Smoking Habits, Current Symptoms, and Premorbid Characteristics of Schizophrenic Patients in Nithsdale, Scotland
Abstract
OBJECTIVE: Previous studies of smoking habits of schizophrenic patients have found rates as high as 88%. The authors report the smoking habits of all known schizophrenic patients within a discrete geographical area and compare them with the smoking habits of a general population sample. METHOD: All known schizophrenic patients in Nithsdale in South-West Scotland (N=168) were invited to complete a questionnaire on smoking habits. Also assessed were mental state, drug-related side effects, and premorbid childhood personality and social adjustment. RESULTS: One hundred thirty-five of the 168 patients returned the questionnaires. The rate of smoking among the patients was 58% (N=78), compared with 28% in the general population. Sixty-eight percent of the patients who smoked (N=53) had 25 or more cigarettes per day. The mean age at starting smoking was 17 years in both patients and normal subjects. Ninety percent of the patients who smoked (N=70) started smoking before the onset of schizophrenia. Patients who smoked were younger than nonsmokers, and more of them were male. They had had more hospitalizations, and more were in contact with psychiatric services. More were receiving intramuscular antipsychotic medication. Smokers had poorer childhood social adjustment. Among the female patients, there was a positive correlation between age at starting smoking and age at onset of schizophrenia. CONCLUSIONS: The rate of smoking and level of nicotine addiction are greater in schizophrenic patients than in the general population. Smoking may be a marker for the neurodevelopmental form of the illness and may be another environmental risk factor for schizophrenia in vulnerable individuals.
Cigarette smoking is frequently observed in schizophrenic patients (1–5). One study has reported the rate to be as high as 88% (1), nearly three times the rate in the general population and higher than the elevated rates of smoking among patients with other psychiatric illnesses.
Why schizophrenic patients smoke at high rates remains elusive (6–8). There are three main possibilities. First, the illness itself may lead patients to smoke. Possibly, patients with schizophrenia self-medicate with nicotine to alleviate both positive and negative symptoms as well as to improve cognition (6). These putative beneficial effects of nicotine may be mediated through the regulation of a dysfunctional mesolimbic dopamine system (9). It has been reported that there is a worsening of psychotic symptoms on stopping smoking and subsequent nicotine withdrawal (10, 11). Second, smoking may be an etiological risk factor in schizophrenia. It may be that repeated activation by nicotine of the mesolimbic system over a lengthy period of time precipitates the onset of schizophrenia in vulnerable individuals. Third, genetic or environmental factors might predispose individuals both to develop schizophrenia and to start smoking. Much work in the genetics of both schizophrenia (12) and nicotine addiction (13) has focused on dopamine receptors. Possible pathophysiological links between smoking and schizophrenia have been the subject of a recent review (14).
A drawback to previous studies is that all have reported on restricted samples of schizophrenic patients, for example, inpatients (5) and outpatients (1, 15). None has reported on the smoking habits of all known schizophrenic patients in a discrete geographical area and compared them with the smoking habits of a local general population sample. In the present study we report on the smoking habits of schizophrenic patients living in Nithsdale, South-West Scotland, and compare them with the smoking habits of samples from the general population of South-West Scotland and from a national population. We also report clinical differences between smoking and nonsmoking schizophrenic patients.
METHOD
Setting
Nithsdale is a well-defined area in the Dumfries and Galloway Region, South-West Scotland, United Kingdom. It is largely rural, covering 550 square miles with a population of 57,000. All psychiatric care is provided by Crichton Royal Hospital in Dumfries and its associated hospital and community services.
Patient Identification
The identification of patients has been described in detail elsewhere (16–18). Briefly, there have been regular censuses of patients with schizophrenia in Nithsdale since 1981. Patients in the present study were identified in the 1996 census (April 1, 1996). They included all inpatients, day patients, and outpatients on the census date known to Crichton Royal Hospital who had a diagnosis of schizophrenia and whose home address was in Nithsdale. General practitioners in Nithsdale were given a list of schizophrenic patients in their practice known to the hospital and asked to update it. All general practitioners replied. In addition, community psychiatric nurses, voluntary agencies, and social workers were asked to identify any other schizophrenic patients. Over the years, this “key informant” method has allowed us to identify, trace, and interview schizophrenic patients living in Nithsdale.
Because part of the study was a replication of the initial census undertaken in 1981 (16), patients were defined as having schizophrenia if they fulfilled ICD-9 criteria (19). This is a broad definition; however, a 1997 Nithsdale study (17) showed that 87% of patients with ICD-9 schizophrenia also fulfilled OPCRIT-derived (20) DSM-III-R criteria.
The 1996 census identified 168 patients with an ICD-9 diagnosis of schizophrenia (2.92/1,000 general population). Eighty-seven (52%) were male, mean age=46 years (SD=15), mean length of illness=19 years (SD=13); 81 (48%) were female, mean age=55 years (SD=17), mean length of illness=24 years (SD=14). On the census date, 29 (17%) were inpatients, 45 (27%) day patients, 50 (30%) outpatients, 22 (13%) were receiving mainly community psychiatric nurse support, and 22 (13%) were in contact only with their general practitioner.
Assessment
Patients were asked to complete a questionnaire about their smoking habits; the same questionnaire was used in the Health and Lifestyle Survey of the general population in South-West Scotland (21, 22). Patients completed this self-report questionnaire under supervision of the examining psychiatrist or nurse.
As part of the comparison of the patients identified in 1981 with those identified in the 1996 census, assessment of patients’ mental state was made by using the Manchester Scale (23). On this scale, each of nine symptoms is scored from 0 (absent) to 4 (severe). A symptom was defined as present if it had a score of 2 (mild) or higher.
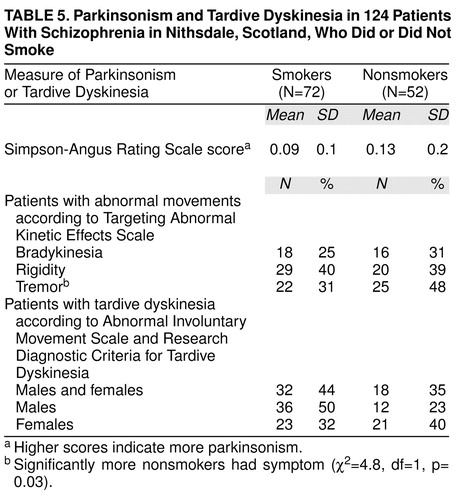
Side effects of medication were also assessed: parkinsonism was explored with the Simpson-Angus Rating Scale (24) and the Targeting Abnormal Kinetic Effects Scale (25) and dyskinesia with the Abnormal Involuntary Movement Scale (AIMS) (26) and Research Diagnostic Criteria for Tardive Dyskinesia (27).
The following demographic and clinical information was known: age at onset of illness (as estimated from first contact with psychiatric services or hospital admission), number of hospital admissions, and current medication.
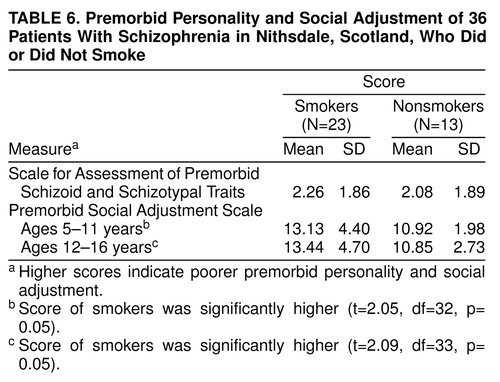
Measures of premorbid social adjustment and personality were available for 36 patients who completed the smoking questionnaire because in a previous study their mothers had been interviewed about their children’s premorbid personality and social adjustment (28). Premorbid personality was assessed by using the Scale for Assessment of Premorbid Schizoid and Schizotypal Traits (29), which examines behavior between 5 and 16 years of age. Premorbid social adjustment was assessed by using the Premorbid Social Adjustment Scale (29), which assesses two age periods, 5–11 years and 12–16 years.
Statistical Analysis
Differences in proportions were measured by using the chi-square test. Normally distributed data are presented with means and standard deviations, and significance was tested by Student’s t test. Correlations were determined by using Pearson’s correlation. Tests were two-tailed.
RESULTS
The smoking questionnaire was returned by 135 (80%) of the 168 patients; 73 (54%) of the respondents were male and 62 (46%) were female. However, seven patients, all smokers, did not complete the questionnaire fully; therefore, numbers vary slightly in different categories.
Comparison With Smoking Habits in the General Population
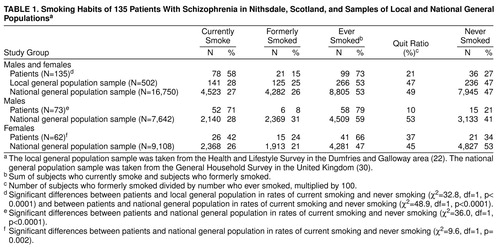
Table 1 shows the smoking habits of the 135 schizophrenic patients, a 1995 general population sample in the same area of South-West Scotland (Dumfries and Galloway) (22), and a 1994 general population sample in the United Kingdom (30). More patients than subjects in the two general population samples reported that they currently smoked, and more had smoked at some time in their lives. The quit ratio (the number who gave up smoking divided by the number who were currently smoking plus those who had given up smoking) was lower in patients.
Seventy-one percent of male and 42% of female patients were current smokers compared with 28% of males and 26% of females in the general population. The quit ratio among male patients was lower than that in the general population, but among female patients and females in the general population it was broadly similar (table 1). Although there were similar numbers of male and female smokers in the general population, more male than female patients smoked (χ2=11.8, df=1, p=0.001). More female than male patients had given up smoking (χ2=6.5, df=1, p=0.01). The mean age at starting smoking was 17 years (SD=5) in patients and normal subjects (22); there were no between-sex differences.
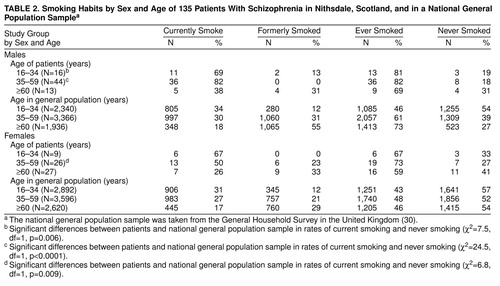
Table 2 shows the smoking habits by age and sex for patients and for the U.K. general population sample. Within each of the three age bands and for both sexes more patients than normal subjects were current smokers. These differences achieved statistical significance in males aged 16–34 and 35–59 and in females aged 35–59.
More patients who smoked were classed as heavy smokers; 53 (68%) of the patients who smoked were smoking 25 or more cigarettes daily (37 [71%] of male and 16 [63%] of female smokers), compared with 11% of the smokers in the local general population (21) (χ2=15.6, df=1, p< 0.0001).
Twenty-six (33%) of the patients who smoked reported that they wanted to give up smoking (22 [42%] of male and four [15%] of female smokers), compared with 60% of smokers in the local general population (22).
Smoking Among the Schizophrenic Patients
Sixty-three percent [N=15] of inpatients, 62% [N=21] of day patients, 65% [N=26] of outpatients, and and 25% [N=9] of community-nurse-supported patients and patients only in contact with their general practitioner were smokers (χ2=8.4, df=3, p=0.04).
Seventy (90%) of the patients had started to smoke before the onset of their schizophrenic illness; the mean age at starting smoking preceded the mean age at onset of illness by 11 years (SD=10). There was a positive correlation between age at starting smoking and age at onset of illness in all patients (r=0.29, df=69, p=0.04). This relationship remained significant for female patients (r=0.47, df=24, p=0.02) but not for male patients (r=0.08, df=47, p=0.58).
Smokers and Nonsmokers Among the Schizophrenic Patients
Among the schizophrenic patients, there were more males among the 78 smokers (N=52 [67%]) than among the 36 nonsmokers (N=15 [42%]) (χ2=11.8, df=1, p=0.001). Smokers were younger (mean age=45, SD=13) than nonsmokers (mean age=56, SD=18) (t=3.80, df=131, p<0.001). The age at onset of illness was lower in smokers (mean=27, SD=10; males mean=27, SD=9; females mean=28, SD=11) than in nonsmokers (mean 31=years, SD=13; males mean=30, SD=13; females mean=32, SD=13), but these differences were not statistically significant. Smokers had had more hospital admissions (mean=7.5, SD=7) than nonsmokers (mean=5, SD=5) (t=2.09, df=131, p=0.04). Both male (mean=7, SD=6) and female (mean=8.4, SD=8.6) smokers had had more admissions than male (mean=4.6, SD=3.4) and female (mean=5.5, SD=6.2) nonsmokers, but the difference was statistically significant only in males (t=2.17, df=70, p=0.03, versus t=1.48, df=59, p=0.13).
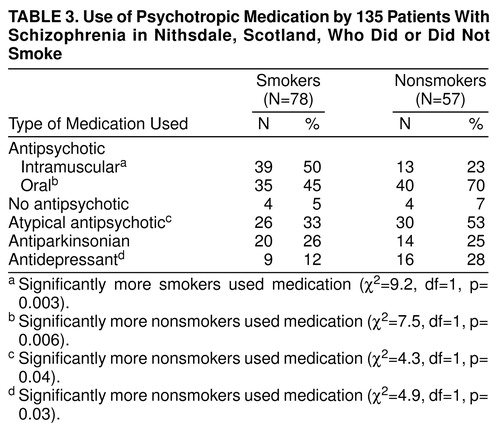
Table 3 shows the relationship between smoking and medication use among the schizophrenic patients. Smokers were significantly more likely to be receiving intramuscular antipsychotic medication and less likely to be receiving oral and atypical antipsychotics and antidepressants.
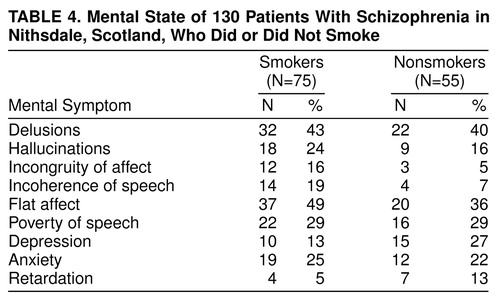
Table 4 shows the mental state findings for the smoking and nonsmoking schizophrenic patients. There were no significant between-group differences in the individual symptoms rated.
Table 5 shows the drug side effect profile for smoking and nonsmoking schizophrenic patients. Significantly more nonsmokers exhibited tremor. Twice as many male smokers had tardive dyskinesia than nonsmoking males, but the difference was not statistically significant.
The mean scores on the Premorbid Social Adjustment Scale for ages 5–11 and for ages 12–16 and the mean scores on the Scale for Assessment of Premorbid Schizoid and Schizotypal Traits for smoking and nonsmoking schizophrenic patients are shown in table 6. Smokers scored significantly higher than nonsmokers on the Premorbid Social Adjustment Scale for ages 5–11 and for ages 12–16, which indicates they had a poorer premorbid social adjustment at both ages 5–11 and ages 12–16.
DISCUSSION
We have found that the rate of cigarette smoking in schizophrenic patients from a discrete geographical area is more than twice that of a local population sample (58% versus 28%). The rate of 58% is lower than that found in other studies, but these studies looked at samples of patients who were more likely to be young and male (1–5), who have higher rates of smoking. To our knowledge, our study is the first to examine the smoking habits of patients with schizophrenia independent of selection bias. The differences between smoking rates in patients and in the general population narrow when we compare subjects who have smoked at some time in their lives (73% versus 53%). However, we found that many fewer schizophrenic patients than subjects in the general population are able to quit, especially among male patients (10% versus 53%).
Within the general population, there are groups where the smoking rate is high. For example, in 1991, the National Child Development Survey in the United Kingdom (31) found that the rate of smoking in 33-year-old men who had no academic qualifications and lived in subsidized housing was 67%. This is a rate similar to that found in our young male patients, many of whom also had no academic qualifications and lived in subsidized housing. However, we believe our comparison with a general population sample is more valid because at the time our patients first started smoking (in their teenage years), they were distributed widely through a variety of educational and social backgrounds.
The rate of smoking in the general population sample, 28%, is similar to that found in other countries in the developed world. For example, in 1989, in six countries (Australia, Canada, Norway, Sweden, the United Kingdom, and the United States) the rate in males ranged from 24% to 41%, and in females, 27% to 33% (32). There is little information about the rates in developing countries.
Earlier in this article, we considered three possibilities to explain the association between high rates of smoking and schizophrenia. In the Nithsdale patients, it cannot be the symptoms of the illness itself or the effects of “institutionalization” that drove patients to smoke because 90% of the patients started smoking before the first episode of illness. However, patients who smoke were more poorly socially adjusted in their childhood than nonsmokers. Perhaps premorbid traits, rather than florid symptoms, lead schizophrenic individuals to smoke. Poor social adjustment in childhood is a feature of the neurodevelopmental form of schizophrenia (33); other features include male sex, early age at onset of illness, and a more severe form of illness (33). These features were found in our smoking patients, whose severity of illness was measured by contact with psychiatric services and numbers of admissions.
These results suggest that either premorbid characteristics typically found in neurodevelopmental schizophrenia lead to smoking or genetic and environmental factors lead both to neurodevelopmental schizophrenia and smoking. However, a third possibility is raised by our finding in women that the earlier the age at starting smoking, the earlier was the onset of schizophrenia. Might heavy nicotine consumption over a prolonged period of time, especially in vulnerable individuals, be another environmental risk factor in schizophrenia, through its action on the mesolimbic dopamine system?
The other principal difference between patients and the general population sample was that the level of addiction among patients was much higher: 68% of the smoking patients smoked 25 or more cigarettes per day, compared with 11% of general population smokers. There are several possible explanations for why patients smoke so much. Nicotine, like other drugs of addiction such as cocaine and amphetamine, activates the mesolimbic dopamine system (34); this appears to be of critical importance for the reinforcing and reward properties of the drug (35). However, patients were also receiving antipsychotic drugs, which produce marked dopamine receptor blockade. Possibly a very high level of smoking is necessary to overcome this blockade and produce the reward effects. It has been shown that, compared with baseline, patients with chronic schizophrenia smoke more after starting haloperidol (36). It is also possible that schizophrenic patients smoke at high levels to self-medicate both positive and negative symptoms, although in our patients there was no difference between smokers and nonsmokers in the level of such symptoms. Because we believe that our smoking patients were drawn from a group that was more severely ill, however, we cannot rule out the possibility that nicotine is helping the patients’ mental states.
There were differences between smoking and nonsmoking patients in antipsychotic medication patterns. More smokers were receiving intramuscular antipsychotic medication and fewer were receiving oral and atypical antipsychotics. We find this hard to explain, but once again we could postulate that smoking is a marker for neurodevelopmental schizophrenia; schizophrenic patients who are more severely ill and more difficult to manage may be more likely to receive intramuscular drugs in the United Kingdom. More nonsmokers than smokers were receiving antidepressant medication. Nicotine’s positive effects on enhancing mood have been well described in the general population, and it may be that our smoking patients were successfully modifying their mood state with their heavy and regular consumption of nicotine.
Although more nonsmokers than smokers were receiving atypical antipsychotics, said to produce less extrapyramidal symptoms (37), side effects were largely similar for both groups. Several studies have reported a lower rate of drug-induced extrapyramidal side effects in smokers (2, 10, 38), others have reported no between-group differences (3, 39, 40), but none has reported higher rates; this includes a study finding that smokers on average were receiving twice the dose of neuroleptics of nonsmokers (2). Smoking increases neuroleptic metabolism by inducing hepatic microsomal enzymes (41).
Although the difference between smokers and nonsmokers was not statistically significant, more male smokers (50%) had tardive dyskinesia. In three previous studies, two (39, 40) found no relationship between smoking and tardive dyskinesia and one (42) found tardive dyskinesia to be more common among smokers. A study of smoking in a population of normal men, few of whom had been exposed to neuroleptics (43), found that 21% of men who smoked 20 or more cigarettes per day had dyskinesia. Cigarette smoke is rich in free radicals, which may cause structural damage to catecholaminergic neurons and, thus, tardive dyskinesia (44).
It is clear from our study and previous work that heavy cigarette smoking and severe nicotine addiction are intimately associated with the schizophrenic illness. More patients with schizophrenia start to smoke, and few give up smoking. The patients’ high level of addiction almost certainly contributes to their reduced life expectancy (45). Schizophrenic patients who smoke are an even more disadvantaged group within a very vulnerable population.
Presented at the annual meeting of the Royal College of Psychiatrists, Belfast, Northern Ireland, June 22–25, 1998, and at the 21st Collegium Internationale Neuro-Psychopharmacologicum Congress, Glasgow, Scotland, July 12–16, 1998. Received Dec. 11, 1998; revision received March 23, 1999; accepted March 26, 1999. From the Department of Psychological Medicine, Academic Centre, Gartnavel Royal Hospital, Glasgow, Scotland; and the Department of Clinical Research, Crichton Royal Hospital, Dumfries, Scotland. Address reprint requests to Dr. McCreadie, Department of Clinical Research, Crichton Royal Hospital, Bankend Road, Dumfries DG1 4TG, U.K.; [email protected] (e-mail)
 |
 |
 |
 |
 |
 |
1. Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA: Prevalence of smoking among psychiatric outpatients. Am J Psychiatry 1986; 143:993–997Link, Google Scholar
2. Goff DC, Henderson DC, Amico E: Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Am J Psychiatry 1992; 149:1189–1194Google Scholar
3. Chong SA, Choo HL: Smoking among Chinese patients with schizophrenia. Aust NZ J Psychiatry 1996; 30:350–353Crossref, Medline, Google Scholar
4. Tanskanen A, Viinamaki H, Koivumaa-Honkanen H-T, Jaaskelainen J, Lehtonen J, Heli-Tuulie KH: Smoking among psychiatric patients. Eur J Psychiatry 1997; 11:179–188Google Scholar
5. de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM: Schizophrenia and smoking: an epidemiological survey in a state hospital. Am J Psychiatry 1995; 152:453–455Link, Google Scholar
6. Lavin MR, Siris SG, Mason SE: What is the clinical importance of cigarette smoking in schizophrenia? Am J Addict 1996; 5:189–208Google Scholar
7. Lohr JB, Flynn K: Smoking and schizophrenia. Schizophr Res 1992; 8:93–102Crossref, Medline, Google Scholar
8. Glassman AH: Cigarette smoking: implications for psychiatric illness. Am J Psychiatry 1993; 150:546–553Link, Google Scholar
9. Olincy A, Young DA, Freedman R: Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry 1997; 42:1–5Crossref, Medline, Google Scholar
10. Hartman N, Leong GB, Glynn SM, Wilkins JN, Jarvik ME: Transdermal nicotine and smoking behavior in psychiatric patients. Am J Psychiatry 1991; 148:374–375Link, Google Scholar
11. Dalack GW, Meador-Woodruff JH: Smoking, smoking withdrawal and schizophrenia: case reports and a review of the literature. Schizophr Res 1996; 22:133–141Crossref, Medline, Google Scholar
12. Maier W, Schwab S: Molecular genetics of schizophrenia. Current Opinion in Psychiatry 1998; 11:19–25Crossref, Google Scholar
13. Clarke PBS: Tobacco smoking, genes and dopamine. Lancet 1998; 353:84–85Crossref, Google Scholar
14. Dalack GW, Healy DJ, Meador-Woodruff JH: Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am J Psychiatry 1998; 155:1490–1501Google Scholar
15. Ziedonis DM, Kosten TR, Glazer WM, Frances RJ: Nicotine dependence and schizophrenia. Hosp Community Psychiatry 1994; 45:204–206Abstract, Google Scholar
16. McCreadie RG: The Nithsdale schizophrenia survey, I: psychiatric and social handicaps. Br J Psychiatry 1982; 140:582–586Crossref, Medline, Google Scholar
17. McCreadie RG, Leese M, Tilak-Singh D, Loftus L, MacEwan T, Thornicroft G: Nithsdale, Nunhead and Norwood: similarities and differences in prevalence of schizophrenia and utilisation of services in rural and urban areas. Br J Psychiatry 1997; 170:31–36Crossref, Medline, Google Scholar
18. Kelly C, McCreadie RG, MacEwan T, Carey S: Nithsdale schizophrenia surveys, 17: fifteen year review. Br J Psychiatry 1998; 172:513–517Crossref, Medline, Google Scholar
19. World Health Organization: Mental Disorders: Glossary and Guide to Their Classification in Accordance With Ninth Revision of the International Classification of Diseases. Geneva, WHO, 1978Google Scholar
20. McGuffin P, Farmer AE, Harvey I: A polydiagnostic application of operational criteria in studies of psychotic illness: development and reliability of the OPCRIT system. Arch Gen Psychiatry 1991; 48:764–770Crossref, Medline, Google Scholar
21. Dumfries and Galloway Health and Lifestyle Survey 1990: A Picture of Health? Dumfries, Scotland, Dumfries and Galloway Health Board, 1990Google Scholar
22. Waldron G, Chalmers J, Bone A, Strickland J, Johnstone D: Health and Lifestyles in Dumfries and Galloway in 1995. Dumfries, Scotland, Dumfries and Galloway Health Board, 1995Google Scholar
23. Krawiecka M, Goldberg D, Vaughan M: A standardised psychiatric assessment scale for rating chronic psychotic patients. Acta Psychiatr Scand 1997; 55:299–308Crossref, Google Scholar
24. Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11–19Crossref, Medline, Google Scholar
25. Wojcik JD, Gelenberg AJ, La Brie RA, Mieske M: Prevalence of tardive dyskinesia in an outpatient population. Compr Psychiatry 1980; 21:370–380Crossref, Medline, Google Scholar
26. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 534–537Google Scholar
27. Schooler NR, Kane JM: Research diagnoses for tardive dyskinesia (letter). Arch Gen Psychiatry 1982; 39:486–487Medline, Google Scholar
28. McCreadie RG, Connolly MA, Williamson DJ, Athawes RWB, Tilak-Singh D: The Nithsdale schizophrenia surveys, XII: “neurodevelopmental” schizophrenia: a search for clinical correlates and putative aetiologic factors. Br J Psychiatry 1994; 165:340–346Crossref, Medline, Google Scholar
29. Foerster A, Lewis S, Owen M, Murray R: Premorbid adjustment and personality in psychosis: effects of sex and diagnosis. Br J Psychiatry 1991; 158:171–176Crossref, Medline, Google Scholar
30. Bennett N, Jarvis L, Rowlands O, Singleton N, Haselden L: Living in Britain: Results From the 1994 General Household Survey. London, Office of Population Censuses and Surveys, Her Majesty’s Stationery Office, 1996, pp 81–112Google Scholar
31. March A, McKay S: Poor Smokers. London, Policy Studies Institute, 1994, pp 42–47Google Scholar
32. Fiore MC, Newcomb P, McBride P: Natural history and epidemiology of tobacco use and addiction, in Nicotine Addiction: Principles and Management. Edited by Orleans CT, Slade J. New York, Oxford University Press, 1993, pp 89–104Google Scholar
33. Murray RM, O’Callaghan E: Neurodevelopmental schizophrenia. Schizophrenia Monitor 1991; 1:1–3Google Scholar
34. Pontieri FE, Tanda G, Orzi F, DiChiara G: Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 1996; 382:255–256Crossref, Medline, Google Scholar
35. Iversen LL: Smoking…harmful to the brain. Nature 1996; 382:206–207Crossref, Medline, Google Scholar
36. McEvoy JP, Freudenreich O, Levin ED, Rose JE: Haloperidol increases smoking in patients with schizophrenia. Psychopharmacology (Berl) 1995; 119:124–126Crossref, Medline, Google Scholar
37. Kerwin RW: The new atypical antipsychotics. Br J Psychiatry 1994; 164:141–148Crossref, Medline, Google Scholar
38. Decina P, Caracci G, Sandik R, Berman W, Mukherjee S, Scapicchio PL: Cigarette smoking and neuroleptic-induced parkinsonism. Biol Psychiatry 1990; 28:502–508Crossref, Medline, Google Scholar
39. Chiles JA, Cohen S, Roland M, Wright R: Smoking and schizophrenic psychopathology. Am J Addict 1993; 2:315–319Crossref, Google Scholar
40. Menza MA, Grossman N, Van Horn M, Cody R, Forman N: Smoking and movement disorders in psychiatric patients. Biol Psychiatry 1991; 30:109–115Crossref, Medline, Google Scholar
41. Salokangas RKR, Saarijarvi S, Taiminen T, Lehto H, Niemi H, Ahola V, Syvalahti E: Effect of smoking on neuroleptics in schizophrenia. Schizophr Res 1997; 23:55–60Crossref, Medline, Google Scholar
42. Yassa R, Lal S, Korpassy A, Ally J: Nicotine exposure and tardive dyskinesia. Biol Psychiatry 1987; 22:67–72Crossref, Medline, Google Scholar
43. Nilsson A, Waller L, Rosengren A, Adlerberth A, Wilhelmsen L: Cigarette smoking is associated with abnormal involuntary movements in the general male population. Biol Psychiatry 1997; 41:717–723Crossref, Medline, Google Scholar
44. Church DF, Pryor WA: Free radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect 1985; 64:111–126Crossref, Medline, Google Scholar
45. Mortensen PB, Juel K: Mortality and causes of death in first admitted schizophrenic patients. Br J Psychiatry 1993; 163:183–189Crossref, Medline, Google Scholar



