Impact of Stressful Life Events, Depression, Social Support, Coping, and Cortisol on Progression to AIDS
Abstract
OBJECTIVE: This study examined prospectively the effects of stressful events, depressive symptoms, social support, coping methods, and cortisol levels on progression of HIV-1 infection. METHOD: Eighty-two homosexual men with HIV type-1 infection without AIDS or symptoms at baseline were studied every 6 months for up to 7.5 years. Men were recruited from rural and urban areas in North Carolina, and none was using antiretroviral medications at entry. Disease progression was defined as CD4+ lymphocyte count <200/μl or the presence of an AIDS indicator condition. RESULTS: Cox regression models with time-dependent covariates were used adjusting for race, baseline CD4+ count and viral load, and cumulative average antiretroviral medications. Faster progression to AIDS was associated with higher cumulative average stressful life events, coping by means of denial, and higher serum cortisol as well as with lower cumulative average satisfaction with social support. Other background (e.g., age, education) and health habit variables (e.g., tobacco use, risky sexual behavior) did not significantly predict disease progression. The risk of AIDS was approximately doubled for every 1.5-unit decrease in cumulative average support satisfaction and for every cumulative average increase of one severe stressor, one unit of denial, and 5 mg/dl of cortisol. CONCLUSIONS: Further research is needed to determine if treatments based on these findings might alter the clinical course of HIV-1 infection.
Recently, we reported one of the first prospective findings that stressful events and social support were related to HIV-1 disease progression to AIDS (1). In addition, other studies have shown that depression may have an impact on HIV-1 disease progression (2–4). The mechanisms linking psychosocial variables to changes in disease status in HIV-infected individuals are not well understood. Stressful events and negative affect may be associated with elevated cortisol levels (5, 6), which, in turn, may hasten HIV-1 disease progression (7–9). Furthermore, studies have shown that how people cope with stress may alter the course of HIV-1 infection (3, 10, 11). Therefore, the primary goal of the present study was to examine how cortisol and several coping strategies (e.g., denial), in addition to stressful events, social support, and depressive symptoms, may affect disease progression to AIDS. This current aim extends our previous observations (1) in several ways. We have added neuroendocrine function and coping factors as predictors, we have added baseline viral load and a larger set of health habit control variables, and we have studied the same cohort for up to 7.5 years, 2 years longer than previously.
Past analyses of our ongoing cohort have consistently pointed to the importance of stressful events in HIV-1 infection. We found that more stressful events were associated with greater reductions in killer lymphocytes, especially among those with depressive symptoms (4, 12), and greater risk of disease change (after 3.5 years) and progression to AIDS (after 5.5 years) (1, 13). Likewise, Kemeny and Dean (14) reported that bereavement before study entry was associated with more rapid decline in CD4+ count during 3–4-year follow-up.
Considerable interest has been given to the hypothesis that elevated cortisol levels might hasten HIV-1 progression by altering T-lymphocyte production of cytokines (e.g., shift from TH1 to TH2 cytokines), thereby triggering destruction of CD4+ lymphocytes through programmed cell death and bolstering HIV-1 viral replication (8, 9, 15). These hypotheses are somewhat controversial. For example, some research indicates that HIV-1 does not promote a shift in cytokines from TH1 to TH2(16, 17). The role of stress in producing changes in cortisol level, and how such changes might impact immune status, has not been established clinically in HIV-1 infection. Goodkin et al. (6) found that elevations in plasma cortisol in bereaved HIV-1 infected men were associated with lower lymphocyte proliferative responses to a mitogen. Gorman and colleagues found that urinary free cortisol levels were positively correlated with depressive symptoms and number of HIV-related medical symptoms at baseline (18) but not to CD4+ count or HIV-1 symptoms at 2-year follow-up (19). To the contrary, Antoni et al. (20) found that plasma cortisol level was negatively associated with dysphoric mood and positively correlated with proliferative mitogen response after positive serostatus notification. At the time of entry into this cohort, we found that HIV-positive men with high basal cortisol levels had significantly greater stress-associated changes in immune status than subjects with low basal cortisol levels (21). Therefore, here we examined whether elevated cortisol secretion patterns across time may be associated with an increased rate of HIV disease progression.
The role of depression in HIV-1 disease progression has been examined in several longitudinal studies. A 9-year study of seropositive men showed that baseline depression was associated with faster progression to AIDS (2) and that elevated depression at every visit increased the risk of mortality (22). Data from the Multicenter AIDS Cohort Study showed no relationship between baseline depression and AIDS progression (23); however, self-reported depressive symptoms increased 1.5 years before AIDS diagnosis (24). Mixed findings might be explained by the reliance on a baseline measure of depression and by the need to consider other moderating factors (e.g., coping, social support).
Studies have also reported a link between passive coping strategies (e.g., denial) and HIV-1 disease progression. Coping by means of denial was found to correlate with lower CD4+/CD8+ ratios 1 year after serostatus notification (10) and with a greater probability of disease progression 2 years later (3). Less denial and more active coping strategies (e.g., fighting spirit) were associated with a lower probability of developing HIV-related symptoms after 1 year (11, 25).
On the basis of previous findings by our group and others, we hypothesized that an increased risk of AIDS would be associated with more cumulative average stressful events, coping by means of denial, depressive symptoms, and higher cortisol levels as well as less cumulative average satisfaction with social support. We also examined how cortisol and coping might explain or modify the effects of stressful events on disease change. Our study design has several notable features: 1) stressful life events were measured by interviewer-based contextual ratings, 2) all subjects were asymptomatic and not using antiretroviral medications at entry, 3) analyses controlled for baseline viral load and a wide set of health habits that could explain our findings, 4) subjects were studied every 6 months for up to 7.5 years, and 5) protease inhibitors were not taken before AIDS progression. This study should shed light on possible psychological and pharmacological interventions for HIV-1 infection.
Method
Subjects
Data were collected in North Carolina as part of an ongoing longitudinal study, the Coping in Health and Illness Project. The study group included 82 homosexual men with HIV-1 infection recruited from rural and urban areas of North Carolina. At study entry, all subjects were clinically asymptomatic with CD4+ lymphocytes ³200/μl (defined as clinical stage A by the Centers for Disease Control and Prevention [CDC]). No subject was using antiretroviral medications at entry. Men were assessed at 6-month intervals for up to 7.5 years (16 visits). All subjects had at least two visits (mean number of visits=10.63, SD=4.49).
To avoid confounding the measurement of the immune and neuropsychological measures, we excluded subjects with 1) less than 10 years of education, 2) ages less than 18 or greater than 51, 3) previous intravenous drug use, 4) significant medical illness (e.g., heart, lung, or kidney disease), 5) preexisting neurological disorder/trauma, 6) past treatment for alcoholism or current intake of more than 60 alcoholic drinks/month, 7) present or past heavy recreational drug use, and 8) use of antiretroviral medications at entry. Determination of homosexuality was based on self-report. The study was approved by the University of North Carolina School of Medicine’s Committee for the Protection of the Rights of Human Subjects. After complete description of the study to the subjects, written informed consent was obtained.
Procedure
Subjects were evaluated every 6 months in the General Clinical Research Center at the University of North Carolina, Chapel Hill. All subjects were asked to abstain from over-the-counter medications known to affect immune response, recreational drugs, and any alcohol consumption for at least 2 weeks before each study visit.
Subjects took nothing by mouth starting at 12:00 midnight before blood drawing the following morning. Subjects were recumbent, and an intravenous line was inserted at 8:30 a.m. in an antecubital vein and was maintained patent with a slow, normal saline drip. After a 30-minute acclimation period, four blood samples were drawn at 20-minute intervals. Cortisol measurements were made at each time point to obtain an integrated basal cortisol determination (26). The mean of the four values was used in analyses.
Measurement
All variables were measured every 6 months except social support, which was assessed yearly. More details on the measures have been presented previously (4, 12, 13).
Stressful life events
To obtain a list of stressful life events and difficulties, we modified the Psychiatric Epidemiology Research Interview (27). Stressors were objectively rated by using a manual of norms and vignettes, a methodology similar to that developed by Brown and Harris (28). Norms for each stressful event were based on the degree of threat that most people would experience given the particular circumstances (e.g., financial impact, life threat, personal involvement). The objective threat rating was made independently of the subject’s rating in order to reduce the possibility that worsening disease might lead to higher stressful event scores.
One of two trained raters (previously shown to have high interrater reliability) (12) used the manual to rate the impact of each stressor from 0 (no threat) to 4 (severe threat). All ratings were summed at each visit, except that we removed stressors that were likely to be caused by disease progression (e.g., drop in CD4+ count, retirement due to worsening of HIV-1 infection).
Depressive symptoms
Severity of depressive symptoms was measured with the interviewer-based Hamilton Depression Rating Scale (29), with previously reported high intraclass correlation (0.99) (4). We eliminated six of the 17 medical symptom items (e.g., somatic symptoms, weight loss, retardation) that overlapped with symptoms of HIV-1 disease to help avoid confounding the measure with disease progression.
Support satisfaction
To assess satisfaction with social support, we administered the Brief Social Support Questionnaire of Sarason et al. (30) on a yearly basis (Cronbach’s alpha at baseline=0.89) (31). Ratings signify the individual’s level of satisfaction with support received and could range from 1 (very dissatisfied) to 6 (very satisfied). Support scores at the previous yearly interval were used to estimate scores at each 6-month visit. Correlations between visits 1 year apart ranged from 0.38 to 0.80.
Coping
Coping was assessed by using selected scales from the COPE (32). Subjects were asked to indicate on a 4-point scale (where 1=“not at all” and 4=“very much”) how they “generally cope with or handle the threat of getting AIDS.” For this study, we examined three coping strategies (each consisting of four items): 1) planning (e.g., “I try to come up with a strategy about what to do”), 2) positive reinterpretation and personal growth (e.g., “I try to see it in a different light, to make it seem more positive”), and 3) denial (e.g., “I pretend that it hasn’t really happened”). We previously reported high Cronbach’s alpha reliabilities for these scales (range=0.82–0.88) (33).
Cortisol
Serum cortisol concentrations were measured by using a commercial radioimmunoassay kit (Cortisol Solid Phase Component System, Becton Dickinson, Mountain View, Calif.). Samples obtained at each time-point were assayed in duplicate. Following the kit instructions, a logit-log transformation was graphed and the concentration of cortisol was determined by interpolation from the standard curve of the percent trace level binding (B/Bo) versus mg/dl cortisol. The sensitivity of the assay was 0.07 mg/dl.
HIV-1 disease stage
All of the men knew their HIV-1 status at baseline, which was confirmed by HIV antibody screening through enzyme-linked immunosorbent assay and Western blot testing. We defined disease progression as the first time-point when a subject met the CDC AIDS surveillance case definition (34), i.e., reduction in CD4+ lymphocytes to below 200/μl or the presence of an AIDS indicator condition. A comprehensive physical examination was completed every 6 months by a clinician trained to assess HIV-1 disease stages. Peripheral blood CD4+ lymphocyte counts were performed by flow cytometry that used commercially prepared monoclonal antibodies (Becton Dickinson). Absolute CD4+ count was derived from CD4+ cell percentages × total lymphocytes × 100.
Control variables
To adjust for disease status at entry, we controlled for CD4+ count and viral load at baseline. Serum HIV RNA viral load was determined from archived samples by using the Roche Amplicor Monitor assay (Branchburg, N.J.). The lower limit of quantification for this assay is 400 copies/μl. Logarithmic transformation (base 10) was performed on viral load because the distribution was skewed and contained outliers. We were unable to get archived serum samples on two subjects. For one subject, we used his 6-month serum sample to estimate viral load at entry. The other subject was given a viral load value that was based on the median value of subjects with a similar CD4+ count at entry, since the correlation of CD4+ and viral load was –0.49 (df=1, p≤0.0001).
The number of antiretroviral medications (e.g., zidovudine, lamivudine, or didanosine) used during a 6-month interval ranged from 0 to 3. The amount of cigarette smoking ranged from 0 to three or more packs per day. The Structured Clinical Interview for DSM-III-R (35) was used to assess drug and alcohol abuse/dependence, with diagnoses assigned by consensus conferences and the presence of a diagnosis dichotomously coded (0=no, 1=yes). High-risk sexual behavior (coded 0–15) was measured at each visit by multiplying two items: 1) extent of condom use during receptive anal sex (0=no receptive anal sex to 3=never used condoms) and 2) number of receptive anal sex partners (coded 0–5). Exercise was rated on a 1–6 scale (1=never, 6=daily) on the basis of responses to the following question: “In the last 6 months, how often have you engaged in some form of physical exercise (…for at least 20 minutes)?”
Statistical Methods
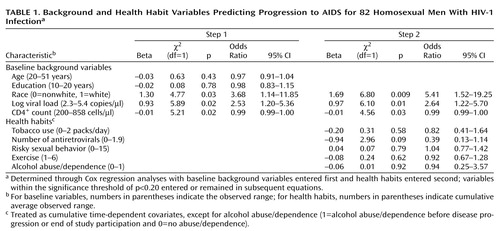
Survival analysis with Cox regression models (with EXACT method for handling ties) (36) was used to calculate the risk of AIDS associated with control variables. Since the background variables were preexisting and health habits change over time, we entered the variables in two steps. Baseline background measures (age, education, race, and baseline CD4+ T lymphocytes and viral load) were entered first, followed by health habits (cigarette smoking, number of antiretrovirals, risky sexual behavior, exercise, and alcohol abuse/dependence). All health habit variables, except alcohol abuse/dependence, were treated as time-dependent covariates by using cumulative average values at regular intervals. Cumulative averages were used because we hypothesized that health habits might have an additive effect on disease progression. The time-dependent scores at each visit were based on the average of scores 6 months before that visit. For example, scores at visit 5 were based on the cumulative average of visits 1–4. Because so few men had alcohol abuse/dependence at any visit (N=14), we used bivariate coding: diagnosis before disease progression or end of study, coded 1, versus no diagnosis ever in the study, coded 0. Control variables were kept in equations during subsequent steps only if within the significance threshold of p<0.20.
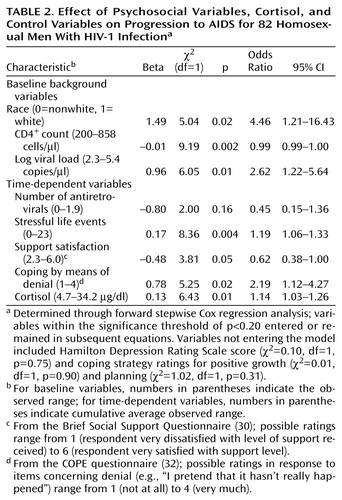
We ran a forward stepwise Cox regression analysis to calculate the risk of AIDS associated with the psychosocial variables (stressful life events, depressive symptoms, social support, three coping strategies) and cortisol, adjusting for control variables that remained in the model. Psychosocial variables and cortisol levels were treated as time-dependent covariates by using cumulative average values at regular intervals, the same method used for health habits (e.g., cumulative averages 6 months before each visit). We allowed any variable to enter and stay in the model if it was within the significance threshold of p<0.20.
Missing data points were estimated from the previous 6-month data for the small number of men who missed visits but continued study participation (eight men missed one visit, and three men missed from three to six visits). The background (time-independent) variables met the proportionality assumption (i.e., nonsignificance of the interactions between background variables and follow-up time). We checked the linearity of the covariate effects by adding in the squared term of each psychosocial covariate; the squared term was always nonsignificant. Outliers did not account for our results, which was determined by performing square root transformations of the psychosocial variables.
We computed descriptive statistics for all baseline control variables. For descriptive purposes, Kaplan-Meier estimates and Wilcoxon tests were computed to plot the survival probability for psychosocial variables, which were grouped by dividing subjects at the median on their average scores for these variables. Averages were computed for all time points 6 months before the visit when AIDS was diagnosed, or the average of all available time points 6 months before dropping out or last visit for those without AIDS.
Number of antiretroviral medications and the other health habit variables were not significantly associated with decreased risk of progression. (Only the former was kept in equations on the basis of our p<0.20 criterion.) No one used protease inhibitors before developing AIDS. In addition, we could not evaluate the effects of drug abuse/dependence, since only three men (10%) had these diagnoses at a time point before disease progression, and only five men (10%) who did not progress had these diagnoses during their study participation.
Results
Subjects
At entry, the 82 men were an average age of 30.3 years (SD=5.9), had 14.3 years of education (SD=2.4), and 79% (N=65) were white; all but one of the nonwhites were African American. At baseline, all men were clinically asymptomatic with a mean CD4+ lymphocyte count of 397.4/μl (SD=133.6). During the study, 37% (N=30) progressed to AIDS; the average time to AIDS progression was 2.98 years (SD=1.66). Almost all of the men who progressed to AIDS (90%, N=27) met AIDS criteria based solely on CD4+ decline to <200/μl. The average length of time in the study was 4.46 years (SD=2.49) for those without AIDS. The number of men studied each year (from entry through the seventh year), respectively, was 82, 77, 69, 63, 57, 48, 43, and 11 (the low number for year 7 resulted from many men not yet being scheduled for this visit).
For all visits, mean stressful life event scores ranged from 2.00 to 9.91 (individual scores ranged from 0 to 28), mean depressive symptom scores ranged from 1.09 to 2.93 (individual scores ranged from 0 to 21), mean social support scores ranged from 4.89 to 5.34, mean scores for denial as a coping strategy ranged from 1.15 to 1.53, and mean cortisol levels ranged from 9.08 to 13.24 mg/dl (individual scores ranged from 2.82 to 40.55, normal range=7–25 mg/dl).
Effects of Control Variables on Progression to AIDS
We used survival analysis to predict progression to AIDS. Table 1 shows the simultaneous effects of control variables, entered in two steps (baseline background first, and health habits second). Race, baseline CD4+ lymphocyte count, and baseline viral load were significant predictors of AIDS diagnosis. White men had 3.68 times the risk of developing AIDS compared to nonwhite men. For every 150 cell/μl decrease in baseline CD4+ T-helper count, men had about a twofold increased risk of AIDS. The risk of AIDS was increased 2.5-fold for every one log-unit increase in baseline viral load (or every 10-fold increase in actual viral load [e.g., from 1000 to 10,000 copies/μl]). Age and years of education were not significant predictors of disease progression and were dropped from subsequent analyses.
Effects of Psychosocial Variables and Cortisol Levels on Progression to AIDS
Table 2 shows the results of the forward stepwise Cox regression model with psychosocial variables and serum cortisol, adjusting for controls. Note that stressful events, support satisfaction, coping by means of denial, and cortisol levels were all significantly associated with progression to AIDS. For each 1-point increase in cumulative average stressful life events, the risk of AIDS increased by 19%. For every 4-point increase in cumulative average stressful life events, the equivalent of one severe stressor or two moderate stressors, the risk of AIDS was doubled. For each 1-point increase in cumulative average social support satisfaction, the risk of AIDS progression decreased by 62%. Stated another way, for each 1.5-point decrease in cumulative average support satisfaction, the risk of AIDS was about twofold. For every one-unit increase in cumulative average coping through denial, the risk of AIDS was doubled. Finally, for each one mg/dl increase in cumulative average cortisol, the risk of AIDS increased respectively by 14%; or was nearly doubled for each 5 mg/dl increase in cortisol. When the time-dependent predictors were measured as cumulative averages during the 1 year (as opposed to 6 months) before disease status, findings were nearly identical to those reported in Table 2 (e.g., stressful events [beta=0.17, χ2=4.78, df=1, p=0.03], support satisfaction [beta=–0.50, χ2=3.47, df=1, p=0.06], coping by means of denial [beta=0.64, χ2=2.43, df=1, p=0.12], cortisol [beta=0.14, χ2=5.80, df=1, p=0.02]; N=72, model df=8). Depressive symptoms and coping by means of positive growth or planning were not significantly related to disease progression in the stepwise Cox regression model.
We examined the effects of stressful life events on disease progression with cortisol (Table 2) and without cortisol in the model (stress beta=0.14, χ2=6.09, df=1, p=0.01, model df=7) to test whether the effects of stressful events might be explained by cortisol level. To the contrary, the effects of stressful life events were slightly augmented and not diminished by the inclusion of cortisol. The inclusion of cortisol in the equation also slightly increased the effects of the other psychosocial variables. Cortisol levels were not significantly correlated with stressful life events or depressive symptoms at most study visits. Furthermore, there were no significant interactions between cortisol levels and stressful events or between cortisol levels and other psychosocial variables in predicting AIDS. Interactions between all psychosocial variables were also not significant in predicting progression to AIDS.
In order to display the effects of psychosocial variables on disease progression, we plotted survival probabilities based on Kaplan-Meier estimates of the distributions of time until AIDS diagnosis, dividing subjects into those above or below the median score on stressful life events (5.67) and the median ratings for social support satisfaction (5.10) and coping by means of denial (1.31). A higher rate of progressing to AIDS was seen among subjects above the median number of stressful events (47% versus 27% of those below the median; χ2=8.69, df=1, p=0.003) and coping by means of denial (45% versus 28%; χ2=3.98, df=1, p=0.05). In addition, more subjects who were below the median for support satisfaction progressed to AIDS than did those above the median (49% versus 24%, respectively; χ2=4.43, df=1, p=0.04). As seen in Figure 1, at 90 months, those below the median on stressful life events had a 36% higher probability of being free of AIDS compared to those above the median. Likewise, those above the median on support satisfaction had a 39% higher probability of being free of AIDS compared to those below the median at 90 months. Finally, those below the median for coping by means of denial had a 14% higher probability of being free of AIDS at 90 months than those above the median.
Discussion
These findings extend our previous studies by showing that men with higher average serum cortisol and those who cope with the threat of AIDS by using strategies of denial have faster disease progression when followed for up to 7.5 years. Previously, we showed that cumulative stressful life events and social support were predictive of disease progression at 5.5 years. Two years later our data confirm these original findings with additional controls for baseline viral load (to approximate disease status at entry along with CD4+ count) and a more extensive list of health habits. The risk of AIDS was about doubled for every cumulative average increase in one severe stressful event, increase in one unit of the coping strategy of denial, increase in 5 mg/dl of cortisol, or decrease of 1.5 units of support satisfaction. Almost half the men above the median for stressful events and denial and below the median for social support satisfaction had progressed to AIDS during the study, compared to about one-quarter of those with fewer stressful events, less denial, and more support satisfaction. Finally, in attempting to understand a possible neuroendocrine mechanism underpinning the relationship of stressful events and disease progression, we did not find that cortisol levels were related to stressful events or that cortisol levels mediated or modified the increased risk of AIDS associated with stressful events (or any psychosocial variable). There were also no significant interactions between stressful events and coping or between any of the psychosocial variables.
Although cortisol levels did not mediate or modify the increased risk of AIDS due to stressful events, it is of interest that cortisol levels independently predicted disease progression. There are several pathways by which cortisol could affect change in immune function and disease progression. Cortisol may stimulate HIV-1 viral replication (8), modify programmed cell death, and alter the pattern of cytokines secreted (8, 9, 37, 38). Such changes have been associated with HIV-1 disease progression (9, 16). Our findings are consistent with those in depressed patients that have shown a negative relationship between cortisol levels and several measures of cellular immune status (39, 40). It should be emphasized, however, that there are conflicting reports throughout the literature regarding the neuroimmunomodulatory effects of glucocorticoids.
We must be cautious in interpreting the data from our study. First, we do not know whether our findings on homosexual men in North Carolina are generalizable to other populations (e.g., women, injecting drug users, other geographic areas). African Americans had slower disease progression, despite controlling for education and a variety of health habits. The race effect in our study may be limited to our group, since most other studies have not shown that African Americans have slower HIV-1 progression (2, 41). Third, we could not control for length of time with HIV-1 infection, since date of infection was unknown. However, our findings remained after controlling for baseline CD4+ count and viral load (two approximations of disease stage). Consistent with other studies (2, 41), we found that men with low CD4+ count and high viral load at baseline had faster disease progression. Neither poor health habits or number of antiretroviral medications accounted for the relationships evidenced in this study.
A fourth caution for interpreting the data is the problem of establishing causal direction of relationships of psychosocial variables and cortisol levels with disease progression. Do social support and denial contribute to disease progression, or do these variables reflect response bias resulting from disease progression? Is an increased cortisol level a result of advancing HIV-1 disease? To address causal direction, we used cumulative averages of predictor variables 6 months before AIDS was assessed. We also examined predictor variables averaged 1 year before assessing AIDS, and the results were similar. The measure of stressful life events was less likely to be confounded with disease progression because it was rated independently from the subject’s ratings and excluded events that might be caused by disease progression. Previously, we found that this measure of stressful events was consistently related to many indicators of disease progression (1, 4, 12, 13) and thus may provide the best evidence for the impact of psychosocial factors on morbidity in HIV-1.
In conclusion, these data provide prospective evidence that psychosocial factors and neuroendocrine function may accelerate HIV-1 disease progression. Men with more cumulative stressful life events, greater use of denial as a coping mechanism, less social support, and higher cortisol levels may be at greater risk for HIV-1 disease progression. Further research is needed to determine if psychological interventions (e.g., cognitive behavioral stress management) and pharmacological treatments can modify the effects of stressful events, denial, poor social support, and elevated cortisol levels, thereby altering the course of HIV-1 disease progression.
 |
 |
Received Oct. 6, 1999; revision received Feb. 15, 2000; accepted March 9, 2000. From the Department of Psychiatry, University of North Carolina School of Medicine; and the Department of Psychiatry, University of Pennsylvania School of Medicine, Philadelphia. Address reprint requests to Dr. Leserman, Department of Psychiatry, CB 7160, University of North Carolina School of Medicine, Chapel Hill, NC 27599-7160; [email protected] (e-mail).Supported in part by NIMH grants MH-44618 and MH-33127, NIH grant RR-00046, and NIH grant HD-37260 (UNC Center for AIDS Research).The authors thank Dr. Susan A. Fiscus for her help in obtaining viral load data.
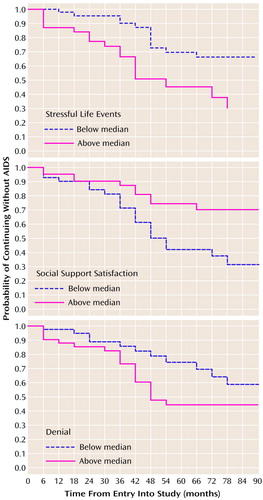
Figure 1. Effects of Stressful Life Events, Social Support Satisfaction, and Denial Coping on Time Until AIDS Diagnosis for 82 Homosexual Men With HIV-1 Infectiona
aThe median ratings for stressful events, social support satisfaction, and denial coping were 567, 5.10, and 1.31, respectively.
1. Leserman J, Jackson ED, Petitto JM, Golden RN, Silva SG, Perkins DO, Cai J, Folds JD, Evans DL: Progression to AIDS: the effects of stress, depressive symptoms, and social support. Psychosom Med 1999; 61:397–406Crossref, Medline, Google Scholar
2. Page-Shafer K, Delorenze GN, Satariano W, Winkelstein W Jr: Comorbidity and survival in HIV-infected men in the San Francisco Men’s Health Survey. Ann Epidemiol 1996; 6:420–430Crossref, Medline, Google Scholar
3. Ironson G, Friedman A, Klimas N, Antoni M, Fletcher MA, LaPerriere A, Simoneau J, Schneiderman N: Distress, denial, and low adherence to behavioral interventions predict faster disease progression in gay men infected with human immunodeficiency virus. Int J Behavioral Med 1994; 1:90–105Crossref, Medline, Google Scholar
4. Leserman J, Petitto JM, Perkins DO, Folds JD, Golden RN, Evans DL: Severe stress, depressive symptoms, and changes in lymphocyte subsets in human immunodeficiency virus-infected men. Arch Gen Psychiatry 1997; 54:279–285Crossref, Medline, Google Scholar
5. van Eck M, Berkhof H, Nicolson N, Sulon J: The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom Med 1996; 58:447–458Crossref, Medline, Google Scholar
6. Goodkin K, Feaster DJ, Tuttle R, Blaney NT, Kumar M, Baum MK, Shapshak P, Fletcher MA: Bereavement is associated with time-dependent decrements in cellular immune function in asymptomatic human immunodeficiency virus type 1-seropositive homosexual men. Clin Diagn Lab Immunol 1996; 3:109–118Medline, Google Scholar
7. Sapse AT: Cortisol, high cortisol diseases and anti-cortisol therapy. Psychoneuroendocrinology 1997; 22(suppl 1):S3–S10Google Scholar
8. Corley PA: Acquired immune deficiency syndrome: the glucocorticoid solution. Med Hypotheses 1996; 47:49–54Crossref, Medline, Google Scholar
9. Clerici M, Trabattoni D, Piconi S, Fusi ML, Ruzzante S, Clerici C, Villa ML: A possible role for the cortisol/anticortisols imbalance in the progression of human immunodeficiency virus. Psychoneuroendocrinology 1997; 22(suppl 1):S27–S31Google Scholar
10. Antoni MH, Goldstein D, Ironson G, LaPerriere A, Fletcher MA, Schneiderman N: Coping responses to HIV-1 serostatus notification predict concurrent and prospective immunologic status. Clin Psychol Psychother 1995; 2:234–248Crossref, Google Scholar
11. Solano L, Costa M, Salvati S, Coda R, Aiuti F, Mezzaroma I, Bertini M: Psychological factors and clinical evolution in HIV-1 infection: a longitudinal study. J Psychosom Res 1993; 37:39–51Crossref, Medline, Google Scholar
12. Evans DL, Leserman J, Perkins DO, Stern RA, Murphy C, Tamul K, Liao D, van der Horst CM, Hall CD, Folds JD, Golden RN, Petitto JM: Stress-associated reductions of cytotoxic T lymphocytes and natural killer cells in asymptomatic HIV infection. Am J Psychiatry 1995; 152:543–550Link, Google Scholar
13. Evans DL, Leserman J, Perkins DO, Stern RA, Murphy C, Zheng B, Gettes D, Longmate JA, Silva SG, van der Horst CM, Hall CD, Folds JD, Golden RN, Petitto JM: Severe life stress as a predictor of early disease progression in HIV infection. Am J Psychiatry 1997; 154:630–634Link, Google Scholar
14. Kemeny ME, Dean L: Effects of AIDS-related bereavement on HIV progression among New York City gay men. AIDS Educ Prev 1995; 7:36–47Medline, Google Scholar
15. Swanson B, Zeller JM, Spear GT: Cortisol upregulates HIV p24 antigen production in cultured human monocyte-derived macrophages. J Assoc Nurses AIDS Care 1998; 9:78–83Crossref, Medline, Google Scholar
16. Maggi E, Mazzetti M, Ravina A, Annunziato F, de Carli M, Piccinni MP, Manetti R, Carbonari M, Pesce AM, del Prete G: Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science 1994; 265:244–248Crossref, Medline, Google Scholar
17. Graziosi C, Pantaleo G, Gantt KR, Fortin JP, Demarest JF, Cohen OJ, Sekaly RP: Lack of evidence for the dichotomy of TH1 and TH2 predominance in HIV-infected individuals. Science 1994; 265:248–252Crossref, Medline, Google Scholar
18. Gorman JM, Kertzner R, Cooper T, Goetz RR, Lagomasino I, Novacenko H, Williams JBW, Stern Y, Mayeux R, Ehrhardt AA: Glucocorticoid level and neuropsychiatric symptoms in homosexual men with HIV infection. Am J Psychiatry 1991; 148:41–45Link, Google Scholar
19. Kertzner RM, Goetz R, Todak G, Cooper T, Lin S-H, Reddy MM, Novacenko H, Williams JBW, Ehrhardt AA, Gorman JM: Cortisol levels, immune status, and mood in homosexual men with and without HIV infection. Am J Psychiatry 1993; 150:1674–1678Google Scholar
20. Antoni MH, Schneiderman N, Klimas N, LaPerriere A, Ironson G, Fletcher MA: Disparities in psychological, neuroendocrine, and immunologic patterns in asymptomatic HIV-1 seropositive and seronegative gay men. Biol Psychiatry 1991; 29:1023–1041Google Scholar
21. Petitto JM, Leserman J, Perkins DO, Stern RA, Silva SG, Gettes D, Zheng B, Folds JD, Golden RN, Evans DL: High versus low basal cortisol secretion in asymptomatic, medication-free HIV infected men: differential effects of severe life stress on parameters of immune status. Behav Med 2000; 25:143–151Crossref, Medline, Google Scholar
22. Mayne TJ, Vittinghoff E, Chesney MA, Barrett DC, Coates TJ: Depressive affect and survival among gay and bisexual men infected with HIV. Arch Intern Med 1996; 156:2233–2238Google Scholar
23. Lyketsos CG, Hoover DR, Guccione M, Senterfitt W, Dew MA, Wesch J, VanRaden MJ, Treisman GJ, Morgenstern H: Depressive symptoms as predictors of medical outcomes in HIV infection. JAMA 1993; 270:2563–2567Google Scholar
24. Lyketsos CG, Hoover DR, Guccione M, Dew MA, Wesch JE, Bing EG, Treisman GJ: Changes in depressive symptoms as AIDS develops. Am J Psychiatry 1996; 153:1430–1437Google Scholar
25. Mulder CL, Antoni MH, Duivenvoorden HJ, Kauffmann RH, Goodkin K: Active confrontational coping predicts decreased clinical progression over a one-year period in HIV-infected homosexual men. J Psychosom Res 1995; 39:957–965Crossref, Medline, Google Scholar
26. Miller AH, Asnis GM, Lackner C, Halbreich U, Norin AJ: Depression, natural killer cell activity, and cortisol secretion. Biol Psychiatry 1991; 29:878–886Crossref, Medline, Google Scholar
27. Dohrenwend BS, Krasnoff L, Askenasy AR, Dohrenwend BP: Exemplification of a method for scaling life events: the PERI Life Events Scale. J Health Soc Behav 1978; 19:205–229Crossref, Medline, Google Scholar
28. Brown GW, Harris T: Social Origins of Depression: A Study of Psychiatric Disorder in Women. New York, Free Press, 1978Google Scholar
29. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
30. Sarason IG, Sarason BR, Shearin EN, Pierce GR: A brief measure of social support: practical and theoretical implications. J Social and Personal Relationships 1987; 4:497–510Crossref, Google Scholar
31. Leserman J, DiSantostefano R, Perkins DO, Murphy C, Golden RN, Evans DL: Longitudinal study of social support and social conflict as predictors of depression and dysphoria among HIV-positive and HIV-negative men. Depression 1994; 2:189–199Crossref, Google Scholar
32. Carver CS, Scheier MF, Weintraub JK: Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol 1989; 56:267–283Crossref, Medline, Google Scholar
33. Leserman J, Perkins DO, Evans DL: Coping with the threat of AIDS: the role of social support. Am J Psychiatry 1992; 149:1514–1520Google Scholar
34. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep 1992; 41(RR-17):1–19Google Scholar
35. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Edition (SCID-NP, Version 1.0). Washington, DC, American Psychiatric Press, 1990Google Scholar
36. Allison PD: Survival Analysis Using the SAS System: A Practical Guide, 2nd ed. Cary, NC, SAS Institute, 1995, pp 61–184Google Scholar
37. Daynes RA, Meikle AW, Araneo BA: Locally active steroid hormones may facilitate compartmentalization of immunity by regulating the types of lymphokines produced by helper T cells. Res Immunol 1991; 142:40–45Crossref, Medline, Google Scholar
38. Daynes RA, Araneo BA, Hennebold J, Enioutina E, Mu HH: Steroids as regulators of the mammalian immune response. J Invest Dermatol 1995; 105:14S–19SCrossref, Medline, Google Scholar
39. Maes M, Vandoolaeghe E, Ranjan R, Bosmans E, Van Gastel A, Bergmans R, Desnyder R: Increased serum soluble CD8 or suppressor/cytotoxic antigen concentrations in depression: suppressive effects of glucocorticoids. Biol Psychiatry 1996; 40:1273–1281Google Scholar
40. Maes M, Bosmans E, Suy E, Minner B, Raus J: A further exploration of the relationships between immune parameters and the HPA-axis activity in depressed patients. Psychol Med 1991; 21:313–320Crossref, Medline, Google Scholar
41. Poole WK, Fulkerson W, Lou Y, Kvale P, Hopewell PC, Hirschtick R, Glassroth J, Rosen M, Mangura B, Wallace J, Markowitz N (Pulmonary Complications of Human Immunodeficiency Virus Infection Study Group): Overall and cause-specific mortality in a cohort of homo-/bisexual men, injecting drug users, and female partners of HIV-infected men. AIDS 1996; 10:1257–1264Google Scholar



