Relationship Between Dopamine D2 Occupancy, Clinical Response, and Side Effects: A Double-Blind PET Study of First-Episode Schizophrenia
Abstract
OBJECTIVE: Since all antipsychotics block dopamine D2 receptors, the authors investigated how well D2 receptor occupancy in vivo predicts clinical response, extrapyramidal side effects, and hyperprolactinemia. METHOD: In a double-blind study, 22 patients with first-episode schizophrenia were randomly assigned to 1.0 or 2.5 mg/day of haloperidol. After 2 weeks of treatment, D2 receptor occupancy was determined with [11C]raclopride and positron emission tomography, and clinical response, extrapyramidal side effects, and prolactin levels were measured. Patients who showed adequate responses continued taking their initial doses, those who did not respond had their doses increased to 5.0 mg/day, and evaluations were repeated at 4 weeks for all patients. RESULTS: The patients showed a wide range of D2 occupancy (38%–87%). The degree of receptor occupancy predicted clinical improvement, hyperprolactinemia, and extrapyramidal side effects. The likelihood of clinical response, hyperprolactinemia, and extrapyramidal side effects increased significantly as D2 occupancy exceeded 65%, 72%, and 78%, respectively. CONCLUSIONS: The study confirms that D2 occupancy is an important mediator of response and side effects in antipsychotic treatment. The data are consistent with a “target and trigger” hypothesis of antipsychotic action, i.e., that the D2 receptor specificity of antipsychotics permits them to target discrete neurons and that their antagonist properties trigger within those neurons intracellular changes that ultimately beget antipsychotic response. While limited to haloperidol, the relationship between D2 occupancy and side effects in this study helps explain many of the observed clinical differences between typical and atypical antipsychotics.
While the precise mechanism of antipsychotic response is not known, antagonism of dopamine transmission is likely to play an important role. The dopamine system is a neuromodulatory system that arises from cells in the midbrain and has discrete projections to the mesolimbic, striatal, and cortical areas of the brain. These midbrain neurons release dopamine, which acts by means of actions on dopamine receptors. There are five known dopamine receptors, classified into “families” on the basis of similarities in gene-sequence and functional effects. The D1 family includes the D1 and D5 receptors, while the dopamine D2 family includes D2, D3, and D4 receptors (1). Of these, the dopamine D2 family, and in particular the dopamine D2 receptor, has been of sustained interest from the point of view of antipsychotics. In vitro studies show that all antipsychotics bind to the dopamine D2 receptor (2, 3), and in most cases the clinical doses correlate with their affinity for the dopamine D2 receptor (2, 3). This correlation is not seen for any other receptor, dopamine or otherwise. At the same time, the blockade of dopamine D2 receptors is thought to be responsible for the antipsychotic-induced parkinsonian side effects (4, 5) and prolactin elevation (6). Over two dozen studies using positron emission tomography (PET) (7–12) and single photon emission computed tomography (SPECT) (13, 14) with benzamide (8, 11, 13, 14) and spiperone (7, 9, 10) ligands have confirmed that all antipsychotics block dopamine D2 receptors in patients, albeit to varying degrees. However, all these reports, with few exceptions (10, 12), are based on cross-sectional or uncontrolled study designs, with open rating of clinical response and side effects and with high levels of D2 occupancy. Thus, while there can be no doubt that antipsychotics block dopamine D2 receptors, the quantitative relationship between in vivo blockade of dopamine D2 receptors, clinical response, extrapyramidal side effects, and prolactin elevation is still unclear.
The primary objective of this study was to examine whether D2 receptor occupancy, as induced by haloperidol, predicts three aspects of clinical outcome:1) clinical response, 2) extrapyramidal side effects and akathisia, and 3) prolactin elevation. We were also interested in evaluating whether one could identify the quantitative relationship between D2 occupancy and the clinical outcomes such that this information could guide routine treatment. The ability to detect the relationship between D2 occupancy and response is reduced if there is a sizable proportion of nonresponders in the study group. Since populations of chronically treated patients with schizophrenia have a high representation of treatment-resistant patients and have already been exposed to a D2-blocking antipsychotic, we chose to ask this question regarding patients with a first episode of schizophrenia. Furthermore, almost all previous studies relating D2 occupancy to outcome variables have been cross-sectional with open ratings, and we wanted to increase the validity of our conclusions by opting for a prospective design with double-blind ratings.
METHOD
The study was undertaken at the Clarke Division of the Centre for Addiction and Mental Health, Toronto. Eligible subjects, inpatients as well as outpatients, were included after they provided written consent using forms and procedures approved by the University of Toronto Human Subjects Review Committee. The study was restricted to patients between the ages of 18 and 50 who 1) met the DSM-IV criteria for schizophrenia or provisional schizophreniform disorder, 2) had never received antipsychotic treatment or had received antipsychotic treatment for less than 6 weeks and had been drug free for at least 2 weeks at inclusion, and 3) had no major medical or neurological illness or history of major head injury. Subjects were excluded if they had a lifetime history of substance dependence or a current diagnosis of substance abuse according to DSM-IV criteria. The diagnoses were established by using a structured interview of the patient by means of the Structured Clinical Interview for DSM-IV (SCID) (15), supplemented with information from the family and the clinical staff.
The study was carried out in a double-blind fashion, and the patients were randomly assigned to doses of 1.0 or 2.5 mg of haloperidol per night. These doses were chosen on the basis of previous PET occupancy data (16, 17) to give a wide range of dopamine D2 occupancy without floor or ceiling effects. Clinical status was assessed by using the Clinical Global Impression Scale (CGI) (18) for rating improvement, Positive and Negative Syndrome Scale (19) for assessing clinical symptoms, Extrapyramidal Symptom Rating Scale (20) for assessing drug-induced extrapyramidal side effects, and the Barnes Akathisia Rating Scale (21) for assessing akathisia.
Only lorazepam and benztropine were allowed as adjunctive medications. The decisions regarding their use were made by the treating physicians on the basis of clinical considerations. The use of lorazepam (up to 6 mg/day) was indicated for daytime anxiety, agitation, akathisia, and insomnia. Benztropine (up to 6 mg/day) was used for extrapyramidal side effects and for akathisia.
After 2 weeks of continuous treatment, the patients each had a PET scan with [11C]raclopride to determine their levels of D2 occupancy. All scans were carried out 12 to 13 hours after the administration of the nightly dose of haloperidol. Clinical status and side effects were rated at this time by a rater blind to occupancy results, using the aforementioned scales. Patients who scored 2 or lower on the CGI improvement measure (i.e., were much improved or very much improved) were classified as responders, while those who scored 3 or higher (minimal improvement, no change, or worsening) were considered nonresponders. The responders had their doses held at the initial levels, while the nonresponders had their doses increased to 5.0 mg/day of haloperidol for the next 2 weeks. The purpose of this second stage of the experiment was to evaluate whether the nonresponders could be converted to responders when their doses were increased to give greater than 80% D2 occupancy. The dose of 5.0 mg/day was chosen because previous data suggested that it would lead to greater than 80% dopamine D2 occupancy for almost all patients (16). The treatment was then continued, at the initial dose (for responders) or at the increased dose (for nonresponders), for a further period of 2 weeks. Ratings on the clinical scales were repeated at the end of this phase.
D2 receptor occupancy was determined by using 9.7 mCi (SD=0.5) of high-specific-activity (970 Ci/mmol, SD=420) [11C]raclopride administered as a bolus plus continuous infusion. Imaging was performed with a GE-2048-15B head scanner as described in previous publications (8, 22). A magnetic resonance imaging (MRI) scan was obtained on each of the patients (GE Signa 1.5-T scanner, proton density maps) and was coregistered to the composite [11C]raclopride PET scan by using automatic image registration (23, 24) software, provided by Dr. Roger Woods, University of California, Los Angeles. Since the PET scan has a more limited axial field of view than the MRI scan and the two techniques differ in their resolution and interslice intervals, the coregistration usually resulted in a slight misalignment. Therefore, rather than draw regions of interest on the MRI scan, we drew the striatal (caudate plus putamen) and cerebellar regions of interest on two contiguous PET slices with reference to an overlapping coregistered MRI scan. The cerebellar time-activity curve was taken as an estimate of the free and nonspecific [11C]raclopride binding (25), while the striatal time-activity curve provided an estimate of specific binding to the D2 receptors plus free and nonspecific binding. Under these assumptions, it can be shown that the striatal-cerebellar ratio minus one, at the time when the binding is at equilibrium (30–75 minutes in the aforementioned scans), provides an index proportional to the Bmax/Kd ratio of [11C]raclopride for dopamine D2 receptors (referred to as the binding potential). In previous studies (22) we have demonstrated that this ratio method correlates very well (r>0.95) with analytically derived estimates of D2 binding potential, is highly reliable with a scan-rescan standard deviation of 6%, and has been standardized in our laboratory with interrater and intrarater reliability (intraclass correlation coefficients: r>0.95). There was no significant hemispheric asymmetry in the D2 binding potential; therefore, data from the left and right striatum were pooled for all subsequent calculations. Since we did not have baseline measures of D2 binding potential for these patients, we used an age-corrected estimate from a separate group of 12 antipsychotic-naive patients with schizophrenia and 15 age-matched healthy subjects who functioned as a comparison group. The pooling of patients and healthy subjects was justified as there was no significant difference in D2 binding potential between groups (F=0.36, df=1, 24, p=0.55), nor was there a significant age-by-illness interaction (F=0.58, df=1, 24, p=0.45), findings consistent with those from previous studies that examined D2 binding potential with raclopride in patients with schizophrenia (26, 27).
The haloperidol levels were determined by using a gas chromatograph/mass spectrometer as described previously (16). Prolactin levels were determined by using a two-site chemoluminometric immunoassay with a minimum detectable limit of 0.3 ng/ml and a coefficient of variance of 4.5%.
All subjects who completed 2 weeks of treatment and for whom PET data were available were included in the analysis. Since the clinical decision hinged on the CGI rating, that was taken as the primary response variable and the scores on the Positive and Negative Syndrome Scale subscales were used to provide a more detailed profile of clinical response. Four subjects developed extrapyramidal side effects or akathisia in the first 2 weeks, and adjunctive treatment was instituted for three of these four before the PET scan. As a result of this concomitant treatment, the severity of extrapyramidal side effects at the time of the PET scan was probably underestimated in these cases. For this reason, for the purposes of this analysis, extrapyramidal side effects and akathisia were rated only as present or absent, without an effort to relate the quantitative degree of extrapyramidal side effects to the degree of D2 receptor occupancy. Hyperprolactinemia for the purposes of this study was defined by reference to the normal ranges established for this assay, which are 2.1–17.7 ng/ml for men and 2.8–29.2 ng/ml for nonpregnant women. The statistical analyses were carried out in Statistica for Windows (Stat Soft, Inc., Tulsa, Okla.).
RESULTS
Of the 26 patients who entered the study, three did not complete 2 weeks: two (receiving 1.0 and 2.5 mg/day) because of clinical worsening and the third (2.5 mg/day) because of notable extrapyramidal side effects. PET data could not be obtained for one patient because of equipment failure. Of the remaining 22, five were women and 17 were men; 19 were antipsychotic naive. Their median age was 30 years (range=19–45), and they had a median duration of psychotic symptoms of 52 weeks (range=4–260 weeks). Eighteen of these patients met the criteria for schizophrenia, and four met criteria for provisional schizophreniform disorder at the time of inclusion in the study. Of those initially diagnosed with schizophreniform disorder, three were finally diagnosed with schizophrenia, and one received the diagnosis of delusional disorder.
The patients’ illnesses were of moderate to marked severity according to the CGI severity scale (mean score=4.5, SD=0.6), and positive symptoms predominated (Positive and Negative Syndrome Scale positive syndrome score: mean=20.9, SD=3.8) over negative symptoms (negative syndrome score: mean=15.9, SD=6.5). Thirteen of these patients received 1.0 mg/day of haloperidol, and nine received 2.5 mg/day. The D2 occupancy varied from 38% to 87% and was significantly related to dose (1.0-mg/day group: mean=59%, SD=11%; 2.5-mg/day group: mean=75%, SD=6%) (F=15.9, df=1, 20, p<0.001). Occupancy was highly correlated with plasma haloperidol level (F=15.9, df=1, 19, p<0.001) but was not related to any other baseline variable (age, sex, or severity of illness at baseline). As adjunctive medication four patients received lorazepam, and two others received benztropine. Ten patients were considered responders: three (23%) of the 13 who received 1.0 mg/day and seven (78%) of the nine who received 2.5 mg/day. Twelve patients were judged to be nonresponders: 10 receiving 1.0 mg/day and two receiving 2.5 mg/day. The responders showed a greater improvement in positive symptoms; they had a decrease in the Positive and Negative Syndrome Scale positive syndrome score of 8.5 (SD=4.2), as compared to 1.3 (SD=2.4) for the nonresponders (F=24.2, df=1, 20, p<0.0001). On the other hand, the overall improvement in negative symptoms was low regardless of responder status. The negative syndrome score improved by 2.2 (SD=4.4) for the responders and 1.6 (SD=1.3) for the nonresponders (F=0.2, df=1, 20, p=0.64).
The responders showed significantly higher D2 occupancy (mean=73%, SD=9%) than the nonresponders (mean=60%, SD=12%) (F=8.3, df=1, 20, p<0.009) (figure 1A). The D2 occupancy significantly predicted the degree of clinical response as measured with the CGI (F=9.3, df=1, 20, p=0.006) (figure 1A). The relationship between D2 occupancy and improvement in CGI rating was equally maintained in a multiple regression model that included baseline severity as a covariate (omnibus F=4.4, df=2, 19, p=0.02); the relationship between baseline severity and improvement was nonsignificant (t=0.02, df=19, p=0.98), and the relationship between D2 occupancy and improvement was highly significant (t=2.86, df=19, p=0.009). However, the relationships of D2 occupancy to improvement in the scores on the Positive and Negative Syndrome Scale positive syndrome scale (F=2.8, df=1, 20, beta=0.35, p=0.10) and negative syndrome scale (F=0.0, df=1, 20, beta=0.11, p=0.90) did not achieve significance.
We were interested in determining whether a threshold for clinical response existed in the data. Ideally, a threshold would be a level of D2 occupancy such that there are no responders below it and everyone above that level obtains equivalent response. We did not find such an absolute threshold. However, a cutoff at 65% D2 occupancy provided optimal separation: 80% of the responders were above it while 67% of the nonresponders were below it (p=0.04, Fisher’s exact test). Furthermore, when we restricted the analysis to subjects who were above this threshold of 65%, we did not find a relationship between clinical response and D2 occupancy (multiple regression, baseline severity as measured with the CGI and D2 occupancy as independent variables; omnibus F=0.9, df=2, 9, p=0.45; test for the effect of D2 occupancy: t=1.20, df=9, p=0.25).
Of the 10 responders, nine had maintained or improved responses over the next 2 weeks, while one patient (with 62% D2 occupancy) showed a decline in response. Of the 12 nonresponders, whose doses were increased to 5.0 mg/day, one had an acute dystonic reaction after the first dose of 5.0 mg of haloperidol and left the study. Of the remaining 11, seven achieved responder status while four continued to be nonresponders. Six of the seven patients who had occupancies below 65% before the dose increase showed improvement, while only one of the four who had occupancies above 65% showed improvement.
Only three of the 23 patients who completed 2 weeks showed extrapyramidal side effects at 2 weeks. Patients who showed drug-induced extrapyramidal side effects had significantly higher D2 occupancies (mean=80%, SD=0%, N=3) than patients without extrapyramidal side effects (mean=64%, SD=12%, N=20) (Mann-Whitney U Test: z=2.4, exact p=0.009). For clinical considerations, benztropine was prescribed for two of these three patients, and lorazepam was prescribed for one. Four patients showed symptoms of akathisia; three of them were the same ones who experienced the extrapyramidal side effects just noted. The patients with akathisia as a group showed higher occupancies (mean=81%, SD=4%, N=4) than those without (mean=63%, SD=12%, N=19) (Mann-Whitney U Test: z=3.1, exact p=0.0002). None of the patients with D2 occupancies below 78% showed extrapyramidal side effects or akathisia (figure 1A).
Among the nonresponders whose doses were increased to 5.0 mg/day, there were several new cases of extrapyramidal side effects. One patient developed an acute dystonic reaction, two developed extrapyramidal side effects, two developed akathisia, and another two patients exhibited both extrapyramidal side effects and akathisia at the 4-week mark. Thus, seven of the 12 patients who received 5.0 mg/day of haloperidol manifested either extrapyramidal side effects or akathisia, as compared to only three of the 23 patients who were treated with 1.0 or 2.5 mg/day (p=0.008, Fisher’s exact test).
Finally, D2 occupancy significantly predicted an abnormally high prolactin level at the time of PET scanning (F=7.3, df=1, 20, p<0.01). The likelihood of hyperprolactinemia was 15% with a D2 occupancy below 72%, and the likelihood was 86% at occupancies higher than 72% (p=0.002, Fisher’s exact test) (figure 1B).
DISCUSSION
The results of our study show that striatal D2 occupancy, as induced by haloperidol, predicts antipsychotic response, drug-induced extrapyramidal side effects, akathisia, and prolactin elevation. There is also evidence for a stepped increase in response beyond 65% D2 occupancy; prolactin elevation became prominent beyond 72%, while extrapyramidal side effects and akathisia were evident beyond 78%.
To our knowledge, only one previous study has systematically explored the relationship between D2 occupancy and clinical response. Nordstrom et al. (12) studied 12 patients with schizophrenia treated with an experimental antipsychotic, raclopride, and reported a significant relationship between D2 occupancy and clinical response. Our data confirm this initial report in much larger study group and extend its applicability to a clinically relevant typical antipsychotic. Since 65%–70% D2 occupancy was obtained with 2.5 mg/day of haloperidol in a majority of the patients, our data suggest that a dose in the range of 2–3 mg/day should be the optimal starting dose for first-episode patients. This dose contrasts to those in previous clinical studies (28, 29), which started treatment at haloperidol doses in the range of 10–20 mg/day for first-episode patients. Such doses would lead to occupancies beyond the cutoff for extrapyramidal side effects discerned in this study, and in keeping with that, 78%–85% of the patients in those high-dose studies experienced extrapyramidal side effects (28μ30). To our knowledge, only two controlled studies of first-episode patients have tested lower doses (31, 32). These studies indicated that 1–4 mg/day (31) or 2–5 mg/day (32) of haloperidol is the optimal dose for patients with a first episode of schizophrenia. Higher doses in both studies were associated with a higher incidence of extrapyramidal side effects without a significant improvement in clinical response (30–32).
While previous cross-sectional neuroimaging studies have suggested a relationship between D2 occupancy and extrapyramidal side effects (11, 12, 14, 33), our study not only confirms this relationship with a prospective design but provides evidence that clinical extrapyramidal side effects emerge only beyond 78% D2 occupancy. This situation is similar to idiopathic Parkinson’s disease, in which a significant loss of dopaminergic transmission occurs before clinical symptoms become evident (34, 35). Our data also confirm previous associations between D2 occupancy and prolactin elevation (7, 36) and extend them by showing that a high level of blockade (approximately 72%) is necessary before prolactin elevation becomes manifest. The extrapyramidal side effects and hyperprolactinemia findings suggest that there is a functional reserve in the D2-mediated systems. As a result, these side effects are not a linear function of receptor blockade but become evident only after a high proportion of receptors have been blocked.
The aim of an optimal treatment strategy is to achieve the highest rate of response with the lowest incidence of side effects. The therapeutic window suggested by our data is rather narrow: from 65% (for response) to 72% (when side effects emerge). This window corresponds to less than 0.5 mg/day of haloperidol for a given patient. Given this narrow therapeutic window and the wide interindividual variation in D2 occupancy at a given dose (8, 16), one cannot recommend a single dose of haloperidol that will get most patients above 65% without pushing them into the occupancies associated with side effects. This explains why clinically it has been difficult to obtain clinical response without a high incidence of side effects in the use of haloperidol-like agents.
Several limitations qualify the preceding results. First, mesolimbic D2 receptors are thought to be more relevant to the induction of clinical response (37), and pituitary D2 receptors mediate prolactin levels (6). However, we could not measure D2 occupancy in these regions as extrastriatal regions cannot be reliably quantified with [11C]raclopride and PET. Two studies (38, 39) have shown no significant difference between striatal and extrastriatal D2 occupancy in patients treated with haloperidol. Therefore, striatal occupancy is a reasonable surrogate of mesolimbic D2 occupancy, at least in the case of haloperidol. Second, this study is limited in that patients were given only 2 weeks to respond at the lower doses. This duration reflects a compromise. To increase the validity of this study we wanted to expose patients to low D2 occupancies, lower than what had been tested before. On the other hand, there was concern that low doses/occupancies may not be optimally antipsychotic, and therefore we wanted to keep the duration of such exposure short. The short duration increases the risk of missing a relationship because of limited power. Since we found significant positive relationships in this study, this is less of a concern. However, the question of whether patients with lower D2 occupancies would have responded if given a longer period of time still remains.
In addition to the aforementioned limitations, our study does not address several important questions. Clinical studies suggest that patients who have been previously treated may need higher doses than first-episode patients (31). It is unclear why this is the case. Whether the chronic patients achieve lower occupancies for a given drug/plasma level because of better drug disposition or up-regulation of receptors, or whether chronic treatment alters the basic relationship between occupancy and clinical correlates, is a question for the future. Finally, our data apply mainly to antipsychotics whose predominant mode of action is D2 blockade. Several newer, “atypical” antipsychotics (e.g., clozapine, risperidone, olanzapine) show a higher affinity for the serotonin, adrenergic, and histaminergic receptors than for D2 receptors (40), and these additional properties may enhance their efficacy (41, 42) or diminish the side effects induced by D2 blockade (43–45). Thus, the relationship of D2 blockade to clinical response and side effects in this study may be different with these newer medications (14, 46–48), a hypothesis that has yet to be directly tested. Most of the current studies of typical versus atypical antipsychotics have used doses of haloperidol (10–20 mg/day) that give inordinately high levels of D2 occupancy and have compared these doses to doses of the atypical agent that give 60%–80% D2 occupancy. These studies have routinely shown more extrapyramidal side effects with haloperidol than with the atypical agents, but it is unclear whether this difference reflects the different levels of D2 occupancy induced by the drugs or some other fundamental difference between the typical and atypical agents (48).
The more interesting question that this relationship raises is one of causality and mechanism. How does D2 blockade mediate response? At this stage one can offer only logical speculations. While D2 blockade is essential, it does not temporally correspond with clinical response. The D2 occupancy is achieved within hours after the start of treatment, while response takes days. Conversely, when the drug is stopped, the occupancy declines in days while the rate of relapse has a half-life of many months. This suggests that D2 blockade induces response by invoking downstream changes, which are delayed but enduring. And these alterations lead to the physiological changes that are the ultimate mediator of clinical improvement. These downstream changes are neurochemically nonspecific (e.g., the changes in cAMP, immediate early genes, and glucose metabolism, etc., that can also be observed with other modulators) but are distinguished by their anatomically specificity (happen only in circuits linked to cells with D2 receptors). Furthermore, these intracellular and gene expression changes are associated with changes in D2 occupancy, as opposed to sustained high D2 occupancy, i.e., they are triggered by D2 occupancy rather than being maintained by continuous occupancy. Thus, we believe that blockade of D2 receptors targets the appropriate anatomical circuits and triggers within them intracellular changes that lead to clinical improvement. The precise circuits and the crucial intracellular changes are a matter of active research, but the target-and-trigger conception of the role of D2 occupancy helps in narrowing the possible candidates.
In summary, we find that dopamine D2 occupancy predicts short-term clinical response, extrapyramidal side effects, and hyperprolactinemia. The likelihood of clinical response increases as D2 occupancies exceed 65%–70%, while the risks of hyperprolactinemia and extrapyramidal side effects or akathisia increase at occupancies higher than 72% and 78%, respectively. Therefore, low doses of typical antipsychotics, in the range of 1–3 mg/day of haloperidol, should be used as initial doses for first-episode patients.
Received March 17, 1999; revisions received July 23 and Aug. 16, 1999; accepted Aug. 24, 1999. From the Schizophrenia and Continuing Care Program and the PET Centre, Centre for Addiction and Mental Health, Department of Psychiatry, University of Toronto. Address reprint requests to Dr. Kapur, PET Centre, Clarke Division of the Centre for Addiction and Mental Health, 250 College St., Toronto, ON, Canada M5T 1R8; [email protected] (e-mail). Supported by a grant from the National Alliance for Research on Schizophrenia and Depression, U.S.A. Dr. Kapur was supported by a Clinician Scientist award from the Medical Research Council of Canada and by a Scholar Research Award of the EJLB Foundation. Astra Arcus, A.B., provided the precursor for synthesis of [11C]raclopride. The authors thank the patient volunteers, their families, and the clinical staff involved in patient care, especially Drs. Paul Roy and C.S. Shammi; Erin Toole, Doug Hussey, Kevin Cheung, Armando Garcia, and Jenny Lee for technical assistance; Kalyna Butler and Piri Babos for pharmacy services; and Dr. Alan Wilson for supervising the radiochemical syntheses.
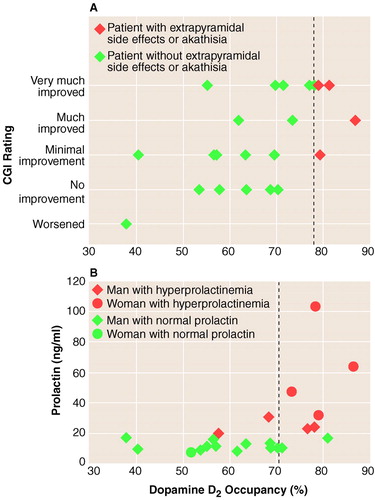
FIGURE 1. Relation of Dopamine D2 Occupancy to CGI-Rated Clinical Response (A) and Prolactin Level (B) Among Patients With First-Episode Schizophrenia Receiving Haloperidola
aDotted line in part A indicates 78% D2 occupancy, which was associated with a significantly greater likelihood of extrapyramidal side effects or akathisia. Dotted line in part B indicates 72% D2 occupancy, which was associated with a significantly greater likelihood of hyperprolactinemia.
1. Palermo-Neto J: Dopaminergic systems: dopamine receptors. Psychiatr Clin North Am 1997; 20:705–721Crossref, Medline, Google Scholar
2. Seeman P, Lee T, Chau-Wong M, Wong K: Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 1976; 261:717–719Crossref, Medline, Google Scholar
3. Seeman P, Corbett R, Van Tol HHM: Atypical neuroleptics have low affinity for dopamine D2 receptors or are selective for D4 receptors—reply. Neuropsychopharmacology 1997; 16:127–135Crossref, Google Scholar
4. Sanberg PR: Haloperidol-induced catalepsy is mediated by postsynaptic dopamine receptors. Nature 1980; 284:472–473Crossref, Medline, Google Scholar
5. Casey DE: Dopamine D1 (SCH 23390) and D2 (haloperidol) antagonists in drug-naive monkeys. Psychopharmacology (Berl) 1992; 107:18–22Crossref, Medline, Google Scholar
6. Moore KE, Lookingland KJ: Dopaminergic neuronal systems in the hypothalamus, in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom FE, Kupfer DJ. New York, Raven Press, 1994, pp 245–256Google Scholar
7. Baron JC, Martinot JL, Cambon H, Boulenger JP, Poirier MF, Caillard V, Blin J, Huret JD, Loc’h C, Maziere B: Striatal dopamine receptor occupancy during and following withdrawal from neuroleptic treatment: correlative evaluation by positron emission tomography and plasma prolactin levels. Psychopharmacology (Berl) 1989; 99:463–472Crossref, Medline, Google Scholar
8. Kapur S, Remington G, Jones C, Wilson A, DaSilva J, Houle S, Zipursky R: High levels of dopamine D2 receptor occupancy with low-dose haloperidol treatment: a PET study. Am J Psychiatry 1996; 153:948–950Link, Google Scholar
9. Goyer PF, Berridge MS, Morris ED, Semple WE, Compton-Toth BA, Schulz SC, Wong DF, Miraldi F, Meltzer HY: PET measurement of neuroreceptor occupancy by typical and atypical neuroleptics. J Nucl Med 1996; 37:1122–1127Google Scholar
10. Wolkin A, Barouche F, Wolf AP, Rotrosen J, Fowler JS, Shiue C-Y, Cooper TB, Brodie JD: Dopamine blockade and clinical response: evidence for two biological subgroups of schizophrenia. Am J Psychiatry 1989; 146:905–908Link, Google Scholar
11. Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G: Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine: relation to extrapyramidal side effects. Arch Gen Psychiatry 1992; 49:538–544Crossref, Medline, Google Scholar
12. Nordstrom AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C, Uppfeldt G: Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects—a double-blind PET study of schizophrenic patients. Biol Psychiatry 1993; 33:227–235Crossref, Medline, Google Scholar
13. Brucke T, Roth J, Podreka I, Strobl R, Wenger S, Asenbaum S: Striatal dopamine D2-receptor blockade by typical and atypical neuroleptics (letter). Lancet 1992; 339:497Crossref, Medline, Google Scholar
14. Pilowsky LS, Costa DC, Ell PJ, Murray RM, Verhoeff NP, Kerwin RW: Antipsychotic medication, D2 dopamine receptor blockade and clinical response: a 123I IBZM SPET (single photon emission tomography) study. Psychol Med 1993; 23:791–797Crossref, Medline, Google Scholar
15. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician Version. Washington, DC, American Psychiatric Press, 1996Google Scholar
16. Kapur S, Zipursky R, Roy P, Jones C, Remington G, Reed K, Houle S: The relationship between D-2 receptor occupancy and plasma levels on low dose oral haloperidol: a PET study. Psychopharmacology (Berl) 1997; 131:148–152Crossref, Medline, Google Scholar
17. Nyberg S, Farde L, Halldin C, Dahl M-L, Bertilsson L: D2 dopamine receptor occupancy during low-dose treatment with haloperidol decanoate. Am J Psychiatry 1995; 152:173–178Link, Google Scholar
18. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218–222Google Scholar
19. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
20. Chouinard G, Ross-Chouinard A, Annable L, Jones BD: Extrapyramidal Symptom Rating Scale (ESRS) (abstract). Can J Neurol Sci 1980; 7:233Google Scholar
21. Barnes TRE: A rating scale for drug-induced akathisia. Br J Psychiatry 1989; 154:672–676Crossref, Medline, Google Scholar
22. Kapur S, Zipursky RB, Jones C, Remington GJ, Wilson AA, DaSilva J, Houle S: The D-2 receptor occupancy profile of loxapine determined using PET. Neuropsychopharmacology 1996; 15:562–566Crossref, Medline, Google Scholar
23. Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC: Automated image registration, I: general methods and intrasubject, intramodality validation. J Comput Assist Tomogr 1998; 22:139–152Crossref, Medline, Google Scholar
24. Woods RP, Grafton ST, Watson JDG, Sicotte NL, Mazziotta JC: Automated image registration, II: intersubject validation of linear and nonlinear models. J Comput Assist Tomogr 1998; 22:153–165Crossref, Medline, Google Scholar
25. Farde L, Hall H, Ehrin E, Sedvall G: Quantitative analysis of D2 dopamine receptor binding in the living brain by PET. Science 1986; 231:258–261Crossref, Medline, Google Scholar
26. Farde L, Wiesel FA, Stone-Elander S, Halldin C, Nordstrom AL, Hall H, Sedvall G: D2 dopamine receptors in neuroleptic-naive schizophrenic patients. Arch Gen Psychiatry 1990; 47:213–219Crossref, Medline, Google Scholar
27. Hietala J, Syvalahti E, Vuorio K, Nagren K, Lehikoinen P, Ruotsalainen U, Rakkolainen V, Lehtinen V, Wegelius U: Striatal D2 dopamine receptor characteristics in neuroleptic-naive schizophrenic patients studied with positron emission tomography. Arch Gen Psychiatry 1994; 51:116–123Crossref, Medline, Google Scholar
28. The Scottish First Episode Schizophrenia Study, II: treatment: pimozide versus flupenthixol: the Scottish Schizophrenia Research Group. Br J Psychiatry 1987; 150:334–338Crossref, Medline, Google Scholar
29. Lieberman J, Jody D, Geisler S, Vital-Herne J, Alvir JM, Walsleben J, Woerner MG: Treatment outcome of first episode schizophrenia. Psychopharmacol Bull 1989; 25:92–96Medline, Google Scholar
30. Remington G, Kapur S, Zipursky RB: Pharmacotherapy of first-episode schizophrenia. Br J Psychiatry Suppl 1998; 172:66–70Crossref, Medline, Google Scholar
31. McEvoy JP, Hogarty GE, Steingard S: Optimal dose of neuroleptic in acute schizophrenia: a controlled study of neuroleptic threshold and higher haloperidol dose. Arch Gen Psychiatry 1991; 48:739–745Crossref, Medline, Google Scholar
32. Ahang-Wong J, Zipursky RB, Beiser M, Bean G: Optimal haloperidol dosage for first-episode psychosis. Can J Psychiatry 1999; 44:164–167Crossref, Medline, Google Scholar
33. Scherer J, Tatsch K, Schwarz J, Oertel WH, Konjarczyk M, Albus M: D2-dopamine receptor occupancy differs between patients with and without extrapyramidal side effects. Acta Psychiatr Scand 1994; 90:266–268Crossref, Medline, Google Scholar
34. Marek KL, Seibyl JP, Zoghbi SS, Zea-Ponce Y, Baldwin RM, Fussell B, Charney DS, van Dyck C, Hoffer PB, Innis RP: [123I] beta-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson’s disease. Neurology 1996; 46:231–237Crossref, Medline, Google Scholar
35. Frost JJ, Rosier AJ, Reich SG, Smith JS, Ehlers MD, Snyder SH, Ravert HT, Dannals RF: Positron emission tomographic imaging of the dopamine transporter with 11C-WIN 35,428 reveals marked declines in mild Parkinson’s disease. Ann Neurol 1993; 34:423–431Crossref, Medline, Google Scholar
36. Schlegel S, Schlosser R, Hiemke C, Nickel O, Bockisch A, Hahn K: Prolactin plasma levels and D-2-dopamine receptor occupancy measured with IBZM-SPECT. Psychopharmacology (Berl) 1996; 124:285–287Crossref, Medline, Google Scholar
37. Davis KL, Kahn RS, Ko G, Davidson M: Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 1991; 148:1474–1486Google Scholar
38. Farde L, Suhara T, Nyberg S, Karlsson P, Nakashima Y, Hietala J, Halldin C: A PET study of [C-11]FLB 457 binding to extrastriatal D-2-dopamine receptors in healthy subjects and antipsychotic drug-treated patients. Psychopharmacology (Berl) 1997; 133:396–404Crossref, Medline, Google Scholar
39. Hietala J, Vilkman H, Syvã«¡hti E, Nagren K: Extrastriatal and striatal dopamine receptor occupancy by haloperidol and clozapine measured with PET, in Abstracts of the XXIst CINP Congress. Glasgow, UK, Collegium Internationale Neuro-Psychopharmacologicum, 1998, SW0104Google Scholar
40. Schotte A, Janssen PFM, Gommeren W, Luyten WHML, VanGompel P, Lesage AS, DeLoore K, Leysen JE: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996; 124:57–73Crossref, Medline, Google Scholar
41. Wadenberg ML, Salmi P, Jimenez P, Svensson T, Ahlenius S: Enhancement of antipsychotic-like properties of the dopamine D2 receptor antagonist, raclopride, by the additional treatment with the 5-HT2 receptor blocking agent, ritanserin, in the rat. Eur Neuropsychopharmacol 1996; 6:305–310Crossref, Medline, Google Scholar
42. Prinssen EP, Ellenbroek BA, Cools AR: Combined antagonism of adrenoceptors and dopamine and 5-HT receptors underlies the atypical profile of clozapine. Eur J Pharmacol 1994; 262:167–170Crossref, Medline, Google Scholar
43. Kapur S, Remington G: Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatry 1996; 153:466–476Link, Google Scholar
44. Gerlach J, Peacock L: New antipsychotics: the present status. Int Clin Psychopharmacol 1995; 10:39–48Medline, Google Scholar
45. Meltzer HY, Matsubara S, Lee JC: The ratios of serotonin-2 and dopamine-2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol Bull 1989; 25:390–392Medline, Google Scholar
46. Nordstr�-L, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G: D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry 1995; 152:1444–1449Google Scholar
47. Pilowsky LS, Costa DC, Ell PJ, Murray RM, Verhoeff NPLG, Kerwin RW: Clozapine, single photon emission tomography, and the D2 dopamine receptor blockade hypothesis of schizophrenia. Lancet 1992; 340:199–202Crossref, Medline, Google Scholar
48. Kapur S, Zipursky RB, Remington G: Comparison and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry 1999; 156:286–293Abstract, Google Scholar



