Electrophysiological Evidence of Serotonergic Impairment in Long-Term MDMA (“Ecstasy”) Users
Abstract
OBJECTIVE: “Ecstasy,” or 3,4-methylenedioxymethamphetamine (MDMA), causes long-term impairment to the serotonin (5-HT) system in rats, dogs, and nonhuman primates. 5-HT dysfunction has also been observed in human recreational users of the drug, but whether 5-HT dysfunction in humans is caused by MDMA has not been established, since dysfunction may have preceded MDMA exposure. This ambiguity about causation is particularly important in MDMA research, because 5-HT deficiency is a predictor of risky behavior. METHOD: The 5-HT function of 22 long-term MDMA users was compared to that of 20 drug-naive comparison subjects and 19 cannabis users. 5-HT function was assessed with the intensity dependence paradigm, a tool that measures 5-HT-related attenuation of neural response to auditory stimuli (measured with EEG). RESULTS: Long-term MDMA users exhibited 5-HT dysfunction, relative to both cannabis users and drug-naive comparison subjects. This dysfunction was related to total MDMA consumption (after removing the effect of frequency of use) but not to frequency of use (after removing the effect of total consumption). CONCLUSIONS: These data show that 5-HT dysfunction occurs in MDMA users, is related to users’ MDMA consumption, and is independent of cannabis use. The results do not suggest that self-medication explains this relationship, because the deficit was related to total MDMA consumption but not frequency of consumption. The results are thus consistent with the thesis that MDMA consumption causes 5-HT impairment in humans.
With its street name “ecstasy” deriving from the characteristic euphoria that the drug produces, (+/–)3,4-methylenedioxymethamphetamine (MDMA) is currently one of the most popular recreational drugs in the Western world. Administration of the drug results in a massive increase in extracellular serotonin (5-HT), likely owing to an efflux of cytoplasmic 5-HT and concurrent 5-HT transporter facilitation (1). In a corresponding effect, MDMA has been shown to impair 5-HT neuron integrity in rats (2–4) and nonhuman primates (5–7).
MDMA has a severe effect on 5-HT in nonhuman primates. Repeated administration causes dose-related depletion of 5-HT in the somatosensory cortex (44% reduction at 2.5 mg/kg; 90% at 5 mg/kg), as well as depletion to a lesser extent in the caudate nucleus, putamen, hippocampus, hypothalamus, and thalamus (5)—a function of damage to 5-HT nerve endings and axons (1). Many of these deficits have been found to remain for 2 years (6) and 7 years (7) after MDMA exposure. These findings raise serious concern about possible damage that the drug may cause in humans, because the doses used in the studies were similar to those used recreationally. However, conclusions made on the basis of animal research do not necessarily generalize to humans. Thus these studies should be viewed as cause for concern but not evidence that recreational use of MDMA impairs serotonergic function. This point was demonstrated recently when MDMA intoxication was shown to cause opposing sensorimotor gating effects in rats and in humans (8).
Consequently, many researchers have attempted to determine if MDMA adversely affects brain 5-HT in humans. MDMA users have been found to exhibit alterations in several indexes suggestive of 5-HT dysfunction, including decreases in cerebrospinal fluid concentrations of 5-HT metabolites (9, 10), alterations in nociception (9), prolactin responses to l-tryptophan (9), prolactin and cortisol responses to d-fenfluramine (11), and alterations in neuroendocrine and behavioral responses to meta-chlorophenylpiperazine (12). Further, positron emission tomography has provided direct evidence of 5-HT impairment in MDMA users, relative to comparison subjects (13). So it is clear that MDMA users do exhibit 5-HT impairment.
However, such studies were not able to show that MDMA caused 5-HT impairment, since they could not account for the possibility that long-term MDMA users might have used the drug because they suffered from 5-HT dysfunction. This ambiguity is particularly relevant to the issue of MDMA-related impairment owing to the connection that has been demonstrated between 5-HT impairment and the “novelty-seeking personality” (14). That is, people with low levels of brain 5-HT are more likely to engage in novelty-seeking activities, such as illicit drug taking, than those with normal 5-HT levels (15).
In the quasi-experimental designs of these studies, correlations between MDMA use and 5-HT dysfunction are necessary but insufficient to demonstrate a causal relation because of the confounding effects of self-medication among drug users (16–18). However, causation is supported where a deficit is related to the cumulative dose of a drug independent of frequency of use, because frequency of use may be controlled by the user in response to 5-HT function, but cumulative dose (independent of frequency) cannot be controlled in this way. For instance, the self-medication hypothesis would predict that subjects with low 5-HT levels would gain greater positive reinforcement from MDMA use than those with normal 5-HT levels and would use MDMA more regularly, resulting in a relation between frequency of use and 5-HT function. Conversely, it would predict that cumulative dose (independent of frequency) would not be related to 5-HT function, because cumulative dose, independent of frequency of use, is effectively a random variable, dependent only on the stage of the subject’s drug use history when they volunteer for testing. This delineation allows a resolution to the impasse regarding causation in quasi-experimental drug research: causation is suggested by demonstrating a relation between impairment and total drug use that is independent of frequency of use.
Accordingly, to determine whether MDMA causes 5-HT impairment, we examined the relation between an index of 5-HT integrity (the intensity dependence of auditory evoked potentials [15, 19], described in the Method section) and both frequency of MDMA use and cumulative amount of MDMA used. A failure to find any relations would suggest that any group differences in 5-HT indexes would not have been associated with MDMA. Evidence of a relation between the 5-HT index and either frequency of use or total amount used would demonstrate association, but the nature of the association would remain ambiguous, e.g., it may be that the 5-HT deficit made subjects more likely to consume MDMA. Thus, to support the thesis that MDMA had caused the deficit, a correlation between the 5-HT index and total MDMA consumed that is independent of frequency of use would be required.
Another aim of the study was to determine whether relations between MDMA and 5-HT could equally be explained by subjects’ cannabis use. This is important because many MDMA users consume cannabis when the effect of MDMA is diminishing. In addition, because the main psychoactive constituent of cannabis, Δ9-tetrahydrocannabinol (Δ9-THC), markedly affects various neurotransmitter systems, it may confound the relation between MDMA and 5-HT. It has been shown, for example, that cannabis may be a confound in studies of MDMA-related cognitive deficits (20).
Method
Subjects
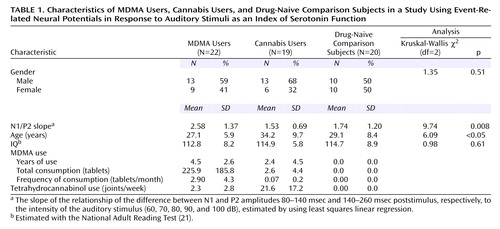
Twenty-two long-term MDMA users, 19 long-term cannabis users, and 20 drug-naive comparison subjects participated in the study. Table 1 shows the subjects’ demographic characteristics. After complete description of the study to the subjects, written informed consent was obtained.
Data Acquisition
Tin electrodes were used to record EEGs. EEGs were recorded from vertex and referenced to the left mastoid. Subjects were grounded on the scalp over the frontal cortex. Eye movements were monitored with a vertical electrooculogram (EOG), and measurements were calculated as the difference between voltages from electrodes above and below the left eye. A gain of 1000 was used for each channel; the bandpass was 0.016 to 35 Hz. Impedances were kept below 5 kΩ, and data were digitized at 500 Hz.
Stimuli
Stimuli for the intensity dependence task consisted of 100 binaural 1000 Hz tones (30 msec plus 10 msec rise and fall time) at each of 60-, 70-, 80-, 90-, and 100-dB (SPL) intensities (19). The stimuli were presented through headphones in a pseudorandom fashion with variable interstimulus intervals (1800–2200 msec).
Serotonin (5-HT) Index
The auditory intensity dependence function was employed as an electrophysiological index of 5-HT function (15, 19). 5-HT in the primary auditory cortex is thought to operate as a protective mechanism by attenuating cortical response to loud auditory stimuli. This mechanism decreases evoked neural potentials to high-intensity auditory stimuli, such that the response to a 100-dB tone will not be proportionately larger than the response to a 60-dB tone. Because this attenuation is greater in primary auditory cortex in which 5-HT is intact, we can infer 5-HT function from the relation between auditory intensity dependence and the evoked neural potentials. This method is particularly suitable for assessing 5-HT function in MDMA users, since neocortex is one of the primary sites of MDMA-related damage in nonhuman primates (5–7).
Procedure
Subjects were screened in a telephone interview and excluded if they reported having suffered from neurological disturbances or having taken prescribed psychotropic medication. Subjects were assigned to the MDMA group if they had used MDMA on at least 20 occasions, to the comparison group if they had never used MDMA and had not used cannabis regularly, and to the cannabis group if they had used cannabis at least twice a week for at least a 2-year period and MDMA only irregularly (Table 1). On arrival at the laboratory, subjects completed consent forms, were administered the National Adult Reading Test (21) to obtain an estimated IQ, and were fitted with an electrode cap. They were seated in an armchair in an electrically shielded sound-attenuated booth, where their hearing thresholds were checked. Subjects then completed a series of personality and drug use questionnaires while the intensity dependence paradigm was run (duration=15 minutes).
Data Analysis
Epochs were defined as –100 to 300 msec poststimulus. Epochs containing vertical EOGs greater than 50 μV relative to baseline were excluded from further analysis. Five event-related potentials were created for each subject by averaging the epochs in which stimuli with the same intensity were presented. For each subject, N1 and P2 amplitudes were calculated as the maximum absolute amplitude (relative to baseline) in the 80–140 msec and 140–260 msec time windows, respectively. The N1/P2 complex was defined as the difference between the N1 and P2 amplitudes. For each subject, the slope of the relationship between the N1/P2 complex and intensity (dB level) of the auditory stimulus was estimated by using least squares linear regression, in which N1/P2 amplitude was the criterion variable and intensity of the stimulus (coded 1–5) was the predictor variable.
Statistical Analysis
The Kruskal-Wallis nonparametric test was used to test for differences between the intensity dependence slopes of the three goups. Nondirectional Mann-Whitney tests were used for post hoc analyses (with Bonferroni adjustment). To determine whether any group differences were related to the MDMA group’s history of MDMA use, data for the MDMA group were analyzed with a multiple regression in which the N1/P2 slope was the criterion and total MDMA consumption (tablets), frequency of MDMA use (tablets per month), age, and sex were the predictor variables (all tests nondirectional). The variable “total MDMA tablets used” was transformed to normality by using the square root function (yielding t_total), and “frequency of use” was transformed by using the natural log (yielding t_frequency). A correlation analysis was performed to determine if t_total and t_frequency were related.
Results
Grand mean event-related potential waveforms for the three groups are shown in Figure 1. The three groups’ intensity dependence slopes were significantly different (χ2=9.74, df=2, p=0.008). Post hoc analyses found that while the N1/P2 slopes of the comparison and cannabis groups did not differ (z=0.16, p=0.88), the MDMA group had larger a N1/P2 slope than either the cannabis group (z=2.88, p=0.004) or comparison group (z=2.44, p<0.02) (Figure 2).
The regression analysis found that t_total and sex predicted the N1/P2 slope (b=0.12, SE=0.04, p<0.02, and b=1.84, SE=0.48, p=0.001, respectively), but that t_frequency and age did not (b=0.33, SE=0.25, p=0.19, and b=0.07, SE=0.045, p=0.13, respectively) (Figure 3). That is, in support of a causative relation, significant positive relations were found between MDMA users’ N1/P2 slopes and total MDMA consumption, independent of frequency of use, but not frequency of MDMA consumption independent of total consumption. In addition, t_total did not correlate with t_frequency (r=0.05, df=22, p=0.83).
Discussion
This study demonstrated that regular users of MDMA exhibit impaired 5-HT function relative to drug-naive comparison subjects and regular users of cannabis, which suggests 5-HT impairment in this population of MDMA users. In keeping with a causative relation, a strong relation was found between total MDMA consumption and 5-HT impairment that was independent of frequency of use. As this relation accounted for 25% of the variance in the 5-HT index (independent of frequency of use, age, and gender), a substantive deficit in human 5-HT function as a result of MDMA use is indicated. This study also found that cannabis use, a possible confound to MDMA research, was not related to the 5-HT deficit.
These results are consistent with the findings of other research demonstrating 5-HT impairment in MDMA users (9–12). Most important, however, the results are consistent with findings in animal studies showing that MDMA causes 5-HT impairment (6–8). Our results, combined with the animal studies results, provide strong evidence that the 5-HT deficits observed in earlier studies in humans were caused by subjects’ MDMA use (9–12).
Several issues relating to this conclusion should be considered. First, the possible confounding effect of catecholamines on the intensity dependence paradigm (22) is not likely to be a problem in the current study, because animal research has shown that MDMA has no lasting effect on catecholamines (5, 7). Second, because self-report measures were used to obtain subjects’ drug histories, inaccuracies in estimated drug use and the time since the last dose are likely. However, such inaccuracies would not affect interpretation, because inaccurate estimates of drug use would likely result in random error and would only decrease the observed relation. Inaccurate estimates of the time since last use of MDMA—and thus possible residual toxicity—would affect relations only with frequency of use and not relations with total use. The third issue relates to the current treatment of causation. It is important to clarify that, because a full experimental protocol was not used, causation was attributed on the basis of the delineation of 5-HT relations with frequency of MDMA use and total MDMA consumption. However, as frequency of MDMA use did not relate to either total consumption or to measures of subjects’ 5-HT levels, we believe causation to be the most parsimonious explanation of the results.
Because 5-HT functions predominantly as a neuromodulator throughout the brain, concern has been raised about the possible cognitive and psychiatric consequences of MDMA use. Research to date suggests that this concern is warranted, since mild cognitive impairment has been demonstrated in MDMA users, relative to comparison subjects, in several studies (10, 23–28). However, this interpretation should be made with caution, because the reported deficits may not be the result of MDMA-related 5-HT impairment but may be related to premorbid cognitive abilities or to other characteristics of the users’ lives, such as polydrug use (20).
In contrast, the causal relation between 5-HT and affect is well established. The role of 5-HT in depression, aggression, and violent suicide has been deduced theoretically (15), and the predicted 5-HT deficits in groups with these characteristics have been demonstrated empirically (29, 30). Further, 5-HT neurotransmission has been shown to increase in all clinically efficacious antidepressant treatments (tricyclic antidepressants, electroconvulsive therapy, monoamine oxidase inhibitors, 5-HT-uptake inhibitors, and 5-HT agonists) (30). The depression that follows 5 days after MDMA consumption (31) and that has been found in long-term MDMA users in general (32) appears to be a result of the drug’s impairment of the 5-HT system. Further, reports of psychopathology after acute (33, 34) and long-term (35–37) exposure to MDMA suggest that other psychiatric conditions may also be related to MDMA use.
This study has demonstrated electrophysiologically that MDMA users suffer substantial 5-HT impairment proportional to their cumulative dose of the drug, irrespective of frequency of use. These results are consistent with previous research demonstrating 5-HT impairment in MDMA users and go further by providing evidence consistent with the thesis that this damage is caused by the MDMA. Owing to the link that has been demonstrated between 5-HT dysfunction and psychopathology, and also owing to the cognitive and affective dysfunction that has been demonstrated in MDMA users, it is concluded that regular use of this drug may carry with it serious consequences.
 |
Received June 14, 2000; revision received Feb. 13, 2001; accepted March 22, 2001. From the Department of Cognitive Neuroscience & Behaviour, Imperial College School of Medicine, London. Address reprint requests to Dr. Gruzelier, Department of Cognitive Neuroscience & Behaviour, Imperial College School of Medicine, St. Dunstan’s Road, London, W6 8RF, U.K.; [email protected] (e-mail). The authors thank Andrea Kübler of the Institute of Medical Psychology and Behavioral Neurobiology, University of Tübingen, Germany, for assistance in data collection.
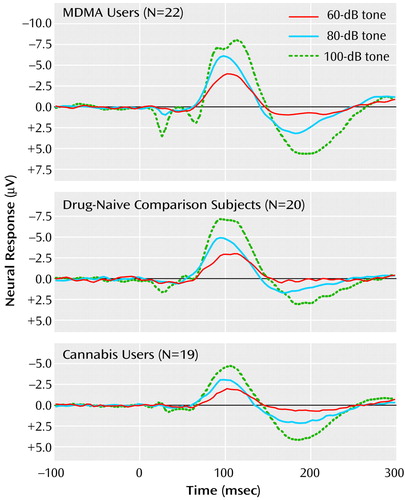
Figure 1. Grand Mean Waveforms for Event-Related Neural Potentials in Response to 60-, 80-, and 100-dB Tones for MDMA Users, Drug-Naive Comparison Subjects, and Cannabis Usersa
aEvent-related potentials were elicited with 100 binaural 1000 Hz tones at each of 60-, 70-, 80-, 90-, and 100-dB intensities. The artifact related to subjects’ blinking in the 100-dB trace for the MDMA group at about 100 msec is consistent with the observation that 5-HT reduces the startling effect of loud stimuli (8) and the current finding of 5-HT impairment in the MDMA group.
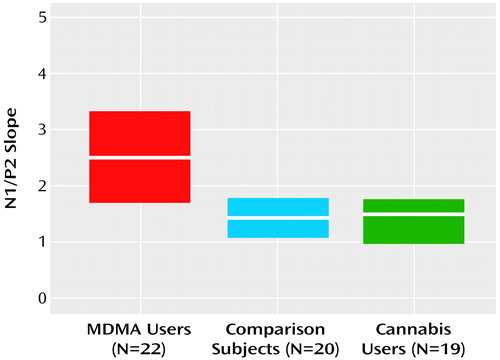
Figure 2. Medians and Interquartile Ranges of the N1/P2 Slopesa in Event-Related Neural Potentials for MDMA Users, Drug-Naive Comparison Subjects, and Cannabis Users
aThe slope of the relationship of the difference between N1 and P2 amplitudes 80–140 msec and 140–260 msec poststimulus, respectively, to the intensity of the auditory stimulus (60, 70, 80, 90, and 100 dB, coded 1–5), estimated by using least squares linear regression.
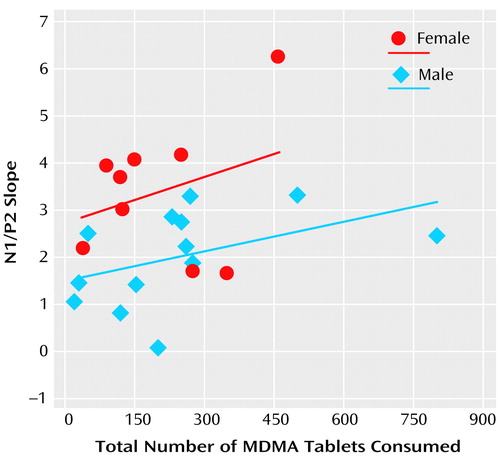
Figure 3. Relationship Between Total Number of MDMA Tablets Consumed (Independent of Frequency) and the N1/P2 Slopea in Event-Related Neural Potentials for Male and Female MDMA Usersb
aThe slope of the relationship of the difference between N1 and P2 amplitudes 80–140 msec and 140–260 msec poststimulus, respectively, to the intensity of the auditory stimulus (60, 70, 80, 90, and 100 dB, coded 1–5), estimated by using least squares linear regression.
bRegression lines show the least squares estimate of the relationships.
1. Huether G, Zhou D, Ruther E: Causes and consequences of the loss of serotonergic presynapses elicited by the consumption of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) and its congeners. J Neural Transm 1997; 104:771-794Crossref, Medline, Google Scholar
2. Commins DL, Vosmer G, Virus RM, Woolverton WL, Schuster CR, Seiden LS: Biochemical and histological evidence that methylenedioxymethamphetamine (MDMA) is toxic to neurons in the rat brain. J Pharmacol Exp Ther 1987; 241:338-345Medline, Google Scholar
3. Mokler DJ, Robinson SE, Rosecrans JA: (+/-)3,4-Methylenedioxymethamphetamine (MDMA) produces long-term reductions in brain 5-hydroxytryptamine in rats. Eur J Pharmacol 1987; 138:265-268Crossref, Medline, Google Scholar
4. O’Shea E, Granados R, Esteban B, Colado MI, Green AR: The relationship between the degree of neurodegeneration of rat brain 5-HT nerve terminals and the dose and frequency of administration of MDMA (“ecstasy”). Neuropharmacology 1998; 37:919-926Crossref, Medline, Google Scholar
5. Ricaurte GA, Forno LS, Wilson MA, DeLanney LE, Irwin I, Molliver ME, Langston JW: (+/-)3,4-Methylenedioxymethamphetamine selectively damages central serotonergic neurons in nonhuman primates. JAMA 1988; 260:51-55Crossref, Medline, Google Scholar
6. Scheffel U, Szabo Z, Mathews WB, Finley PA, Dannals RF, Ravert HT, Szabo K, Yuan J, Ricaurte GA: In vivo detection of short- and long-term MDMA neurotoxicity—a positron emission tomography study in the living baboon brain. Synapse 1998; 29:183-192Crossref, Medline, Google Scholar
7. Hatzidimitriou G, McCann UD, Ricaurte GA: Altered serotonin innervation patterns in the forebrain of monkeys treated with (+/-)3,4-methylenedioxymethamphetamine seven years previously: factors influencing abnormal recovery. J Neurosci 1999; 19:5096-5107Crossref, Medline, Google Scholar
8. Vollenweider FX, Remensberger S, Hell D, Geyer MA: Opposite effects of 3,4-methylenedioxymethamphetamine (MDMA) on sensorimotor gating in rats versus healthy humans. Psychopharmacology (Berl) 1999; 143:365-372Crossref, Medline, Google Scholar
9. Price LH, Ricaurte GA, Krystal JH, Heninger GR: Neuroendocrine and mood responses to intravenous l-tryptophan in 3,4-methylenedioxymethamphetamine (MDMA) users: preliminary observations. Arch Gen Psychiatry 1989; 46:20-22Crossref, Medline, Google Scholar
10. McCann UD, Ridenour A, Shaham Y, Ricaurte GA: Serotonin neurotoxicity after (+/-)3,4-methylenedioxymethamphetamine (MDMA; “Ecstasy”): a controlled study in humans. Neuropsychopharmacology 1994; 10:129-138Crossref, Medline, Google Scholar
11. Gerra G, Zaimovic A, Giucastro G, Maestri D, Monica C, Sartori R, Caccavari R, Delsignore R: Serotonergic function after (+/-)3,4-methylene-dioxymethamphetamine (“Ecstasy”) in humans. Int Clin Psychopharmacol 1998; 13:1-9Crossref, Medline, Google Scholar
12. McCann UD, Eligulashvili V, Mertl M, Murphy DL, Ricaurte GA: Altered neuroendocrine and behavioral responses to m-chlorophenylpiperazine in 3,4-methylenedioxymethamphetamine (MDMA) users. Psychopharmacology (Berl) 1999; 147:56-65Crossref, Medline, Google Scholar
13. McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA: Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet 1998; 352:1433-1437Crossref, Medline, Google Scholar
14. Hegerl U, Karnauchow I, Herrmann WM, Muller-Oerlinghausen B: Intensity dependence of auditory evoked N1/P2 component and personality. Neuropsychobiology 1992; 26:166-172Crossref, Medline, Google Scholar
15. Spoont MR: Modulatory role of serotonin in neural information processing: implications of human psychopathology. Psychol Bull 1992; 112:330-350Crossref, Medline, Google Scholar
16. Jansen KL: Ecstasy (MDMA) dependence. Drug Alcohol Depend 1999; 53:121-124Crossref, Medline, Google Scholar
17. Krystal JH, D’Souza DC, Madonick S, Petrakis IL: Toward a rational pharmacotherapy of comorbid substance abuse in schizophrenic patients. Schizophr Res 1999; 35(suppl):35-49Google Scholar
18. Gruber AJ, Pope HG Jr, Brown ME: Do patients use marijuana as an antidepressant? Depression 1996; 4:77-80Crossref, Medline, Google Scholar
19. Juckel G, Molnar M, Hegerl U, Csepe V, Karmos G: Auditory-evoked potentials as indicator of brain serotonergic activity—first evidence in behaving cats. Biol Psychiatry 1997; 41:1181-1195Crossref, Medline, Google Scholar
20. Croft RJ, Mackay AJ, Mills ATD, Gruzelier JGH: The relative contributions of ecstasy and cannabis to cognitive impairment. Psychopharmacology (Berl) 2001; 153:373-379Crossref, Medline, Google Scholar
21. Nelson H: The National Adult Reading Test (NART): Test Manual. Windsor, UK, National Foundation for Educational Research-Nelson, 1982Google Scholar
22. Dierks T, Barta S, Demisch L, Schmeck K, Englert E, Kewitz A, Maurer K, Poustka F: Intensity dependence of auditory evoked potentials (AEPs) as biological marker for cerebral serotonin levels: effects of tryptophan depletion in healthy subjects. Psychopharmacology (Berl) 1999; 146:101-107Crossref, Medline, Google Scholar
23. Klugman A, Hardy S, Baldeweg T, Gruzelier J: Toxic effect of MDMA on brain serotonin neurons. Lancet 1999; 353:1269-1270Crossref, Medline, Google Scholar
24. Morgan MJ: Memory deficits associated with recreational use of “ecstasy” (MDMA). Psychopharmacology (Berl) 1999; 141:30-36Crossref, Medline, Google Scholar
25. McCann UD, Mertl M, Eligulashvili V, Ricaurte GA: Cognitive performance in (+/-)3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) users: a controlled study. Psychopharmacology (Berl) 1999; 143:417-425Crossref, Medline, Google Scholar
26. Bolla KI, McCann UD, Ricaurte GA: Memory impairment in abstinent MDMA (“Ecstasy”) users. Neurology 1998; 51:1532-1537Crossref, Medline, Google Scholar
27. Krystal JH, Price LH, Opsahl C, Ricaurte GA, Heninger GR: Chronic 3,4-methylenedioxymethamphetamine (MDMA) use: effects on mood and neuropsychological function? Am J Drug Alcohol Abuse 1992; 18:331-341Crossref, Medline, Google Scholar
28. Parrott AC, Lees A, Garnham NJ, Jones M, Wesnes K: Cognitive performance in recreational users of MDMA of “ecstasy”: evidence for memory deficits. J Psychopharmacol 1998; 12:79-83Crossref, Medline, Google Scholar
29. Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE, Goodwin FK: Aggression, suicide and serotonin: relationships to CSF amine metabolites. Am J Psychiatry 1982; 139:741-746Link, Google Scholar
30. Owens MJ, Nemeroff CB: Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem 1994; 40:288-295Medline, Google Scholar
31. Curran HV, Travill RA: Mood and cognitive effects of (+/-)3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”): week-end “high” followed by mid-week low. Addiction 1997; 92:821-831Medline, Google Scholar
32. Gamma A, Frei E, Lehmann D, Pascual-Marqui RD, Hell D, Vollenweider FX: Mood state and brain electric activity in ecstasy users. Neuroreport 2000; 11:157-162Crossref, Medline, Google Scholar
33. Ellis P, Schimmel P: Ecstasy abuse (letter). NZ Med J 1989; 102:358Medline, Google Scholar
34. Whitaker-Azmitia PM, Aronson TA: “Ecstasy” (MDMA)-induced panic (letter). Am J Psychiatry 1989; 146:119Medline, Google Scholar
35. McGuire P, Fahy T: Chronic paranoid psychosis after misuse of MDMA (“ecstasy”). Br Med J 1991; 302:697Crossref, Medline, Google Scholar
36. Schifano F: Chronic atypical psychosis associated with MDMA (“ecstasy”) abuse (letter). Lancet 1991; 338:1335Crossref, Medline, Google Scholar
37. Benazzi F, Mazzoli M: Psychiatric illness associated with “ecstasy” (letter). Lancet 1991; 338:1520Crossref, Medline, Google Scholar



