=97), 15 mg by 18.5% (N=35), and 20 mg by 20.1% (N=38).
Extrapyramidal Symptoms
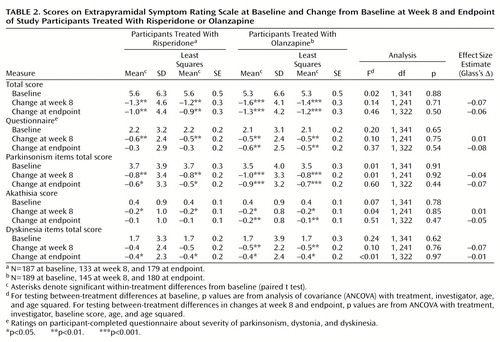
Extrapyramidal symptoms (including hyperkinesia, extrapyramidal disorder, hypokinesia, tremor, dyskinesia, ataxia, and dystonia) were reported as an adverse event for 23.9% of the risperidone participants (N=45) and 20.1% of the olanzapine participants (N=38) (Cochran-Mantel-Haenszel χ2=0.59, df=1, p=0.44). Severity of extrapyramidal symptoms (Extrapyramidal Symptom Rating Scale total scores) was significantly reduced from baseline to week 8 and endpoint in both treatment groups, with no significant between-group differences (Table 2). Antiparkinsonian medications were received by 32.4% of the risperidone participants (N=61) and 28.0% of the olanzapine participants (N=53) during the trial (Cochran-Mantel-Haenszel χ2=1.30, df=1, p=0.26).
Efficacy
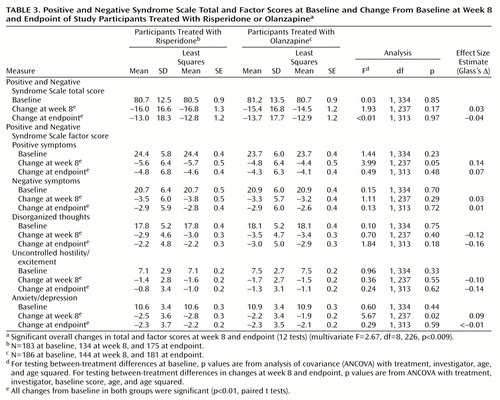
In both treatment groups, significant improvements from baseline to week 8 and endpoint were seen in total Positive and Negative Syndrome Scale scores and on each of the five Positive and Negative Syndrome Scale factors (p<0.001) (Table 3). On all Positive and Negative Syndrome Scale measures, score reductions from baseline were significant at treatment week 2 and throughout the study (p<0.01) (Figure 1). Potential overall differences in efficacy between risperidone and olanzapine were significant (F=2.67, df=8, 226, p<0.009). Comparison of individual factor differences between treatments were not significant at endpoint (i.e., using last-observation-carried-forward analysis to create data for dropouts); however, among those who completed 8 weeks of the study (that is, observed cases), significantly greater improvement on two Positive and Negative Syndrome Scale factors (positive symptoms and anxiety/depression) was seen in participants receiving risperidone than in those receiving olanzapine (p<0.05) (Table 3, Figure 1). There were no factor comparisons in which participants receiving olanzapine had significantly greater improvement than those receiving risperidone.
Clinical improvement, defined as a ≥20% reduction in Positive and Negative Syndrome Scale total scores, was seen in 50.7% of the risperidone group (N=69) and 47.6% of the olanzapine group (N=68) at week 8 and in 45.4% and 44.5%, respectively, at endpoint (N=79 for the risperidone group; N=81 for the olanzapine group). (Data were not available for all patients on each measure.) Percentage reductions in the Positive and Negative Syndrome Scale positive symptoms score for the two groups are shown in Figure 2. A higher proportion of risperidone than of olanzapine participants achieved 20%–40% reductions in the Positive and Negative Syndrome Scale positive symptoms scores at both week 8 and endpoint, and these between-group differences were significant at the 40% reduction level at week 8 (25.4% [N=34] for the risperidone participants versus 16.0% [N=23] for the olanzapine participants; and at endpoint (24.0% [N=42] for the risperidone participants versus 15.5% [N=28] for the olanzapine participants; Cochran-Mantel-Haenszel χ2=4.79, df=1, p<0.03). Few patients achieved 50% symptom reduction.
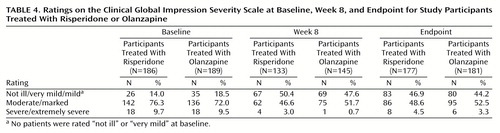
Participants in both treatment groups showed substantial improvements on the CGI severity scale (Table 4) and the CGI change scale (Figure 3), with no significant between-group differences. At week 8, 45.1% of the risperidone group (N=60) and 40.0% of the olanzapine group (N=58) were rated as much or very much improved. At endpoint, 40.1% (N=71) and 35.9% (N=65), respectively, were so rated.
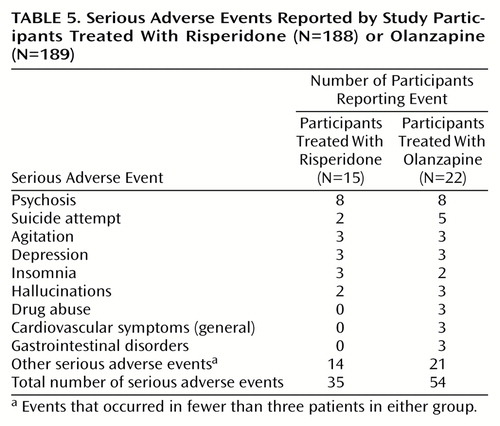
Adverse Events
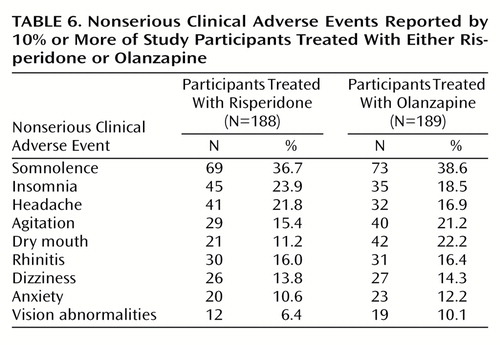
Data on the incidence and severity of extrapyramidal symptoms are reported near the beginning of the Results section. Serious adverse events were experienced by 8.0% of the risperidone participants (N=15) and 11.6% of the olanzapine participants (N=22) (Table 5). The most frequently recorded serious adverse events were psychosis (eight participants in each group), suicide attempt (two risperidone and five olanzapine participants), agitation (three participants in each group), depression (three participants in each group), and insomnia (three risperidone and two olanzapine participants). Data on clinical adverse events that occurred in ≥10% of the participants in either treatment group are shown in Table 6. The most commonly reported event in both groups was somnolence. The largest difference observed between the two groups was in the incidence of dry mouth (11.2% of the risperidone participants [N=21] and 22.2% of the olanzapine participants [N=42]). Lorazepam and other benzodiazepines were received by 49.5% of risperidone participants (N=93) and 51.9% of olanzapine participants (N=98) during the trial (Cochran-Mantel-Haenszel χ2=0.05, df=1, p=0.82). The duration of lorazepam use was similar in the two groups (mean=23.9 days, SD=20.2, in the risperidone group and mean=20.3 days, SD=20.7, in the olanzapine group) (F=0.32, df=1, 83, p=0.57).
Weight Gain
Gains in weight and body mass index (a height-adjusted measure of body weight, defined as kilograms per square meter) were significantly greater in participants treated with olanzapine than in those treated with risperidone. At endpoint, the mean weight gain was 7.2 lb (SD=11.2) in the olanzapine group versus 3.4 lb (SD=7.8) in the risperidone group (F=14.26, df=1, 282, p<0.001), and the mean body mass index increase was 1.1 kg/m2 (SD=1.7) in the olanzapine group versus 0.5 kg/m2 (SD=1.2) in the risperidone group (F=15.72, df=1, 282, p<0.001). An increase in body weight of ≥7% was seen in 27.3% of the olanzapine participants (N=44 of 161) and 11.6% of the risperidone participants (N=18 of 155) (Cochran-Mantel-Haenszel χ2=13.47, df=1, p<0.001).
When participants were categorized by their body mass index stratum at baseline, greater weight gains were seen in the olanzapine group than in the risperidone group at each of the three body mass index strata (Figure 4).
Laboratory Test Results
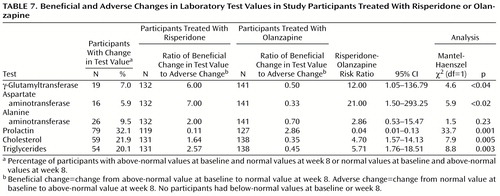
Few or no clinical adverse events were reported that were clearly attributable to any serum laboratory test value relating to liver enzymes, prolactin, or lipids. Among participants experiencing a beneficial or adverse change in laboratory test values, risk ratios for change were worse for olanzapine in relation to liver transaminases and lipid profiles and worse for risperidone in relation to prolactin (Table 7). Reports of side effects potentially related to prolactin were actively solicited; symptoms were common, but the differences between treatment groups were not significant (Table 8).
As for the ECG results, the mean change in the QTc interval from baseline to endpoint was −1.3 msec (SD=25.7) in the risperidone group and 1.2 msec (SD=20.2) in the olanzapine group. The between-group difference was not significant (F=2.09, df=1, 287, p=0.15).
Discussion
The selection of an antipsychotic agent to treat people with schizophrenia or schizoaffective disorder is a complex decision for which the physician must weigh individual patient factors and numerous drug factors, including efficacy, safety, tolerability, and cost. In this large prospective study of risperidone and olanzapine in clinically relevant doses, both antipsychotic drugs were generally safe and efficacious in treating people with schizophrenia or schizoaffective disorder. Significant reductions in the severity of symptoms of schizophrenia and schizoaffective disorder, as measured by scores on the Positive and Negative Syndrome Scale, were seen at 2 weeks after initiation of treatment, and further improvements were noted throughout the 8-week trial.
The mean modal doses of risperidone (4.8 mg/day) and olanzapine (12.4 mg/day) received by the participants in this study are similar to those used in current clinical practice (4.5 mg/day of risperidone and 12.7 mg/day of olanzapine) (national dosing data for schizophrenia only) (14). In contrast, in a previous comparative study of risperidone and olanzapine (7), risperidone doses (mean modal dose=7.2 mg/day) were 60% higher than those selected for use in current clinical practice, as reflected by the same national dosing data. Optimal dosing is important to study interpretation. In addition to reducing tolerability, excessive doses may actually reduce efficacy, perhaps because of the loss of an optimal receptor occupancy (e.g., D2) range or of an optimal binding pattern across different receptors (e.g., across 5-HT, D2, and α2) (15, 16). Severity of extrapyramidal symptoms was reduced over the course of the study in both groups, with no significant between-group differences. The presence of extrapyramidal symptoms also may limit clinical response.
With respect to efficacy, a multivariate analysis of Positive and Negative Syndrome Scale scores revealed significant between-treatment differences. This may be attributable to the finding that on two Positive and Negative Syndrome Scale factors (positive symptoms and anxiety/depression) reductions in symptom severity were greater with risperidone than with olanzapine among participants who completed the 8-week study (p<0.05). In most comparisons of Positive and Negative Syndrome Scale factors, risperidone and olanzapine were equally efficacious. The finding that risperidone may have superior positive symptom efficacy compared with olanzapine is consistent with previously published research, including both an open direct-comparison study (11) and large controlled trials (3, 4) in which risperidone, but not olanzapine, was superior to haloperidol in reduction of positive symptoms. Potential superiority of risperidone in relief of mood symptoms in this population is an interesting finding, possibly related to large differences between the drugs in α2 receptor affinity. Further research is needed to confirm this finding.
Few participants experienced serious adverse events, and somnolence was the only adverse event reported by 30% or more of the participants. There were greater increases in weight in participants treated with olanzapine (weight gain averaged about 1 lb per week in these participants) than in those treated with risperidone. Moreover, as shown in Figure 4, weight gain with olanzapine was observed irrespective of participants’ baseline body mass index; in contrast, weight gain with risperidone was predominantly seen in participants with a low body mass index. More than 25% of the olanzapine participants (and 12% of risperidone participants) experienced a weight gain of 7% or more, an increase that is believed to pose health risks (17). It is noteworthy that during this 8-week study some participants gained >20% of body weight (3% of olanzapine participants and no risperidone participants). Substantial health risks are associated with weight gain, a factor deserving careful consideration in long-term therapy. These health risks may include coronary heart disease, stroke, osteoarthritis, and breast, prostate, and colon cancers (17), as well as possible adverse effects on compliance, self-esteem, and cost. Osser et al. (18) have reported increases in weight and serum triglyceride levels with olanzapine. Wirshing et al. (19), Goldstein et al. (20), and Gatta et al. (21) have reported new-onset diabetes and diabetic ketoacidosis in patients treated with olanzapine. Of particular concern may be the incremental risk with other risk factors common in this population (e.g., smoking) (22). Factors that affect tolerability, such as extrapyramidal symptoms or liver transaminase or prolactin elevation, generally resolve rapidly with treatment modification, but weight gain may be persistent and refractory to treatment even after drug discontinuation. Substantial weight increases with olanzapine have been reported previously (23, 24).
Prolactin levels were elevated in participants receiving risperidone. However, results of a questionnaire that solicited reports of symptoms potentially related to prolactin showed no difference between treatments. A similar lack of association between prolactin levels and adverse events potentially related to prolactin was found by Kleinberg et al. (25) in their analysis of data from more than 800 men and women treated with risperidone. Although risperidone-induced elevated prolactin levels have been reported to produce clinical effects (26–28), we found such effects to be rare. This is not to say that symptoms are uncommon, merely that the association of such symptoms with prolactin levels may be the exception rather than the rule.
The ratio of beneficial-to-adverse change in laboratory test values (Table 7) suggested potentially greater risk for undesirable changes in liver enzymes and serum lipid levels associated with olanzapine treatment, but as with the prolactin laboratory test values, these findings should be interpreted with caution in light of the low proportion of participants affected by such changes and the lack of related spontaneously reported clinical adverse events observed in relation to any laboratory test values in this trial.
Conclusions
Both risperidone and olanzapine were generally well tolerated and efficacious in the treatment of patients with schizophrenia and schizoaffective disorder. The frequency and severity of extrapyramidal symptoms were similar in the two treatment groups. Positive and Negative Syndrome Scale scores on two factors—positive symptoms and anxiety/depression—were better with risperidone than with olanzapine among participants who completed the 8-week trial. Olanzapine treatment was associated with a magnitude of weight gain that may constitute a meaningful health hazard.
Acknowledgments
The other principal investigators in this multicenter study were the following: Robert W. Baker, M.D., Jackson, Miss.; Ileana Berman, M.D., Taunton, Mass.; Ronald Brenner, M.D., Far Rockaway, N.Y.; David Brown, M.D., Austin, Tex.; John S. Carman, M.D., Smyrna, Ga.; K.N. Roy Chengappa, M.D., Pittsburgh; Cal K. Cohn, M.D., Houston; John G. Csernansky, M.D., St. Louis; David G. Daniel, M.D., Falls Church, Va.; Michael DePriest, M.D., Salt Lake City; Louis Fabre, M.D., Houston; David Feifel, M.D., San Diego; James Ferguson, M.D., Salt Lake City; Michael Flaum, M.D., Iowa City, Iowa; John Gurkis, M.D., Pittsburgh; Evelyn Howanitz, M.D., East Orange, N.J.; Richard Josiassen, M.D., Norristown, Pa.; Philip Kanof, M.D., Tucson, Ariz.; Mary Ann Knesevich, M.D., Dallas; Alex Kopelowicz, M.D., Mission Hills, Calif.; John Lauriello, M.D., Albuquerque, N.Mex.; William Lawson, M.D., Indianapolis; Michael Lesem, M.D., Bellaire, Tex.; Douglas F. Levinson, M.D., Philadelphia; Jean-Pierre Lindenmayer, M.D., Wards Island, N.Y.; H.E. Logue, M.D., Birmingham, Ala.; Raymond C. Love, Pharm.D., Perry Point, Md.; Subramoniam Madhusoodanan, M.D., Far Rockaway, N.Y.; Theodore Manschreck, M.D., Fall River, Mass.; Joseph McEvoy, M.D., Butner, N.C.; Steven R. Marder, M.D., Los Angeles; Herbert Y. Meltzer, M.D., Nashville, Tenn.; Alan Mendelowitz, M.D., Glen Oaks, N.Y.; Hussam Mihtar, M.D., San Diego; Alexander Miller, M.D., San Antonio, Tex.; Raj Nakra, M.D., Chesterfield, Mo.; Jorg J. Pahl, M.D., Oklahoma City; Anand K. Pandurangi, M.D., Richmond, Va.; Michael Plopper, M.D., San Diego; John Prosser, M.D., Dallas; Narayana Reddy, M.D., Peoria, Ill.; P. Roy-Byrne, M.D., Seattle; Nina R. Schooler, Ph.D., Pittsburgh; S. Charles Schultz, M.D., Cleveland; Steven Strakowski, M.D., Cincinnati; Robert Stern, M.D., Bronx, N.Y.; Alan Swan, M.D., Houston; Steven Targum, M.D., Upland, Pa.; Dane Wingerson, M.D., Seattle; Donna A. Wirshing, M.D., Los Angeles.
 |
 |
 |
 |
 |
 |
 |
 |
Received April 14, 2000; revisions received Aug. 28 and Dec. 28, 2000; accepted Jan. 11, 2001. From the Maryland Psychiatric Research Center, University of Maryland; and Janssen Pharmaceutica Research Foundation, Titusville, N.J. Address reprint requests to Dr. Conley, Maryland Psychiatric Research Center, P.O. Box 21247, Redwood Circle/White Building, Baltimore, MD 21228. Supported by the Janssen Research Foundation. Dr. Conley was the principal investigator at the lead study site for this trial but was not compensated for the preparation of this report.
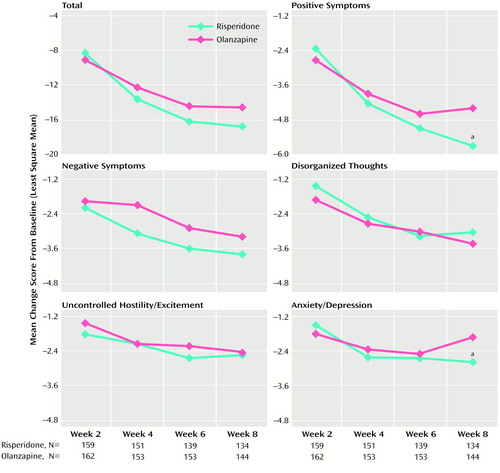
Figure 1. Positive and Negative Syndrome Scale Total and Factor Scores at Weeks 2–8 of Participants Treated With Risperidone or Olanzapine
aSignificant difference between groups (p<0.05; analysis of covariance with treatment, investigator, baseline score, age, and age squared).
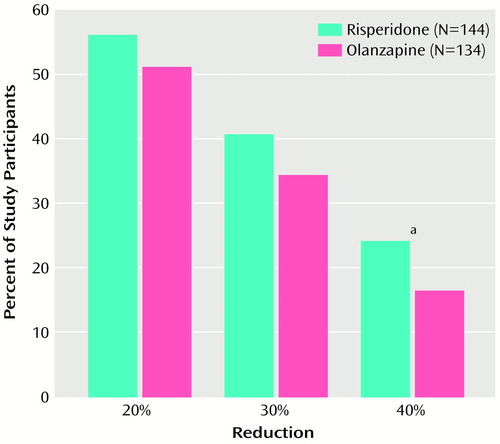
Figure 2. Percentages of Participants Treated With Risperidone or Olanzapine With a 20% to 40% Reduction in the Positive and Negative Syndrome Scale Positive Symptom Score at Week 8
aSignificant difference between groups (Cochran-Mantel-Haenszel χ2=6.54, df=1, p<0.02).
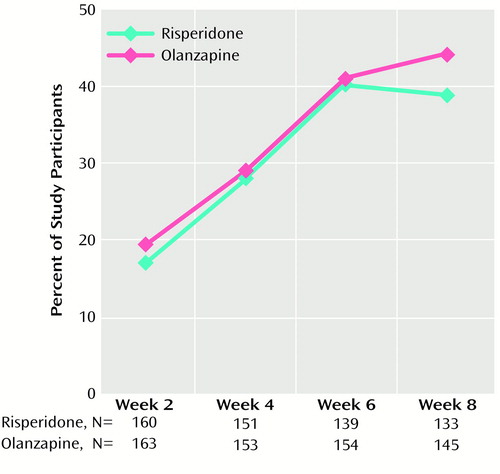
Figure 3. Percentages of Participants Treated With Risperidone or Olanzapine Who Were Rated Much or Very Much Improved on the Clinical Global Impression Change Scale at Weeks 2–8
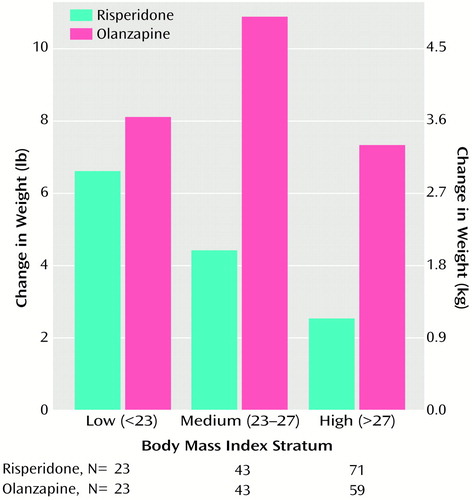
Figure 4. Changes in Body Weight at Week 8 in Participants Treated With Risperidone or Olanzapine by Body Mass Indexa Stratum at Baseline
aA height-adjusted measure of body weight (kilograms per square meter).
1. Chouinard G, Jones B, Remington G, Bloom D, Addington D, MacEwan GW, Labelle A, Beauclair L, Arnott W: A Canadian multicenter placebo-controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. J Clin Psychopharmacol 1993; 13:25–40Crossref, Medline, Google Scholar
2. Marder SR, Meibach RC: Risperidone in the treatment of schizophrenia. Am J Psychiatry 1994; 151:825–835Link, Google Scholar
3. Marder SR, Davis JM, Chouinard G: The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry 1997; 58:538–546Crossref, Medline, Google Scholar
4. Beasley CM Jr, Tollefson GD, Tran P, Satterlee W, Sanger T, Hamilton S (Olanzapine HGAD Study Group): Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology 1996; 14:111–123Crossref, Medline, Google Scholar
5. Tollefson GD, Beasley CM Jr, Tran PV, Street JS, Krueger JA, Tamura RN, Graffeo KA, Thieme ME: Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective disorders: results of an international collaborative trial. Am J Psychiatry 1997; 154:457–465Link, Google Scholar
6. Kane JM: Pharmacologic treatment of schizophrenia. Biol Psychiatry 1999; 46:1396–1408Google Scholar
7. Tran PV, Hamilton SH, Kuntz AJ, Potvin JH, Anderson SW, Beasley C Jr, Tollefson GD: Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol 1997; 17:407–418Crossref, Medline, Google Scholar
8. Schooler NR: Comments on article by Tran and colleagues, “Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders” (letter). J Clin Psychopharmacol 1998; 18:174–176Crossref, Medline, Google Scholar
9. Gheuens J, Grebb JA: Comments on article by Tran and colleagues, “Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders” (letter). J Clin Psychopharmacol 1998; 18:176–179Crossref, Medline, Google Scholar
10. Kasper S, Kuefferle B: Comments on “Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders” by Tran and associates (letter). J Clin Psychopharmacol 1998; 18:353–356Crossref, Medline, Google Scholar
11. Ho BC, Miller D, Nopoulos P, Andreasen NC: A comparative effectiveness study of risperidone and olanzapine in the treatment of schizophrenia. J Clin Psychiatry 1999; 60:658–663Crossref, Medline, Google Scholar
12. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
13. Chouinard G, Ross-Chouinard A, Annable L, Jones BD: Extrapyramidal Symptom Rating Scale (abstract). Can J Neurol Sci 1980; 7:233Google Scholar
14. National Disease and Therapeutic Index. Plymouth Meeting, Penn., IMS America, June 1999Google Scholar
15. Kapur S, Zipursky RB, Remington G: Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry 1999; 156:286–293Abstract, Google Scholar
16. Kasper S: Risperidone and olanzapine: optimal dosing for efficacy and tolerability in patients with schizophrenia. Int Clin Psychopharmacol 1998; 13:253–262Crossref, Medline, Google Scholar
17. National Institutes of Health, National Lung, Heart and Blood Institute: Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. NIH Publication 98-4083. Bethesda, Md, National Institutes of Health, 1998Google Scholar
18. Osser DN, Najarian DM, Dufresne RL: Olanzapine increases weight and serum triglyceride levels. J Clin Psychiatry 1999; 60:767–770Crossref, Medline, Google Scholar
19. Wirshing DA, Spellberg BJ, Erhart SM, Marder SR, Wirshing WC: Novel antipsychotics and new onset diabetes. Biol Psychiatry 1998; 44:778–783Crossref, Medline, Google Scholar
20. Goldstein LE, Sporn J, Brown S, Kim H, Finkelstein J, Gaffey GK, Sachs G, Stern TA: New-onset diabetes mellitus and diabetic ketoacidosis associated with olanzapine treatment. Psychosomatics 1999; 40:438–443Crossref, Medline, Google Scholar
21. Gatta B, Rigalleau V, Gin H: Diabetic ketoacidosis with olanzapine treatment. Diabetes Care 1999; 22:1002–1003Google Scholar
22. Rosengren A, Wedel H, Wilhelmsen L: Body weight and weight gain during adult life in men in relation to coronary heart disease and mortality: a prospective population study. Eur Heart J 1999; 20:269–277Crossref, Medline, Google Scholar
23. Beasley CM Jr: Safety of olanzapine. J Clin Psychiatry Monogr 1997; 15:19–21Google Scholar
24. Gupta S, Droney T, Al-Samarrai S, Keller P, Frank B: Olanzapine-induced weight gain (letter). Ann Clin Psychiatry 1998; 10:39Crossref, Medline, Google Scholar
25. Kleinberg DL, Davis JM, de Coster R, Van Baelen B, Brecher M: Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol 1999; 19:57–61Crossref, Medline, Google Scholar
26. Dickson RA, Dalby JT, Williams R, Edwards AL: Risperidone-induced prolactin elevations in premenopausal women with schizophrenia (letter). Am J Psychiatry 1995; 152:1102–1103Google Scholar
27. Popli A, Gupta S, Rangwani SR: Risperidone-induced galactorrhea associated with prolactin elevation. Ann Clin Psychiatry 1998; 10:31–33Crossref, Medline, Google Scholar
28. Dickson RA, Glazer WM: Hyperprolactinemia and male sexual function (letter). J Clin Psychiatry 1999; 60:125Crossref, Medline, Google Scholar



